Professional Documents
Culture Documents
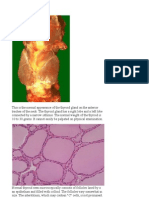
Chapter 15 Lung Pathology Ernie
Chapter 15 Lung Pathology Ernie
Uploaded by
Zandra Lyn AlundayOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 15 Lung Pathology Ernie
Chapter 15 Lung Pathology Ernie
Uploaded by
Zandra Lyn AlundayCopyright:
Available Formats
Chapter 15 – The Lung The Lung 3.
Vascular anomalies
4. Congenital lobar overinflation (emphysema)
1. Congenital Anomalies Developmentally: outgrowth from the ventral wall of
5. Foregut cysts
the foregut
2. Atelectasis (Collapse) o Arise from an abnormal detachment of primitive
Trachea develops two lateral outpocketings – the lung
foregut
3. Pulmonary Edema buds
o Most often located in the hilum or middle
R lung = 3 lobes
4. Acute Lung Injury and Acute Respiratory Distress mediastinum
L lung = 2 lobes
Syndrome (Diffuse Alveolar Damage) o Classified as bronchogenic (most common),
Aspirated foreign materials tend to enter the right lung
esophageal, or enteric
a. ACUTE INTERSTITIAL PNEUMONIA R main stem bronchus is more vertical and more
o LE: columnar epithelium with squamous
directly in line with the trachea
5. Obstructive versus Restrictive Pulmonary Diseases Bronchial mucosa – contains neurosecretory-type
metaplasia
6. Congenital pulmonary airway malformation
6. Obstructive Pulmonary Diseases granules [serotonin, calcitonin, and gastrin-releasing
7. Pulmonary sequestrations
a. EMPHYSEMA peptide (bombesin)]
o Def: Presence of a discrete mass of lung tissue
The entire respiratory tree (larynx, trachea, and
b. CHRONIC BRONCHITIS without normal connection to the airway system
bronchioles) lined by pseudostratified, tall, columnar,
c. ASTHMA o Blood supply: aorta or its branches
ciliated epithelial cells
o Extralobar sequestrations external to the
d. BRONCHIECTASIS Microscopic structure of the alveolar walls
lung and may be located anywhere in the thorax
7. Chronic Diffuse Interstitial (Restrictive) Diseases o Capillary endothelium
or mediastinum
o Basement membrane and surrounding
a. FIBROSING DISEASES o Intralobar sequestrations within the lung,
interstitial tissue
b. PULMONARY EOSINOPHILIA usually in older children and are often
o Alveolar epithelium
associated with recurrent localized infection or
c. SMOKING-RELATED INTERSTITIAL DISEASES o Alveolar macrophages
bronchiectasis
d. PULMONARY ALVEOLAR PROTEINOSIS
Alveolar epithelium
8. Diseases of Vascular Origin A continuous layer of 2 cell types
a. PULMONARY EMBOLISM, HEMORRHAGE, AND Type I pneumocytes - covering 95% of the alveolar 2. Atelectasis (Collapse)
INFARCTION surface D/t incomplete expansion of the lungs (neonatal
b. PULMONARY HYPERTENSION Type II pneumocytes atelectasis) or to the collapse of previously inflated
o Synthesize surfactant lung producing airless pulmonary parenchyma
c. DIFFUSE PULMONARY HEMORRHAGE o Contained in osmiophilic lamellar bodies May be divided into:
SYNDROMES o Involved in the repair of alveolar epithelium o Resorption (or obstruction)
9. Pulmonary Infections through their ability to give rise to type I cells o Compression
a. COMMUNITY-ACQUIRED ACUTE PNEUMONIAS o Contraction atelectasis
*Pores of Kohn - permit the passage of bacteria and
b. COMMUNITY-ACQUIRED ATYPICAL (VIRAL AND exudate between adjacent alveoli
Resorption atelectasis
MYCOPLASMAL) PNEUMONIAS Consequence of complete airway obstruction
c. HOSPITAL-ACQUIRED PNEUMONIA
d. ASPIRATION PNEUMONIA 1. Congenital Anomalies Leads to resorption of the oxygen trapped in the
dependent alveoli, without impairment of blood flow
Developmental defects of the lung include the following: through the affected alveolar walls
e. LUNG ABSCESS
1. Agenesis or hypoplasia of both lungs, one lung, or Mediastinum shifts toward the atelectatic lung
f. CHRONIC PNEUMONIA single lobes Caused principally by excessive secretions
g. PNEUMONIA IN THE IMMUNOCOMPROMISED o Decreased weight, volume, & acini Most often found in: bronchial asthma, chronic
HOST disproportional to the body weight and bronchitis, bronchiectasis, postoperative states,
gestational age aspiration of foreign bodies, or bronchial neoplasm
h. PULMONARY DISEASE IN HUMAN
o Etiology: abnormalities that compress the
IMMUNODEFICIENCY VIRUS INFECTION lung(s) or impede normal lung expansion in Compression atelectasis
10. Lung Transplantation utero (congenital diaphragmatic hernia and The pleural cavity is partially or completely filled by fluid
oligohydramnions) exudate, tumor, blood, tension pneumothorax
11. Tumors 2. Tracheal & bronchial anomalies (atresia, stenosis, Mediastinum shifts away from the affected lung
12. Pleura tracheoesophageal fistula)
Prepared by: egbII, 10-27-11
Contraction atelectasis Hemodynamic Pulmonary Edema
Occurs when local or generalized fibrotic changes in *Increased hydrostatic pressure (Left sided heart failure)
the lung or pleura prevent full expansion - most common hemodynamic cause
Gross: Heavy, wet lungs
*Note: atelectasis is a reversible disorder (except that o Fluid accumulates initially in the basal regions
caused by contraction) of the lower lobes because hydrostatic pressure
is greater in these sites (dependent edema)
Microscopic
3. Pulmonary Edema o
o
Engorged alveolar capillaries
(+) Intra-alveolar granular pink precipitate
Result from hemodynamic disturbances or from direct o (+) Alveolar microhemorrhages and
increases in capillary permeability (microvascular hemosiderin-laden macrophages (“heart
injury) failure” cells)
Long-standing cases (seen in mitral stenosis):
TABLE 15-1 -- Classification and Causes of Pulmonary o Abundant hemosiderin-laden macrophages
Edema o Fibrosis and thickening of the alveolar walls
o Soggy lungs becomes firm and brown (“brown
induration”)
Predisposes to infection
Edema Caused by Microvascular Injury
*Results from primary injury to the vascular endothelium
or damage to alveolar epithelial cells
Direct increases in capillary permeability
Leakage of fluids and proteins first into the interstitial
space & alveoli
Edema is localized and overshadowed by the Pathogenesis:
manifestations of infection Alveolar capillary membrane is formed by two separate
Contributor to ARDS barriers: microvascular endothelium and the alveolar
epithelium
In ARDS - lung injury is caused by an imbalance of pro-
inflammatory and anti-inflammatory mediators
4. Acute Lung Injury and ARDS o Nuclear factor κB (NF-κB) – has pro-
inflammatory effect
(Diffuse Alveolar Damage) o IL-8, IL-1 and TNF - leads to:
ALI – AKA: noncardiogenic pulmonary edema Endothelial activation
Abrupt onset of significant hypoxemia & diffuse Pulmonary microvascular sequestration
pulmonary infiltrates in the absence of cardiac failure Activation of neutrophils (*impt. role in
ARDS – refers to severe ALI pathogenesis of ARDS)
Both (ALI & ARDS) have inflammation-associated How is Neutrophil sequestered?
increase in pulmonary vascular permeability o Activated by IL-8
Histologic feature: diffuse alveolar damage (DAD) o TNF upregulate the expression of adhesion
Most etiologic factor: Sepsis molecules that allow them to bind to their
Complication: Direct injuries to the lungs and systemic ligands on activated endothelial cells
disorders ( Table 15-2 ) o Activated neutrophils become “stiff” and less
deformable and thus get trapped in the narrow
capillary beds of the lung
TABLE 15-2 -- Conditions Associated with Development o Activated neutrophils release a variety of
products (e.g., oxidants, proteases, platelet
of Acute Respiratory Distress Syndrome
Prepared by: egbII, 10-27-11
activating factor, and leukotrienes) that cause
damage to the alveolar epithelium 5. Obstructive vs. 6. Obstructive Pulmonary
Dysregulation of the coagulation system - also a feature
of ARDS
Restrictive Pulmonary Diseases Diseases
o *coagulation pathway is a powerful pro- Obstructive diseases: Restrictive diseases:
inflammatory signal - Increase in resistance to - Reduced expansion of lung
airflow due to partial or parenchyma and decreased
Morphology: complete obstruction at any total lung capacity
ACUTE STAGE level from the trachea
o GROSS: Heavy, firm, red & boggy and larger bronchi to the
o MICROSCOPIC: terminal and respiratory
Congestion, interstitial & and intra-alveolar bronchioles
edema, inflammation, fibrin deposition, and ↓ forced expiratory volume ↓ total lung capacity
DAD at 1 second ↓/Normal expiratory flow
Alveolar walls become lined with waxy rate
hyaline membrane
ORGANIZING STAGE Restrictive defects occur in two general conditions:
o MICROSCOPIC: (1) Chest wall disorders
Type II pneumocytes undergo proliferation Poliomyelitis, severe obesity, pleural diseases, and
(+) granulation tissue kyphoscoliosis
Fibrotic thickening of alveolar septa - (2) Chronic interstitial & infiltrative diseases
caused by proliferation of interstitial cells Pneumoconioses and interstitial fibrosis of unknown
and deposition of collagen etiology
Clinical Course FIGURE 15-5: Schematic representation of overlap between chronic
obstructive lung diseases.
Clinical manifestations: Profound dyspnea and
tachypnea herald ALI, followed by increasing cyanosis
and hypoxemia, respiratory failure, and the appearance 1. EMPHYSEMA
of diffuse bilateral infiltrates on radiographic Def: irreversible enlargement of the airspaces distal to
examination the terminal bronchiole, w/ destruction of their walls &
Functional abnormalities in ALI are not evenly distributed without fibrosis
throughout the lungs Incidence: heavy cigarette smoking, women and
Majority of deaths are attributable to sepsis or multi- African Americans
organ failure and, in some cases, direct lung injury Four major types:
o (1) centriacinar – 95% of cases
ACUTE INTERSTITIAL PNEUMONIA o (2) panacinar
o (3) paraseptal
Term that is used to describe widespread ALI associated
o (4) irregular
with a rapidly progressive clinical course that is of
unknown etiology; AKA: idiopathic ALI-DAD
Clinical Course:
Mean age of 50 years with no sex predilection
C/M: Respiratory failure often following an illness of less Manifestations appear until at least 1/3 of the
than 3 weeks' duration that resembles an URTI functioning pulmonary parenchyma is damaged
Mortality rate: from 33-74% Dyspnea is usually the first symptom
Most deaths occurring within 1-2 months Cough or wheezing is the chief complaint
Weight loss is common and can be severe suggesting a
hidden malignant tumor
Barrel-chested and dyspneic - sits forward in a
hunched-over position
Severe emphysema may overventilate and remain well
oxygenated – called pink puffers
Prepared by: egbII, 10-27-11
Pathogenesis: (+) Inflammation throughout the airways Inflammation on bronchioles < 2 mm in diameter are
Hypothesis: Destruction of alveolar walls is d/t the seen in the ff. changes:
protease antiprotease mechanism, aided and abetted by 1. Goblet cell metaplasia with mucus plug
imbalance of oxidants and antioxidants 2. Inflammatory infiltration of the walls with
Protease – derived from neutrophils neutrophils, macrophages, B cells, CD4 and
Anti protease (protective) - α1-antitrypsin (principal), CD8+ T cells
leukoprotease inhibitor & alpha-1 macroglobulin 3. Thickening of the bronchiolar wall - due to
Thus, result from the destructive effect of high protease smooth muscle hypertrophy and peribronchial
activity in subjects with low antiprotease activity fibrosis
Sequence: Morphology:
1. Neutrophils (the principal source of cellular proteases) Large alveoli separated by thin septa
are normally sequestered in peripheral capillaries, Destruction of alveolar walls
including those in the lung, and gain access to the Respiratory bronchioles & vasculature are deformed &
alveolar spaces compressed
1.] Centriacinar (Centrilobular) Emphysema 2. Any stimulus that increases either the number of
Central or proximal parts of the acini, formed by leukocytes (neutrophils and macrophages) in the lung Cause of Deaths:
respiratory bronchioles, are affected & distal alveoli are or the release of their protease-containing granules (1) Respiratory acidosis & coma
spared/normal increases proteolytic activity (2) Right-sided heart failure
More common & severe in the upper lobes (apical 3. With low levels of serum α1-antitrypsin, elastic tissue (3) Massive collapse of the lungs
segments) destruction is not checked and emphysema results
Occurs predominantly in heavy smokers, often in Treatment:
association with chronic bronchitis Protease-antiprotease imbalance: Bronchodilators, steroids, bullectomy & in some lung
Direct chemoattactant effect of nicotine to neutrophils and volume reduction surgery & transplantation
2.] Panacinar (Panlobular) Emphysema macrophages that accumulate in alveoli
Acini are uniformly enlarged from the level of the ↓
Other forms of Emphysema:
respiratory bronchiole to the terminal blind alveoli Accumulated neutrophils are activated & release their
Compensatory Hyperinflation (Emphysema)
“pan” refers to the entire acinus but not to the entire granules and smoking enhances elastase activity in
Used to designate dilation of alveoli but not destruction
lung macrophages
↓ of septal walls
More common in the lower zones and in the anterior
Lung parenchymal hyperexpansion that follows surgical
margins of the lung Proteases (neutrophil elastase, proteinase 3, and cathepsin
removal of a diseased lung or lobe
Most severe at the bases G, macrophage elastase, matrix metalloproteinases)
Associated with α1-antitrypsin (α1-AT) deficiency ↓
TISSUE DAMAGE Obstructive Overinflation
Lung expands because air is trapped within it
3.] Distal Acinar (Paraseptal) Emphysema Ex: tumor or foreign object, congenital lobar
The proximal portion of the acinus is normal, and the Oxidant-antioxidant imbalance:
Normally, the lung contains a healthy complement of overinflation
distal part is predominantly involved
More striking adjacent to the pleura, along the lobular antioxidants (superoxide dismutase, glutathione)
that keep oxidative damage to a minimum Bullous Emphysema*
connective tissue septa, and at the margins of the A large subpleural blebs or bullae (spaces >1 cm in
lobules Tobacco smoke contains abundant reactive oxygen
species (free radicals), which deplete antioxidant diameter)
More severe in the upper half of the lungs Most often subpleural & occur near the apex,
Findings: multiple, continuous, enlarged airspaces from mechanisms tissue damage
o Oxidative injury causes inactivation of native sometimes in relation to old tuberculous scarring
less than 0.5 cm to more than 2.0 cm in diameter May give rise to pneumothorax
Most cases of spontaneous pneumothorax in young antiproteases resulting in “functional” α1-
adults antitrypsin deficiency
Interstitial Emphysema
Respiratory bronchioles to collapse during expiration – Refers to the entrance of air into the connective tissue
4.] Airspace Enlargement w/ Fibrosis stroma of the lung, mediastinum, or subcutaneous tissue
(Irregular Emphysema) causing airflow obstruction d/t:
Associated with scarring ↓ elastase: loss of elastic tissue in the walls of alveoli
Asymptomatic and clinically insignificant (recoil)
Prepared by: egbII, 10-27-11
Cigarette smoke predisposes to infection in different Asthma is categorized into:
ways: o Atopic (evidence of allergen sensitization, often
o Interferes with ciliary action of the respiratory in a patient with a history of allergic rhinitis,
epithelium eczema)
o Cause direct damage to airway epithelium o Non-atopic (without evidence of allergen
o Inhibits the ability of bronchial and alveolar sensitization)
leukocytes to clear bacteria
Atopic Asthma
Morphology: A classic example of type I IgE-mediated
Gross: hyperemia, swelling, and edema of the mucous hypersensitivity reaction
membranes Begins in childhood and is triggered by environmental
Microscopic: allergens
o Chronic inflammation of the airways Family history of asthma is common
(predominantly lymphocytes) and enlargement Skin test with the offending antigen in these patients
of the mucus-secreting glands of the trachea results in an immediate wheal-and-flare reaction
and bronchi
o Hyperplasia of mucus secreting glands – can be *Non-Atopic Asthma
assessed thru Reid index (normally 0.4) No evidence of allergen sensitization
o Bronchial epithelium exhibit squamous Skin test results are usually negative
metaplasia and dysplasia Family history of asthma is less common
2. CHRONIC BRONCHITIS o Bronchiolitis obliterans - narrowing of Respiratory infections due to viruses are common
Persistent cough with sputum production for at least 3 bronchioles caused by mucus plugging, triggers
months in at least 2 consecutive years, in the absence of inflammation, and fibrosis Lowers the threshold of the subepithelial vagal receptors
any other identifiable cause to irritants
When persistent for years, it may: Clinical Features:
(1) Progress to COPD Cardinal symptom: persistent productive cough w/ Drug-Induced Asthma: Aspirin-sensitive asthma
(2) Lead to cor pulmonale and heart failure sputum Are exquisitely sensitive to small doses of aspirin as well
(3) Cause atypical metaplasia and dysplasia of the Hypercapnia, Hypoxemia, and Mild cyanosis (“blue as other nonsteroidal anti-inflammatory medications
respiratory epithelium, providing a rich soil for cancerous bloaters”) Also experience urticarial
transformation Longstanding severe chronic bronchitis commonly leads Inhibiting the cyclooxygenase pathway of arachidonic
to cor pulmonale with cardiac failure acid metabolism favoring bronchoconstrictor
Pathogenesis: Death may also result from further impairment of leukotrienes
Initiating factor: long-standing irritation by inhaled respiratory function due to superimposed acute
substances such as tobacco smoke (90%), and dust infections Pathogenesis:
from grain, cotton, and silica Initial sensitization to inhaled allergens stimulate
3. ASTHMA induction of TH2 cells which secrete cytokines that
Hypersecretion of mucus in Also a marked Def: Chronic inflammatory disorder of the airways that promote allergic inflammation and stimulate B cells to
the large airways increase in causes recurrent episodes of wheezing, breathlessness, produce IgE and other antibodies that includes:
Proteases from neutrophils goblet cells of chest tightness, and cough, particularly at night and/or o IL-4 - stimulates the production of IgE
(neutrophil elastase, cathepsin, small airways in the early morning o IL-5 - activates locally recruited eosinophils
and matrix metalloproteinases) Hallmarks of the disease are: o IL-13 - stimulates mucus secretion and
stimulate mucus hypersecretion o Increased airway responsiveness promotes IgE production by B cells
↓ o Episodic bronchoconstriction IgE coats submucosal mast cells and repeat exposure to
Causing Hypertrophy of submucosal gland o Inflammation of the bronchial walls the allergen triggers the mast cells to release granule,
A protective reactions o Increased mucus secretion cytokines & other mediators
↓ Status asthmaticus
Mucus secretion = sputum overproduction o State of unremitting attacks Induce the early-phase (immediate hypersensitivity)
o Fatal, usually in patients have had a long history reaction
Secondary role of infection - significant in maintaining it of asthma o Early phase mediators – Leukotrienes
and may be critical in producing acute exacerbations o Asymptomatic C4,D4,E4, Prostaglandin D2, E2, F2-alpha,
Histamine, PAF, Mast cell trptase)
Prepared by: egbII, 10-27-11
o Dominated by bronchoconstriction, increased Class II HLA - tendency to produce IgE antibodies o Other conditions: RA, SLE, inflammatory
mucus production, and variable degrees of against some (pollen) antigens bowel disease, and post-transplantation
vasodilation with increased vascular ADAM-33 polymorphism - accelerate proliferation of
permeability bronchial smooth muscle cells and fibroblasts, thus Morphology:
contributing to bronchial hyperreactivity & fibrosis Affects the lower lobes bilaterally
Late-phase reaction - inflammation with recruitment of β2-adrenergic receptor gene - maps to 5q associated Most severe in the more distal bronchi and bronchioles
leukocytes (eosinophils, neutrophils, & more T cells) with airway hyper-responsiveness Airways are dilated, sometimes up to 4x the normal size
Leukocyte recruitment is stimulated by chemokines IL-4 receptor gene - associated with atopy, elevated
produced by mast cells, epithelial cells, T cells, and other total serum IgE, and asthma Histologic findings vary with the activity and chronicity of the
cytokines Chitinases are enzymes that cleave chitin disease:
o Ex: eotaxin, produced by airway epithelial cells, o acidic mammalian chitinase (w/ enzymatic There is an intense acute and chronic inflammatory
a potent chemoattractant and activator of activity) - is up-regulated in and contributes to exudation within the walls of the bronchi and bronchioles
eosinophils; “the major basic protein of TH2 inflammation With desquamation of the lining epithelium and
eosinophils” o YKL-40 (w/o enzymatic activity) - is associated extensive areas of necrotizing ulceration
with severity of asthma Necrotizing ulceration lead to lung abscess
Chemical Mediators: Develop fibrosis
Putative mediators – role in bronchospasm Morphology:
Leukotrienes C4,D4,E4: bronchoconstriction, increase Curschmann spirals - result either from mucus Etiology and Pathogenesis:
vascular permeability & mucus secretion plugging in subepithelial mucous gland ducts Infection and Obstruction - conditions associated with
Acetylcholine: from intrapulmonary motor nerves Eosinophils (neumerous) bronchiectasis; seen in cystic fibrosis
causes airway smooth muscle constriction directly by Charcot-Leyden crystals - collections of crystalloid OBSTRUCTION - accumulation of thick viscid secretions
stimulating muscarinic receptors made up of an eosinophil lysophospholipase binding that obstruct the airways
protein called galectin-10 INFECTION – increase susceptibility
Mediators at the “scene of crime” – role in acute asthma
effects Clinical Course: Destruction of supporting smooth muscle and elastic
Histamine: potent bronchoconstrictor Classic acute asthmatic attack lasts up to several hours tissue, fibrosis, and further dilatation of bronchi.
PGD2: bronchoconstriction & vasodilation Severe form – Status asthmaticus may lead to death The smaller bronchioles become progressively
PAF: aggregation of platelets & release of histamine & With appropriate therapy, most patients are able to obliterated as a result of fibrosis (bronchiolitis obliterans)
serotonin from their granules maintain a productive life
Other conditions:
“Suspects” – not studied yet 4. BRONCHIECTASIS Primary ciliary dyskinesia – AR; poorly functioning
IL-1, TNF, IL, chemokines (e.g., eotaxin), neuropeptides, cilia contribute to the retention of secretions; absence or
Def: Permanent dilation of bronchi and bronchioles
nitric oxide, bradykinin, and endothelins shortening of the dynein arms that are responsible for
caused by destruction of the muscle and elastic tissue,
resulting from or associated with chronic necrotizing the coordinated bending of the cilia
“Airway remodeling” - repeated bouts of allergen o ½ have Kartagener syndrome (bronchiectasis,
infections
exposure result in structural changes in the bronchial wall: sinusitis, & situs inversus or partial lateralizing
Bronchiectasis develops in association with the
Hypertrophy & Hyperplasia of bronchial smooth muscle abnormality)
conditions:
Epithelial injury o Males – tend to be infertile
o Congenital or hereditary conditions: cystic
Increased airway vascularity Allergic bronchopulmonary aspergillosis - high
fibrosis, intralobar sequestration of the lung,
Increased subepithelial mucus gland serum IgE levels
immunodeficiency states and primary ciliary
hypertrophy/hyperplasia
dyskinesia and Kartagener syndromes
Deposition of subepithelial collagen Clinical course:
o Postinfectious conditions: necrotizing
pneumonia caused by bacteria (Mycobacterium Severe, persistent cough; Expectoration of foul-smelling,
Genetics of Asthma: sometimes bloody sputum
tuberculosis, Staphylococcus aureus,
Multiple susceptibility genes interact with environmental Haemophilus influenzae, Pseudomonas), viruses Dyspnea and Orthopnea; Hemoptysis; Fever
factors: (adenovirus, influenza virus, human Symptoms often precipitated by URTI
Chr 5 polymorphism in the IL13 gene - strongest immunodeficiency virus [HIV]), and fungi Obstructive respiratory insufficiency can lead to
and most consistent associations with asthma or allergic (Aspergillus species) marked dyspnea and cyanosis
disease o Bronchial obstruction Complications: Cor pulmonale, brain abscesses, and
amyloidosis
Prepared by: egbII, 10-27-11
7. Chronic Diffuse Interstitial Caveolin-1 acts as an endogenous 2. Nonspecific Interstitial Pneumonia (NSIP)
inhibitor of pulmonary fibrosis by Dyspnea and cough of several months' duration
(Restrictive) Diseases
limiting TGF-β1
Caveolin-1 is decreased in IPF, which
Between 46-55 years of age
Characterized predominantly by inflammation and Diffuse interstitial lung disease of unknown etiology
inhibits collagen deposition Better prognosis than UIP
fibrosis of the pulmonary connective tissue, principally
the most peripheral and delicate interstitium in the
alveolar walls Morphology:
Reduced expansion of lung parenchyma & decreased Cellular pattern - mild to moderate chronic interstitial
total lung capacity inflammation in a uniform or patchy distribution
C/M: dyspnea, tachypnea, end-inspiratory crackles, and Fibrosing pattern - diffuse or patchy interstitial fibrosis
eventual cyanosis, without wheezing without the temporal heterogeneity that is characteristic
CXR: bilateral infiltrative lesions in the form of small of UIP
nodules, irregular lines, or ground-glass shadows, hence (-) fibroblastic foci and honeycombing
the term infiltrative
Eventually, secondary pulmonary hypertension and 3. Cryptogenic Organizing Pneumonia
right-sided heart failure with cor pulmonale may result Def: with cough and dyspnea and have subpleural or
Advanced forms: scarring and gross destruction of the peribronchial patchy areas of airspace consolidation
lung referred to as end-stage lung or honeycomb lung radiographically
Histologically:
Restrictive Diseases – 2 general conditions: o (+) polypoid plugs of loose organizing
Chest wall disorders connective tissue (Masson bodies) within
o Neuromuscular diseases like poliomyelitis alveolar ducts, alveoli & bronchioles
o Severe obesity o (-) interstitial fibrosis or honeycomb lung
o Pleural diseases
o Kyphoscoliosis 4. Pulmonary Involvement in Connective Tissue
Chronic interstitial & infiltrative diseases
Diseases
Morphology: Rheumatoid arthritis: pulmonary involvement may
A.] FIBROSING DISEASES: The hallmark of UIP is patchy interstitial fibrosis occur in 30% to 40% of patients as
1. Pulmonary Fibrosis (IPF) Fibrosis causes the destruction of alveolar architecture o (1) chronic pleuritis, with or without effusion;
AKA: cryptogenic fibrosing alveolitis and formation of cystic spaces lined by hyperplastic type o (2) diffuse interstitial pneumonitis and fibrosis;
Histologic pattern: usual interstitial pneumonia (UIP) II pneumocytes or bronchiolar epithelium (honeycomb o (3) intrapulmonary rheumatoid nodules;
fibrosis) o (4) pulmonary hypertension
Pathogenesis: Mild to moderate inflammation within the fibrotic areas, Systemic sclerosis (scleroderma): diffuse interstitial
The current concept is that IPF is caused by “repeated consisting: lymphocytes (mostly), and a few plasma fibrosis
cycles” of epithelial activation/injury by some cells, neutrophils, eosinophils, mast cells Lupus erythematosus: patchy, transient parenchymal
unidentified agent Foci of squamous metaplasia and smooth muscle infiltrates
There is inflammation and induction of T H2 cell, hyperplasia
characterized by the presence of eosinophils, mast cells, Pulmonary arterial hypertensive changes (intimal fibrosis 5. Pneumoconioses
IL-4 and IL-13 and medial thickening) Def: A non-neoplastic lung reaction to inhalation of
Abnormal epithelial repair gives rise to exuberant mineral dusts encountered in the workplace, especially in
fibroblastic/myofibroblastic proliferation, leading to the Clinical Course: urban areas
“fibroblastic foci” particularly TGF-β1 as the driver of the Increasing dyspnea on exertion and dry cough
process 40 to 70 years old at the time of presentation Pathogenesis: development of pneumoconiosis depends on
o TGF-β1 is known to be fibrogenic and is released Late course: Hypoxemia, cyanosis, and clubbing (1) Amount of dust retained in the lung and airways;
from injured type I alveolar epithelial cells Mean survival: <3 years (2) Size, shape, and therefore buoyancy of the particles;
o TGF-β1 negatively regulates telomerase activity, (3) Particle solubility and physiochemical reactivity;
thus facilitating epithelial cell apoptosis and the (4) Additional effects of other irritants (tobacco smoking)
cycle of death and repair
o Another molecule regulated by TGF-β1 is
caveolin-1
Prepared by: egbII, 10-27-11
The most dangerous particles range from 1 to 5 μm in Within the macrophages silica causes activation and airstream to be delivered
diameter because they may reach the terminal small release of mediators (IL-1, TNF, fibronectin, lipid deeper into the lungs
airways and air sacs and settle in their linings mediators, oxygen-derived free radicals, and fibrogenic Both are fibrogenic, and increasing doses are associated with
Solubility and Cytotoxicity of particles - modify the cytokines) a higher incidence
nature of the pulmonary response
o The smaller the particle, the more likely it is to Morphology Also act as a tumor initiator and promoter mediated by
appear in the pulmonary fluids and reach toxic Gross: reactive free radicals
levels rapidly Early stage: tiny, barely palpable, discrete pale to
Some Lung Diseases Caused by Air Pollutants: blackened nodules which coalesce into hard, collagenous Morphology
1. Coal Workers' Pneumoconiosis scars Marked by diffuse pulmonary interstitial fibrosis
2. Silicosis Central softening & cavitation – d/t superimposed TB or Presence of multiple asbestos bodies
3. Asbestos-Related Diseases ischemia o Appear as golden brown, fusiform or beaded
Fibrotic lesions in the hilar lymph nodes and pleura – rods with a translucent center and consist of
with eggshell calcification visible on X-ray asbestos fibers coated with an iron-containing
5.1. Coal Workers' Pneumoconiosis (CWP) expansion and coalescence of lesions may produce proteinaceous material
Spectrum of lung findings:
progressive massive fibrosis Ferruginous bodies
(1) Asymptomatic anthracosis
Begins as fibrosis around respiratory bronchioles and
(2) Simple CWP with little or no pulmonary dysfunction
Microscopic features: alveolar ducts
(3) Complicated CWP, or progressive massive fibrosis (PMF)
layers of hyalinized collagen surrounded by a dense (+) honeycombed
capsule of more condensed collagen Begins in the lower lobes and subpleurally
Morphology
On polarized microscopy: a birefringent silica particles Middle and upper lobes of the lungs become affected as
Anthracosis - most innocuous coal-induced pulmonary
fibrosis progresses
lesion
Clinical Course The scarring may trap and narrow pulmonary arteries
Simple CWP - upper lobes and upper zones of the lower
Slow to kill, with impaired pulmonary function and arterioles, causing pulmonary HPN and cor
lobes are more heavily involved; primarily adjacent to
Increased susceptibility to TB pulmonale
respiratory bronchioles; characterized by:
Pleural plaques - most common manifestation of
o coal macules (1 to 2 mm in diameter) -
consists of carbon-laden macrophages 5.3. Asbestos-Related Diseases asbestos exposure
Occupational exposure to asbestos is linked to: o well-circumscribed plaques of dense collagen
o coal nodules - consists collagen fibers
Localized fibrous plaques or, rarely, diffuse pleural often calcium
o centrilobular emphysema - dilation of
fibrosis o develop most frequently on the anterior and
adjacent alveoli
Pleural effusions posterolateral aspects of the parietal pleura
Complicated CWP (progressive massive fibrosis)
Parenchymal interstitial fibrosis (asbestosis) and diaphragm
o Multiple intensely blackened scars (2-10 cm)
o Microscopic: lesions consist of dense collagen Lung carcinoma Both lung carcinomas and mesotheliomas (pleural and
and pigment; center of the lesion is often Mesotheliomas peritoneal) develop in workers exposed to asbestos
Laryngeal & other extrapulmonary neoplasms, including o lung carcinoma (5x risk) & mesotheliomas
necrotic, most likely due to local ischemia
colon carcinomas (1000x)
5.2. Silicosis o smoking increase risk of carcinoma but NOT
Inhalation of crystalline silicon dioxide (silica)
Pathogenesis: mesotheliomas
Silicosis usually presents after decades of exposure as a
slowly progressing, nodular, fibrosing pneumoconiosis 2 distinct forms: serpentine and amphibole
Acute silicosis - a disorder characterized by the Serpentine chrysotile - Amphiboles - more Drug-Induced Lung Diseases
chemical form accounts for pathogenic in induction of Radiation-Induced Lung Diseases
accumulation of abundant lipoproteinaceous material
within alveoli most of the asbestos used malignant pleural tumors Acute Radiation Pneumonitis
(mesotheliomas) o 1-6 months after therapy
more flexible, curled pathogenicity of amphiboles o C/M: Fever, dyspnea, pleural effusion, radiologic
Pathogenesis
structure, are likely to is apparently related to their infiltrates
Silica occurs in both crystalline and amorphous forms,
become impacted in the aerodynamic properties and Chronic Radiation Pneumonitis
but crystalline forms (including quartz, crystobalite, and
upper respiratory passages solubility o AKA: Pulmonary fibrosis
tridymite) are much more fibrogenic
and removed by the o a consequence of the repair of injured
o quartz is most commonly implicated in silicosis
mucociliary elevator endothelial and epithelial cells within the
After inhalation, the particles interact with epithelial cells
More soluble Align themselves in the radiation portal
and macrophages
Prepared by: egbII, 10-27-11
There is no unequivocal evidence that sarcoidosis is 2. Hypersensitivity Pneumonitis (HP)
caused by an infectious agent Def: immunologically mediated predominantly
interstitial, lung disorders in response to intrinsic Ag-
Morphology: immune complex & delayed type of hypersensitivity.
Lungs common site Caused by intense, prolonged exposure to inhaled
Gross: NO demonstrable abnormality or small nodules organic antigens (made up of spores of thermophilic
(1-2cm), noncaseating, noncavitated granulomas bacteria, true fungi, animal proteins, or bacterial
Microscopic: lesions are distributed primarily along the products)
lymphatics, around bronchi & vessels, although alveolar Involves primarily the alveoli – progressing to chronic
lesions may be seen FIBROTIC lung disease
Bronchial lavage: CD4/CD8 ration (>2.5) & CD3/CD4
ration (<0.31) Types of Hypersensitivity Pneumonitis
Farmer’s Lung: dusts from hay with spores of
Lymph nodes actinomycetes
B.] GRANULOMATOUS DISEASES Involved in almost all cases specifically hilar & Pigeon breeder's lung (bird fancier's disease): caused
1. Sarcoidosis mediastinal lymph nodes by proteins from serum, excreta, or feathers of birds
Systemic disease of UNKNOWN cause Humidifier or air-conditioner lung: caused by
Others: thermophilic bacteria in heated water reservoirs
NONCASEATING granulomas
Spleen
C/M:
Liver HP is an immunologically mediated disease
o Bilateral hilar lymphadenopathy or lung
Bone Marrow Bronchoalveolar lavage (BAL) in acute phase - show
involvement
Skin increased levels of proinflammatory chemokines such as
o Eye & skin lesions
Eyes (iritis, iridiocystitis) & salivary glands macrophage inflammatory protein 1α and IL-8
Muscle involvement BAL consistently demonstrate increased numbers of T
Etiology and Pathogenesis:
lymphocytes of both CD4+ and CD8+
Immunological Factors NON CASEATING granulomas Most patients have specific antibodies in their serum, a
Intra-alveolar and interstitial accumulation of CD4+ T Aggregate of tightly clustered epitheliod cells, often with feature that is suggestive of type III (immune complex)
cells, resulting in CD4/CD8 T-cell ratios ranging from 5 : Langhans or Foreign body type giant cells with unusual hypersensitivity
1 to 15 : 1 central necrosis Complement and Ig present within vessel walls by
Increased levels of T cell–derived T H1 cytokines such as Granulomas may become enclosed by fibrous rims or immunofluorescence indicating a type III
IL-2 and IFN-γ replaced by hyaline fibrous scars hypersensitivity
Increased levels of several cytokines in the local Schaumann bodies - laminated concretions composed 2/3 of noncaseating granulomas - suggests the
environment (IL-8, TNF, macrophage inflammatory of calcium and proteins development of a T cell–mediated (type IV)
protein 1α) that favor recruitment of additional T cells Asteroid bodies - stellate inclusions enclosed within
and monocytes giant cells Morphology (centered on bronchioles)
Systemic immunological abnormalities interstitial pneumonitis consisting primarily of
Clinical Course: lymphocytes, plasma cells, and macrophages
Anergy to common skin test antigens such as Candida or
Follows an unpredictable course noncaseating granulomas in 2/3 of patients
tuberculosis purified protein derivative (PPD)
65% - 70% of affected patients recover with minimal interstitial fibrosis, honeycombing, and obliterative
Polyclonal hypergammaglobulinemia, another
or no residual manifestations bronchiolitis (in late stages)
manifestation of helper T-cell dysregulation
20% - have permanent loss of some lung function or >1/2 patients have intra-alveolar infiltrates
some permanent visual impairment
Genetic Factors
10% - 15%, some die of cardiac or central nervous Clinical Features:
Familial and racial clustering of cases
system damage, but most succumb to progressive Symptoms usually appear 4 to 6 hours after exposure
Association with certain HLA genotypes
pulmonary fibrosis and cor pulmonale Acute attacks: consist of recurring episodes of fever,
o (class I HLA-A1 and HLA-B8)
dyspnea, cough, and leukocytosis
Environmental Factors If exposure is continuous and protracted:
Proposed putative microbes: Mycobacteria, o progressive respiratory failure, dyspnea, and
Propionibacterium acnes & Rickettsia species cyanosis
o decrease in total lung capacity and compliance
Prepared by: egbII, 10-27-11
C.] PULMONARY EOSINOPHILIA C/M: insidious onset of dyspnea and dry cough over Congenital PAP
Eosinophils - recruited by IL-5 weeks or months, often associated with clubbing of digits Rare cause of immediate-onset neonatal respiratory
Divided into the following categories: good prognosis & excellent response to steroid distress on a full term with death ensuing at 3-6 months
Associated with mutations in the multiple genes:
Acute eosinophilic pneumonia with respiratory failure Morphology: o ATP-binding cassette protein member A3
acute illness of unknown cause Most striking histologic finding: accumulation of a large (ABCA3)
has a rapid onset with fever, dyspnea, and hypoxemic number of macrophages with abundant cytoplasm o Surfactant protein B (SP-B) – transmitted AR d/t
respiratory failure containing dusty brown pigment (smokers' homozygosity for a frameshift mutation in the
CXR: diffuse infiltrates macrophages) SP-B gene
BAL fluid contains > 25% eosinophils Some of the macrophages contain lamellar bodies o Surfactant protein C (SP-C)
(composed of surfactant) o GM-CSF
Simple pulmonary eosinophilia or Löffler syndrome o Derived from necrotic type II pneumocytes o GM receptor (GM-CSF/IL-3/IL-5) β chain
transient pulmonary lesions, eosinophilia in the blood, Thickened alveolar septa with sparse inflammatory
and a benign clinical course infiltrate of lymphocytes & plasma cells Secondary PAP
CXR: shadows of varying size and shape in any of the o Septa lined by cuboidal pneumocytes Is uncommon
lobes Interstitial fibrosis & emphysema may be present Causes: hematopoietic disorders, malignancies,
Microscopic: alveolar septa are thickened by an infiltrate immunodeficiency disorders, lysinuric protein
composed of eosinophils and occasional interspersed 2. Respiratory Bronchiolitis-Associated Interstitial intolerance, and acute silicosis and other inhalational
giant cells Lung Disease syndromes
o NO vasculitis, fibrosis, or necrosis Found in cigarette smokers
Presence of pigmented intraluminal macrophages within Morphology:
Secondary eosinophilia - occurs in a: first- and second-order respiratory bronchioles Characterized by a peculiar homogeneous, granular
number of parasitic, fungal, and bacterial infections Dyspnea and cough at 40-50 y/o over 30 pack-years of precipitate within the alveoli
hypersensitivity pneumonitis cigarette smoking Causing a focal-to-confluent consolidation of large areas
drug allergies 2 : 1 male predominance? of the lungs with minimal inflammatory reaction
in association with asthma, allergic bronchopulmonary Cut surface: turbid fluid exudes with marked increase in
aspergillosis, or vasculitis Morphology: the size and weight of the lung
Changes are patchy at low magnification and have a Alveolar precipitate is PAS positive and also contains
Chronic eosinophilic pneumonia/ Idiopathic bronchiolocentric distribution cholesterol clefts
focal areas of cellular consolidation of the lung substance contain aggregates of dusty brown macrophages Immunohistochemical stains: show the presence of
distributed chiefly in the periphery of the lung fields (smokers' macrophages) surfactant proteins A and C
heavy aggregates of lymphocytes and eosinophils within patchy submucosal and peribronchiolar infiltrate of Abnormalities in lamellar bodies in type II pneumocytes
both the septal walls and the alveolar spaces can be seen in mutations of SP-B, SP-C, and ABCA3
lymphocytes and histiocytes
C/M: high fever, night sweats, and dyspnea which Centrilobular emphysema - common but NOT severe
respond to corticosteroid therapy
Tropical eosinophilia
E.] PULMONARY ALVEOLAR PROTEINOSIS
Bilateral patchy asymmetric pulmonary opacifications
8. Diseases of Vascular Origin
caused by infection with microfilariae
& accumulation of acellular surfactant in the intra-
alveolar and bronchiolar spaces
A.] PULMONARY EMBOLISM, HEMORRHAGE, &
D.] SMOKING-RELATED INTERSTITIAL DSES. INFARCTION
Grouped into: obstructive diseases and restrictive or Acquired PAP Almost always EMBOLIC in origin – >95% arise from
interstitial diseases 90% of all cases, unknown etiology without any familial thrombi within the large deep veins of the lower legs
2 ends of smoking-associated interstitial lung diseases: predisposition Risk factors:
1. Desquamative interstitial pneumonia (DIP) Considered as autoimmune disorder o Cardiac disease, Cancer, Immobilization,
2. Respiratory bronchiolitis-associated interstitial Anti–GM-CSF antibody is responsible for the Hypercoagulable states
lung disease development of the disease - antibodies inhibit the Pathophysiologic response and clinical significance of
activity of endogenous GM-CSF, leading to a state of pulmonary embolism depends on:
1. Desquamative Interstitial Pneumonia (DIP) functional GM-CSF deficiency 1. extent to which the pulmonary artery blood flow
40-50 y/o, Men > women: 4 : 1 ratio o Normally, GM-CSF – involves in surfactant is obstructed
Found in Cigarette smokers clearance by alveolar macrophage 2. size of the occluded vessel(s)
Prepared by: egbII, 10-27-11
3. number of emboli SECONDARY PULMONARY HPN causes:
4. overall status of the cardiovascular system Endothelial cell dysfunction 1. Goodpasture Syndrome
5. the release of vasoactive factors like Increased shear and mechanical injury associated with autoimmune disease of kidney and lung injury
thromboxane A2 from platelets left-to-right shunts or the biochemical injury produced caused by circulating autoantibodies against the
Emboli result in 2 main pathophysiologic consequences: by fibrin in thromboembolism noncollagenous domain of the α3 chain of collagen IV
o Respiratory compromise due to the ↓ prostacyclin & nitric oxide + ↑ endothelin = antibodies initiate inflammatory destruction of the
nonperfused although ventilated segment vasoconstriction basement membrane in renal glomeruli and pulmonary
o Hemodynamic compromise due to increased Endothelial activation makes endothelial cells alveoli
resistance to pulmonary blood flow w/c leads to thrombogenic and promotes the persistence of fibrin occur in the teens or 20s; male preponderance
pulmonary HPN & R-sided HF Release of GF & cytokines induce the migration & Pathogenesis: genetic predisposition is associated with
replication of vascular smooth muscle cells & elaboration certain HLA subtypes (e.g., HLA-DRB1*1501 and *1502)
2.] PULMONARY HYPERTENSION (PH) of extracellular matrix
Def: occurs when mean pulmonary pressure reaches ¼ Morphology:
of systemic levels – most frequently secondary to Other causes of pulmonary arterial HPN: proliferative, usually rapidly progressive
structural cardiopulmonary conditions that increase Crotalaria spectabilis - used medicinally in bush tea glomerulonephritis and a necrotizing hemorrhagic
pulmonary blood flow or pressure resistance; These Aminorex - appetite depressant interstitial pneumonitis
include the following: Adulterated olive oil Characteristic findings in kidney:
Obstruction of vasculature caused by proliferation of Fenfluramine and phentermine - anti-obesity drugs o Early: focal proliferative glomerulonephritis
endothelial S.M., muscle cell & intimal cells accompanied o Progeressive: crescentic glomerulonephritis
by concentric laminar intimal fibrosis Morphology: reveal linear deposits of immunoglobulins along the
The vessel changes can involve the entire arterial tree, basement membranes of the septal walls
Chronic obstructive or interstitial lung diseases: from the main pulmonary arteries down to the arterioles
Have hypoxia, destruction of lung parenchyma & fewer o Atherosclerosis Clinical Features:
alveolar capillaries. This causes increased pulmonary o Medial hypertrophy & intimal fibrosis* Uremia - most common cause of death
arterial resistance & elevated pressure o Plexogenic pulmonary arteriopathy – tuft of Prognosis for this disease - improved by intensive
capillary formation is present producing a plasmapheresis
Antecedent congenital or acquired heart disease: network of web that spans the lumen of dilated
Occurs w/ mitral stenosis, because of an increase in left thin, walled, small arteries 2. Idiopathic Pulmonary Hemosiderosis
atrial pressure that ↑ pulmonary venous pressure and characterized by intermittent, diffuse alveolar
pulmonary artery pressure Clinical Course: hemorrhage
Idiopathic PH - most common in women 20-40 y/o usually presents with an insidious onset of productive
Autoimmune disorders: cough, hemoptysis, anemia, and weight loss
Clinical signs and symptoms of all forms of hypertension
Due to a reduction in the functional cross-sectional area no anti–basement membrane antibodies
become evident only with advanced disease
of the pulmonary vascular bed brought about by the *Cardinal histologic feature: hemorrhage into the
Initially, the presenting features: dyspnea and fatigue,
obstructing emboli alveolar spaces & hemosisderosis
chest pain
Over time, severe respiratory distress, cyanosis, and
Connective tissue diseases: 3. Wegener Granulomatosis
right ventricular hypertrophy occur, and death from
Most notably systemic sclerosis
decompensated cor pulmonale, often with superimposed most often involves the upper respiratory tract
thromboembolism and pneumonia, within 2 to 5 years in transbronchial lung biopsy - provide the only tissue
Obstructive sleep apnea:
80% of patients available for diagnosis
Associated with obesity
(-) necrosis and granulomatous vasculitis
PRIMARY PULMONARY HPN - encountered sporadically in
C.] DIFFUSE PULMONARY HEMORRHAGE *Important features: capillaritis and scattered,
SYNDROMES poorly formed granulomas (unlike those of
patients in whom all known causes of increased pulmonary sarcoidosis, which are rounded and well-defined)
pressure are excluded Includes:
Mutation in bone morphogenic protein receptor type 2 Goodpasture syndrome
(BMPR2) signaling pathway Idiopathic pulmonary hemosiderosis
o In vascular s.m. cells BMPR2 signaling causes Vasculitis-associated hemorrhage, found in:
inhibition of proliferation and favors apoptosis o Hypersensitivity angiitis
Inactivating BMPR2 causes smooth muscle proliferation o Wegener granulomatosis
o SLE
Prepared by: egbII, 10-27-11
Pulmonary Infections o Typically, the encapsulated form dominates the lobular lobar pneumonia
unencapsulated forms by secreting an antibiotic bronchopneumonia
1) COMMUNITY-ACQUIRED ACUTE PNEUMONIAS called haemocin that kills the unencapsulated H. -Patchy consolidation of the - fibrinosuppurative
2) COMMUNITY-ACQUIRED ATYPICAL (VIRAL AND influenza lung consolidation of a large
MYCOPLASMAL) PNEUMONIAS *Type b (encapsulated) - has a polyribosephosphate - lobe with focal opacities portion of a lobe or of an
3) HOSPITAL-ACQUIRED PNEUMONIA capsule, used to be the most frequent cause of severe -4 stages of inflammatory entire lobe
invasive disease response: congestion, red - lobe is radiopaque
4) ASPIRATION PNEUMONIA Nonencapsulated/ nontypeable forms – spreads in hepatization, gray
5) LUNG ABSCESS URT & produce otitis media (infection of the middle ear), hepatization, and resolution
6) CHRONIC PNEUMONIA sinusitis, and bronchopneumonia
7) PNEUMONIA IN THE IMMUNOCOMPROMISED HOST 4 stages of the inflammatory response:
3. Moraxella catarrhalis 1. Congestion: 1-2 days
8) PULMONARY DISEASE IN HUMAN IMMUNODEFICIENCY
*2nd most common bacterial cause of acute exacerbation Gross: lung is heavy, boggy, and red
VIRUS INFECTION of COPD Microscopic: vascular engorgement, intra-alveolar fluid
constitutes one of the three most common causes of with few neutrophils, and often the presence of
9. Pulmonary Infections otitis media in children numerous bacteria
2. Red hepatization: 2-4 days
Pneumonia - broadly defined as any infection of the lung 4. Staphylococcus aureus Gross: Lungs – red, firm & airless with liver-like
parenchyma important cause of secondary bacterial pneumonia in consistency
Pneumonia can result whenever these local defense children and healthy adults following viral respiratory Microscopic: massive confluent exudation with
mechanisms are impaired or the systemic resistance of illnesses neutrophils, red cells, and fibrin filling the alveolar
the host is lowered associated with a high incidence of complications: lung spaces
abscess and empyema 3. Gray hepatization: 4-6 days
Local defense mechanisms of the lung can be interfered *Intravenous drug abusers are at high risk of Gross: grayish brown, dry surface with liver-like
with: developing staphylococcal pneumonia in association with consistency
Loss or suppression of the cough reflex (coma, endocarditis Microscopic: progressive disintegration of red cells and
anesthesia, neuromuscular disorders, drugs, or chest
the persistence of a fibrinosuppurative exudate
pain) 5. Klebsiella pneumonia 4. Resolution: 8-9 days
Injury to the mucociliary apparatus *Most frequent cause of gram-negative bacterial Microscopic: Consolidated exudate within the alveolar
Accumulation of secretions (cystic fibrosis and bronchial pneumonia; afflicts debilitated and malnourished people, spaces undergoes progressive enzymatic digestion to
obstruction) particularly chronic alcoholics produce granular, semifluid debris that is resorbed,
Interference with the phagocytic or bactericidal action of Thick and gelatinous sputum is characteristic ingested by macrophages, expectorated, or organized by
alveolar macrophages
fibroblasts growing into it
Pulmonary congestion and edema 6. Pseudomonas aeruginosa
*Most commonly causes hospital-acquired infections Complications:
COMMUNITY-ACQUIRED ACUTE PNEUMONIAS Common in patients who are neutropenic with a (1) tissue destruction and necrosis, causing abscess
1. Streptococcus pneumonia or pneumococcus propensity to invade blood vessels with consequent formation (type 3 pneumococci or Klebsiella infections)
*Most common cause of community-acquired acute extrapulmonary spread (2) spread of infection to the pleural cavity, causing the
pneumonia
intrapleural fibrinosuppurative reaction known as empyema
Gm (+), lancet-shaped diplococcic 7. Legionella pneumophila (3) bacteremic dissemination causing metastatic
part of the endogenous flora in 20% of adults Causes: Legionnaires' disease & Pontiac fever abscesses, endocarditis, meningitis, or suppurative arthritis
vaccines containing capsular polysaccharides - used in Flourishes in aquatic environments (ie. water-cooling (4) organization of exudates
patients at high risk towers & tubing system)
Organ transplant recipients are particularly susceptible
2. Haemophilus influenza COMMUNITY-ACQUIRED ATYPICAL (VIRAL
Gm (-), major cause of life-threatening acute lower Morphology:
AND MYCOPLASMAL) PNEUMONIAS
respiratory tract infections and meningitis in young Atypical - applied to an acute febrile respiratory disease
Bacterial pneumonia has two patterns of anatomic
children o *Denotes moderate amount of sputum, no
distribution: lobular bronchopneumonia and lobar
A ubiquitous colonizer of the pharynx, where it exists in physical findings of consolidation, only moderate
pneumonia
two forms: encapsulated (5%) and unencapsulated elevation of white cell count, and lack of alveolar
(95%) exudate
Prepared by: egbII, 10-27-11
Characterized by patchy inflammatory changes in the Avian influenza (antigenic type H5N1) LUNG ABSCESS
lungs, largely confined to the alveolar septa and lethal in humans (approximately 60%) A local suppurative process within the lung,
pulmonary interstitium severity of the disease results from the ability of the characterized by necrosis of lung tissue
Caused by a variety of organisms: virus to cause widespread infection in the human body,
o Mycoplasma pneumonia – most common instead of infection being limited to the lung Etiology and Pathogenesis
o Viruses The tissue tropism of H5N1 influenza is increased due to aerobic and anaerobic streptococci, S. aureus, and a
o Chlamydia pneumonia the unusual structure of its hemagglutinin protein host of gram-negative organisms
o Coxiella burnetii (Q fever) Anaerobic organisms: Bacteroides, Fusobacterium, and
Human Metapneumovirus (MPV) Peptococcus species - are the exclusive isolates in about
Pathogenesis: a paramyxovirus & associated with upper and lower 60% of cases
attachment of the organisms to the upper respiratory respiratory tract infections The causative organisms are introduced by the following
tract epithelium followed by necrosis most commonly in young children, elderly subjects, and mechanisms:
immunocompromised patients o Aspiration of infective material (the most
Morphology: *cause severe infections: bronchiolitis & pneumonia frequent cause) - acute alcoholism, coma,
Lung involvement may be quite patchy or may involve No commercial treatments yet, but anesthesia, sinusitis, gingivodental sepsis,&
whole lobes bilaterally or unilaterally o Ribavirin – shows activity both in vitro and in depressed cough reflex
Histologic: animal models o Antecedent primary lung infection - Post-
o interstitial nature of the inflammatory reaction pneumonic abscess formations are usually
o widened and edematous alveolar septa Severe Acute Respiratory Syndrome (SARS) associated with S. aureus, K. pneumoniae, and
o mononuclear inflammatory infiltrates appeared in November 2002 in the Guangdong Province the type 3 pneumococcus
o pink hyaline membranes lining the alveolar walls of China o Septic embolism
Etiologic agent: coronavirus o Neoplasia
Influenza Infections incubation period: 2 to 10 days
genome of influenza virus is composed of eight helices of C/M: dry cough, malaise, myalgias, fever, and chills w/c *primary cryptogenic lung abscesses – unknown cause
single-stranded RNA may improve and resolve the infection or progress to
each encoding a single gene bound by a nucleoprotein severe respiratory disease Morphology:
MOT: first transmitted to humans through contact with Gross: few millimeters to large cavities of 5 to 6 cm
that determines the type (A,B,C) of virus
wild masked palm civets & subsequently spread person- Pulmonary abscesses due to aspiration are more
Rarely causes interstitial myocarditis or after aspirin
to-person infected respiratory secretions, some cases common on the right (because of the more vertical right
therapy – d/t Reye syndrome
may have been contracted from stool main bronchus) and are most often single
w/ lipid bilayer (envelope) containing the viral
Diagnosed by: Microscopic: cardinal histologic change suppurative
hemagglutinin & neuramidase which determines the
o Detection of virus – PCR destruction of the lung parenchyma within the central
subtype of virus (H1 to H3; N1 to N2)
o Detection of antibodies to the virus area of cavitation
Patients who have died of SARS the lungs show diffuse o In chronic cases - fibroblastic proliferation
*Influenza B&C – do NOT show antigenic drift or shift, infects
alveolar damage and multinucleated giant cells produces a fibrous wall
mostly children
Influenza A
HOSPITAL-ACQUIRED PNEUMONIA Clinical Course:
Hospital acquired pulmonary infections C/M (same w/ bronchiectasis): cough, fever, and copious
Major cause of pandemic and epidemic influenza
the most common isolates: Gram-negative rods amounts of foul-smelling purulent or sanguineous
infection because of antigenic shift or drift
(Enterobacteriaceae and Pseudomonas species) & S. sputum
ANTIGENIC DRIFT – mutations of the hemagglutinin
aureus Complications:
and neuraminidase that allow the virus to escape most
host antibodies; give rise to the EPIDEMICS of o extension of the infection into the pleural cavity
influenza ASPIRATION PNEUMONIA o hemorrhage
ANTIGENIC SHIFT - occur when both the occurs in markedly debilitated patients or those who o development of brain abscesses or meningitis
hemagglutinin and the neuraminidase are replaced aspirate gastric contents either while unconscious (e.g., from septic emboli
through recombination of RNA segments with those of after a stroke) or during repeated vomiting o rarely, secondary amyloidosis (type AA)
animal viruses, making all individuals susceptible to the patients have abnormal gag and swallowing reflexes
new influenza virus; give rise to the PANDEMICS of In those who survive, lung abscess is a common
influenza complication
Prepared by: egbII, 10-27-11
CHRONIC PNEUMONIA 2.] Blastomycosis
Def: localized lesion in the immunocompetent patient, A soil-inhabiting, dimorphic fungus, caused by B.
with or without regional lymph node involvement dermatitidis
the inflammatory reaction is granulomatous There are three clinical forms:
Etiologic agent: o Pulmonary blastomycosis,
o bacteria (e.g., M. tuberculosis) o Disseminated blastomycosis,
o *fungi (e.g., Histoplasma capsulatum, o Primary cutaneous form (rare) - results from
Blastomyces, Coccidioides) direct inoculation of organisms into the skin
CXR: lobar consolidation, multilobar infiltrates, perihilar
1.] Histoplasmosis infiltrates, multiple nodules, or miliary infiltrates
Def: Acquired by inhalation of dust particles from soil Upper lobes - most frequently involved
contaminated with bird or bat droppings that contain
small spores (microconidia), the infectious form of the Morphology:
fungus Lung lesions – are suppurative granulomas
An intracellular parasite of macrophages Macrophages have a limited ability to ingest and kill B.
Clinical presentations and morphologic lesions includes: dermatitidis, and the persistence of the yeast cells leads
1) Self-limited & often latent primary pulmonary to continued recruitment of neutrophils
involvement that result in coin lesions on CXR; Skin and larynx is associated with marked epithelial
2) Chronic, progressive, secondary lung disease, hyperplasia
which is localized to the lung apices and causes
cough, fever, and night sweats; 3.] Coccidioidomycosis
3) Localized lesions in extrapulmonary sites, Etiologic agent: Coccidioides immitis
including mediastinum, adrenals, liver, or PULMONARY DISEASE IN HUMAN
Most of the primary infections with C. immitis are
meninges asymptomatic IMMUNODEFICIENCY VIRUS INFECTION
4) A widely disseminated disease in 10% of people have lung lesions, fever, cough, and Pulmonary disease continues to be the leading cause of
immunocompromised patients pleuritic pains, accompanied by erythema nodosum or morbidity and mortality in HIV-infected individuals
Macrophages are the major target of infection erythema multiforme (the San Joaquin Valley fever
Histoplasma infections are controlled by helper T cells complex) General principles of HIV-associated pulmonary
that recognize fungal cell wall antigens and heat-shock disease:
proteins and subsequently secrete IFN-γ, which Morphology: Bacterial pneumonias in HIV-infected persons are more
activates macrophages to secrete TNF & kill intracellular Primary and secondary lung lesions – are granulomatous common, more severe, & more often associated with
yeasts (same w/Histoplasma) bacteremia than in those without HIV infection
Antigen detection in body fluids is most useful in the o Organisms include: S. pneumoniae, S. aureus,
early stages, because antibodies are formed 2 to 6 H. influenzae, & gram-negative rods
weeks after infection Not all pulmonary infiltrates in HIV-infected individuals
PNEUMONIA IN THE IMMUNOCOMPROMISED
are infectious in etiology
HOST Noninfectious diseases: Kaposi sarcoma,
Morphology: o
Appearance of a pulmonary infiltrate, with or without primary lung cancer
Produce epithelioid cell granulomas that usually undergo signs of infection - one of the most common and serious
caseation necrosis & coalesce to produce large areas of The CD4+ T-cell count can define the risk of infection
complications in patients whose immune defenses are with specific organisms
consolidation & liquefy to form cavities (seen in patients suppressed caused by infection in normal hosts
with COPD) o Bacterial & Tubercular infections – have
lesions undergo fibrosis and concentric calcification higher CD4+ counts (>200 cells/mm)
(tree-bark appearance) o Pneumocystis pneumonia - usually strikes at
In fulminant disseminated histoplasmosis that CD4+ counts below 200 cells/mm3
occurs in immunosuppressed individuals, epithelioid cell o Cytomegalovirus & Mycobacterium avium
granulomas are NOT formed; instead, there are focal complex infections - are uncommon until the
accumulations of mononuclear phagocytes filled with very late stages of immunosuppression (CD4+
fungal yeasts counts <50 cells/mm)
Prepared by: egbII, 10-27-11
SUMMARY of Pulmonary Infections: Morphology:
The transplanted lung is subject to two major
complications: infection & rejection
1] Pulmonary infections
bacterial infections are most common
Most infections occur in the 3rd -12th month after
transplantation
2] Rejections
Acute rejection Chronic rejection
□ Occurs during the early □ By 3 to 5 years
weeks to months or years □ C/M: cough, dyspnea, and
later an irreversible decrease in
□ C/M: fever, dyspnea, lung function tests
cough, and radiologic □ Major morphologic: assoc.
infiltrates with bronchiolitis
□ Morphologic features: obliterans - partial or
inflammatory infiltrates complete occlusion of small
(lymphocytes, plasma cells, airways by fibrosis, with or
and few neutrophils and without active inflammation
eosinophils), either around
small vessels, in the
submucosa of airways, or
both
Acute cellular airway rejection is generally responsive to
therapy, but the treatment of established bronchiolitis
obliterans has been disappointing
Survival rates:
o 1 year – 78%
o 5 years – 50%
o 10 years – 26%
11. Tumors
90-95% are carcinomas
5% are bronchial carcinoids
10. Lung Transplantation 2-5% are mesenchymal & other neoplasms
The most common indications are: (FICE*)
o idiopathic/Familial pulmonary arterial CARCINOMAS:
hypertension Lung cancer - the most frequently diagnosed major
o Idiopathic pulmonary fibrosis cancer in the world
o Cystic fibrosis
o End-stage emphysema Etiology and Pathogenesis:
When bilateral chronic infection is present (e.g., cystic 1] Tobacco Smoking
fibrosis, bronchiectasis), both lungs of the recipient must 87% of lung carcinomas occur in active smokers or those
be replaced to remove the reservoir of infection who stopped recently
Prepared by: egbII, 10-27-11
Statistical association between the frequency of lung 4] Molecular Genetics
cancer and… It has been estimated that 10 to 20 genetic mutations
o (1) the amount of daily smoking, have occurred by the time the tumor is clinically
average smokers of cigarettes have a apparent
10x risk of developing lung cancer Lung cancers can be divided into two clinical subgroups:
heavy smokers (more than 40 small cell carcinoma and non-small cell carcinoma
cigarettes per day for several years) The dominant oncogenes that are frequently involved
have a 60x greater risk in lung cancer include c-MYC, KRAS, EGFR, c-MET, and
o (2) the tendency to inhale, and c-KIT
o (3) the duration of the smoking habit The commonly deleted or inactivated tumor
Cessation of smoking for 10 years suppressor genes include: p53, RB1, p16(INK4a), and
reduces risk but never to control levels multiple loci on chromosome 3p
Only 11% of heavy smokers develop lung cancer in their Small cell lung carcinoma is associated with:
lifetime o C-KIT (40–70%), MYCN and MYCL (20–30%),
Lung tumors of smokers frequently contain a typical, p53 (90%), 3p (100%), RB (90%), and BCL2
though not specific, molecular fingerprint in the form of (75–90%)
G : C > T : A mutations in the p53 gene that are Non-small cell lung carcinoma is associated with:
probably caused by benzo[a]pyrene, one of the many o EGFR (25%), KRAS (10–15%), p53 (50%), p16
carcinogens in tobacco smoke INK4a (70%)
Experimental work: More than 1200 substances of Telomerase activity is increased in over 80% of lung
cigarettes are potential carcinogens w/c contains: tumor tissues
o Initiators: polycyclic aromatic hydrocarbons People with certain alleles of CYP1A1* have an
Morphology:
such as benzo[a]pyrene increased capacity to metabolize procarcinogens derived
o Promoters: phenol derivatives from cigarette smoke and, conceivably, incur the Lung carcinomas arise most often in hilus of the lung
o Radioactive elements: greatest risk of developing lung cancer Carcinomas of the lung arise in the periphery of the lung
polonium-210, from the alveolar septal cells or terminal bronchioles
carbon-14, and 5] Precursor Lesions Gross:
potassium-40 (1) Squamous dysplasia and carcinoma in situ o Irregular warty excrescence
o Other contaminants: (2) Atypical adenomatous hyperplasia o produce an intraluminal mass
arsenic, nickel, molds, & additives (3) Diffuse idiopathic pulmonary neuroendocrine cell o cauliflower-like intraparenchymal mass
The few cancers that have developed have been hyperplasia o neoplastic tissue is gray-white and firm to hard
bronchioloalveolar carcinomas, a type of tumor that Distant spread of lung carcinoma occurs through both
is not strongly associated with smoking in humans *World Health Organization Histologic Classification lymphatic and hematogenous pathways
o Adrenals (50%)
of Malignant Epithelial Lung Tumors o Liver (30-50%)
2] Industrial Hazards
Adenocarcinoma (males 37%, females 47%) o Brain (20%)
High-dose ionizing radiation is carcinogenic
Squamous cell carcinoma (males 32%, females 25%) o Bone (20%)
Uranium - a weak radioactive, but lung cancer rates
Small cell carcinoma (males 14%, females 18%) Tumors often spread early throughout the body
among
Large cell carcinoma (males 18%, females 10%) EXCEPT for squamous cell carcinoma, which
o nonsmoking uranium miners are 4x higher
o smoking miners are about 10x higher metastasizes outside the thorax
Clinical use: Direct extension: pleural cavity, pericardium
Asbestos
*Various histologic types of lung cancer can be clustered
o Asbestos workers who do not smoke have a 5x
into 2 groups on the basis of likelihood of metastases & Clinical Course:
greater risk of developing lung cancer
response to available therapies: *Most patients are in 50s with symptoms of several
o Smoker – 50-90x greater risk
o Small cell carcinomas (almost always months durations
metastatic, high initial response to o Cough (75%)
3] Air Pollution
chemotherapy) o Weight loss (40%)
Attention has been drawn to the potential problem of
o Non-small cell carcinomas (less often o Chest pain (40%)
indoor air pollution, especially by radon
metastatic, less responsive) o Dyspnea (20%)
Radon is a ubiquitous radioactive gas
With strong relationship to smoking is:
o Squamous cell & Small cell carcinoma
Prepared by: egbII, 10-27-11
Bronchioloalveolar carcinomas are noninvasive tumors Nonmucinous Mucinous A single variant of small cell carcinoma is recognized
and do not metastasize; unless resected, they kill by >Consist of a peripheral >Tend to spread aerogenously, Believed to arise from neuroendocrine progenitor cells of
suffocation lung nodule with only forming satellite tumors the lining bronchial epithelium
rare aerogenous spread >Has distinctive, tall, columnar o Secrete polypeptide hormones &
1.] Adenocarcinoma – 25-40% >Has columnar, peg- cells with cytoplasmic and intra- parathormone-like and other hormonally
A malignant epithelial tumor with glandular shaped, or cuboidal cells alveolar mucin, growing along active products
differentiation or mucin production by the tumor >Amenable to surgical the alveolar septa o Presence of neuroendocrine markers:
cells resection with an >Resembling lobar pneumonia & chromogranin, synaptophysin, Leu-7 (in 75% of
Grow in various patterns: acinar, papillary, excellent 5-year survival are less likely to be cured by cases)
bronchioloalveolar, and solid with mucin formation surgery o Neurosecretory granules
o Only pure bronchioloalveolar carcinoma has Mutations:
distinct gross, microscopic, and clinical features 2.] Squamous Cell Carcinoma – 25-40% o P53 (50-80%)
Most common type of lung cancer in women and o RB tumor suppressor genes (80-100%)
Most commonly found in men and is closely correlated
nonsmokers High levels of the anti-apoptotic protein BCL2 (in 90%
with a smoking history
Lesions are usually more peripherally located, and tend of tumors) with a low frequency of pro-apoptotic
With p53 mutations: highest frequency compared to
to be smaller protein BAX
other histologic type
Grow more slowly than squamous cell carcinomas but Loss of protein expression of the tumor suppressor gene
tend to metastasize widely and earlier RB1 4.] Large Cell Carcinoma – 10-15%
Associated with scarring Inactivated cyclin-dependent kinase: *Def: undifferentiated malignant epithelial tumor that
Less frequently associated with a history of smoking o CDK-inhibitor p16(INK4a) lacks the cytologic features of small-cell carcinoma and
(still, greater than 75% in smokers) than are squamous glandular or squamous differentiation
or small cell carcinomas (>98% in smokers) Microscopic findings: One histologic variant: Large cell neuroendocrine
Mutations: Presence of keratinization and/or intercellular bridges carcinoma
o KRAS mutations Keratinization may take the form of squamous pearls or o Recognized by such features:
o p53, RB1, p16 mutations and inactivation individual cells with markedly eosinophilic dense Trabecular,
have the same frequency in adenocarcinoma as cytoplasm Organoid nesting,
in squamous cell carcinoma Higher mitotic activity Rosette-like,
o epidermal growth factor receptor gene (EGFR) Palisading patterns
o c-MET o *These features suggest neuroendocrine
3.] Small Cell Carcinoma – 20-25% differentiation
Def: Highly malignant tumor has a distinctive cell type
Bronchioloalveolar Carcinoma
Most common pattern associated with ectopic hormone
Occurs in the pulmonary parenchyma in the terminal
production
Combined Carcinoma
bronchioloalveolar regions Approximately 10% of all lung carcinomas
Strong relationship with SMOKING
Occurs in 1-9% of all lung cancers Have a combined histology, including two or more of the
Occur both in major bronchi & periphery of the lung
There is NO known preinvasive phase or carcinoma in above types
Gross (always occurs in):
situ
Peripheral portions Prognosis of LUNG CANCER:
Single nodule or multiple diffuse nodules - sometimes Microscopic findings: Overall 5-year survival rate is only 15%
coalesce to produce a pneumonia-like consolidation In general, the adenocarcinoma and squamous cell
Small epithelial cells, with scant cytoplasm, ill-defined
Have a mucinous, gray translucence when secretion is patterns tend to remain localized longer and have a
cell borders, finely granular nuclear chromatin (“salt
present slightly better prognosis than do the undifferentiated
and pepper pattern”), and absent or inconspicuous
cancers, which are usually advanced by the time they
nucleoli
Microscopic findings: are discovered
Cells are round, oval, or spindle-shaped, and nuclear
o Pure bronchioloalveolar growth pattern with no evidence Small cell CA – sensitive to radiochemo therapy &
molding is prominent
of stromal, vascular, or pleural invasion surgical resection is ineffective
The mitotic count is high
o Key feature: “lepidic” – carcinoma grow along o Untreated cases survival rate – 6-17 wks
Cells grow in clusters that exhibit neither glandular nor
preexisting structures without destruction of alveolar o With treatment survival rate – about 1 year
squamous organization
architecture Necrosis is common and often extensive
*Have two subtypes: o Necrotic tumor cells (Azzopardi effect) is
frequently present
Prepared by: egbII, 10-27-11
o C/M:
severe pain in the distribution of the
ulnar nerve
Horner syndrome (enophthalmos,
ptosis, miosis, and anhidrosis)
NEUROENDOCRINE PROLIFERATIONS & TUMORS
Normal lung contains neuroendocrine cells within the
epithelium as single cells or as clusters, the
neuroepithelial bodies
Neoplasms of neuroendocrine cells in the lung include:
1. Benign tumorlets
small, inconsequential, hyperplastic nests of
neuroendocrine cells seen in areas of scarring or chronic
inflammation
2. Carcinoids tumors
Paraneoplastic Syndromes highly aggressive small cell carcinoma and large cell
Lung carcinoma can be associated with several neuroendocrine carcinoma of the lung
paraneoplastic syndromes: represent 1% to 5% of all lung tumors
ADH def. – hyponatremia (small cell CA) younger than 40 years of age
ACTH – Cushing syndrome (small cell CA) incidence is equal for both sexes
Parathormone, Parathyroid hormone related peptide, 20% to 40% of patients are nonsmokers
prostaglanding E & some cytokines – hyperkalemia Carcinoid tumors are low-grade malignant epithelial
(SCCA) neoplasms that are subclassified into typical and atypical
Calcitonin – hypocalcemia carcinoids
Gonadotropins – gynecomastia o Typical carcinoids have no p53 mutations or
Serotonin & Bradykinin – associated with carcinoid abnormalities of BCL2 and BAX expression, while
syndrome atypical carcinoids show these changes
ACTH and ADH are predominantly small cell carcinomas, Morphology:
whereas those that produce hypercalcemia are mostly may arise centrally or may be peripheral
squamous cell tumors GROSS:
o central tumors grow as finger-like or spherical
Other systemic manifestations of lung carcinoma: polypoid masses that commonly project into the
Lambert-Eaton myasthenic syndrome - muscle lumen of the bronchus and are usually covered
weakness is caused by auto-antibodies (possibly elicited by an intact mucosa; exceed 3 to 4 cm in
by tumor ionic channels) directed to the neuronal diameter; produce little intraluminal mass but
calcium channel instead penetrate the bronchial wall to fan out in
Peripheral neuropathy - usually purely sensory the peribronchial tissue – called button lesion
Acanthosis nigricans o Peripheral tumors are solid and nodular
Leukemoid reactions Histologically:
Hypertrophic pulmonary osteoarthropathy - o the tumor is composed of organoid, trabecular,
associated with clubbing of the fingers palisading, ribbon, or rosette-like arrangements
Pancoast tumors of cells separated by a delicate fibrovascular
o Apical lung cancers in the superior pulmonary stroma
~egbii~
sulcus that invade the neural structures around o individual cells are quite regular and have
the trachea, including the cervical sympathetic uniform round nuclei and a moderate amount of
plexus eosinophilic cytoplasm
Prepared by: egbII, 10-27-11
Typical carcinoids
o have fewer than 2 mitoses/ 10 high-power fields
Grossly, the lesion is firm, 3 to 10 cm in diameter, and
grayish white 12. Pleura
and lack necrosis Microscopically, there is proliferation of spindle-shaped
Atypical carcinoids fibroblasts and myofibroblasts, lymphocytes, plasma A] PLEURAL EFFUSION
o have between 2 -10 mitoses/ 10 high-power cells, and peripheral fibrosis A common manifestation of both primary and secondary
fields and/or foci of necrosis anaplastic lymphoma kinase (ALK) gene, located on pleural diseases, which may be inflammatory or
o show increased pleomorphism, have more 2p23, has been implicated in the pathogenesis of this noninflammatory
prominent nucleoli, and are more likely to grow tumor Normally, no more than 15 mL of serous, relatively
in a disorganized fashion and invade lymphatics acellular, clear fluid lubricates the pleural surface
3. Tumors in the mediastinum Accumulation of pleural fluid occurs in the following
On electron microscopy arise in mediastinal structures or may be metastatic settings:
the cells exhibit the dense-core granules characteristic of from the lung or other organs o ↑ hydrostatic pressure (CHF)
other neuroendocrine tumors may also invade or compress the lungs
Immunohistochemistry o ↑ vascular permeability (pneumonia)
Table 15-13 lists the most common tumors in the
found to contain serotonin, neuron-specific enolase, various compartments of the mediastinum
o ↑ intrapleural negative pressure (atelectasis)
bombesin, calcitonin, or other peptides o ↓ osmotic pressure (nephrotic syndrome)
SUPERIOR ANTERIOR o ↓ lymphatic drainage (mediastinal
Clinical Features: A] Lymphoma A] Thymoma carcinomatosis)
cough, hemoptysis, impairment of drainage of B] Thymoma B] Teratoma
respiratory passages with secondary infections, C] Thyroid lesions C] Lymphoma Inflammatory Pleural Effusions
bronchiectasis, emphysema, and atelectasis D] Metastatic carcinoma D] Thyroid lesions
Classic carcinoid syndrome: intermittent attacks of Serofibrinous pleuritis
E] Parathyroid tumors E] Parathyroid tumors o Inflammation in adjacent lung
diarrhea, flushing, and cyanosis MIDDLE POSTERIOR
Most bronchial carcinoids… o Collagen vascular disease
A] Bronchogenic cyst A] Neurogenic tumors Suppurative pleuritis (empyema)
o Do not have secretory activity and do not B] Pericardial cyst (schwannoma, neurofibroma)
metastasize to distant sites but follow a o Results from bacterial or mycotic seeding of the
C] Lymphoma B] Lymphoma pleural space
relatively benign course for long periods, C] Gastroenteric hernia
o and are therefore amenable to resection o Empyema is characterized by loculated, yellow-
green, creamy pus composed of masses of
METASTATIC TUMORS neutrophils admixed with other leukocytes
MISCELLANEOUS TUMORS Lung - the most common site of metastatic neoplasms Hemorrhagic pleuritis
1. Lung hamartoma spread to the lungs via the blood or lymphatics or by o Manifested by sanguineous inflammatory
usually discovered as an incidental, rounded focus of direct continuity exudates
radio-opacity (coin lesion) on a routine chest film Growth of contiguous tumors into the lungs occurs most o Found in hemorrhagic diatheses, rickettsial
less than 3 to 4 cm in diameter often with esophageal carcinomas and mediastinal diseases, and neoplastic involvement of the
chromosomal aberrations involving either 6p21 or lymphomas pleural cavity
12q14–q15
Microscopic findings Morphology: Noninflammatory Pleural Effusions
o Consists of nodules of connective tissue Gross: multiple discrete nodules (cannonball lesions) are Hydrothorax
intersected by epithelial clefts scattered throughout all lobes o Noninflammatory collections of serous fluid
o Epithelial clefts are lined by ciliated columnar Microscopic findings: within the pleural cavities
epithelium or nonciliated epithelium o Confined to peribronchial or perivascular spaces o fluid is clear and straw colored
o Subpleural lymphatics may contain the tumor o The most common cause of hydrothorax is
2. Inflammatory myofibroblastic tumor o Diffuse intralymphatic dissemination dispersed cardiac failure*
more common in children; equal male-to-female ratio throughout the peribronchial & perivascular Hemothorax
Presenting symptoms : fever, cough, chest pain, and channels o Rscape of blood into the pleural cavity
hemoptysis o Microscopic tumor emboli may be present o D/t: ruptured aortic aneurysm & trauma
Imaging studies show a single (rarely multiple) round, Chylothorax
well-defined, usually peripheral mass with calcium o An accumulation of milky fluid, usually of
deposits lymphatic origin, in the pleural cavity
Prepared by: egbII, 10-27-11
o Chyle is milky white because it contains finely Malignant Mesothelioma 1. Epithelioid type
emulsified fats. Arise from either the visceral or the parietal pleura consists of cuboidal, columnar, or flattened cells forming
o Most often caused by thoracic duct trauma or Also arise in peritoneum, pericardium, tunica vaginalis & tubular or papillary structures resembling
obstruction that secondarily causes rupture of genital tract adenocarcinoma
major lymphatic ducts Peritoneal mesotheliomas – particularly related to
heavy asbestos exposure 2. Mesothelioma include:
B] PNEUMOTHORAX o 50% of patients have pulmonary fibrosis 1) (+) staining for acid mucopolysaccharide, which is
o Assoc. w/ intestinal obstruction inhibited by previous digestion by hyaluronidase
Def: refers to air or gas in the pleural cavities and may
2) Lack of staining for carcinoembryonic antigen & other
be spontaneous, traumatic, or therapeutic
C/M: epithelial glycoprotein antigens, markers that are
Spontaneous pneumothorax may complicate any
Chest pain, dyspnea, recurrent pleural effusions generally expressed by adenocarcinoma
form of pulmonary disease that causes rupture of an
Lung is invaded directly with metastatic spread to hilar 3) Strong staining for keratin proteins, with accentuation of
alveolus
lymph nodes & eventually to liver & distant organs perinuclear rather than peripheral staining
o Mostly associated with: emphysema, asthma,
50% die within 12 months of Dx & few survive longer 4) (+) staining for calretinin, Wilms tumor 1 (WT-1),
and tuberculosis
than 2 years cytokeratin 5/6, & D2–40
o Idiopathic form: young people, rupture of small,
5) On electron microscopy: the presence of long microvilli
peripheral, usually apical subpleural blebs &
and abundant tonofilaments but absent microvillous
subsides spontaneously Cytogenic studies:
rootlets and lamellar bodies
Traumatic pneumothorax is usually caused by some 60-80% have deletions in chromosomes 1p, 3p, 6q, 9p,
perforating injury to the chest wall, but sometimes the or 22q
**The mesenchymal type of mesothelioma appears as:
trauma pierces the lung and thus provides two avenues 31% have p16 mutations
o A spindle cell sarcoma, resembling fibrosarcoma
for the accumulation of air within the pleural spaces With low frequency of p53 mutations
(sarcomatoid type).
When the defect acts as a flap valve and permits the presence of SV40 (simian virus 40) viral DNA sequences
***The mixed type of mesothelioma contains both
entrance of air during inspiration but fails to permit its in 60% to 80% of pleural malignant mesotheliomas
epithelioid and sarcomatoid patterns
escape during expiration, it effectively acts as a pump
that creates the progressively increasing pressures of Morphology:
tension pneumothorax, which may be sufficient to Spreads widely in the pleural space and is usually
compress the vital mediastinal structures and the associated with extensive pleural effusion and direct
contralateral lung invasion of thoracic structures
Increased incidence among people with asbestos
C] PLEURAL TUMORS exposure
The most frequent metastatic malignancies arise from o Lifetime risk: 7-10%
primary neoplasms of the lung & breast. o Long latent period: 25-45 years
Gross:
Solitary Fibrous Tumor o Lungs is ensheathed by a layer of soft,
No relationship to asbestos gelatinous, grayish pink tumor tissue
Microscopic findings:
Gross: o 2 types of cells:
1. Epithelium-like lining cells
Small (1-2 cm) or large
2. Mesenchymal stromal cells
Attached to the pleural surface by a pedicle
Dence fibrous tissue w/ occasional cysts filled with viscid
fluid
Microscopic findings:
Whorls of reticulum & collagen fibers among which are
interspersed spindle cells
MALIGNANT variant: with pleomorphism, mitotic activity,
necrosis, large size (>10 cm)
IHC: (+) CD34+ & (-) keratin useful in differentiating
from malignant mesothelioma w/c show opposite results
Prepared by: egbII, 10-27-11
You might also like
- Patho A 1. 5 Hemodynamic Disorders (Bongat, 2015)Document12 pagesPatho A 1. 5 Hemodynamic Disorders (Bongat, 2015)Grant GarcesNo ratings yet
- Migs (With Summary) +paoDocument6 pagesMigs (With Summary) +paoMigs MedinaNo ratings yet
- Robinson Pathology Chapter 20 KidneyDocument11 pagesRobinson Pathology Chapter 20 KidneyElina Drits100% (1)
- Study Notes Respiratory SystemDocument19 pagesStudy Notes Respiratory SystemAnde Mangkuluhur Azhari ThalibbanNo ratings yet
- 3 Surgery - Mediastinum and PleuraDocument6 pages3 Surgery - Mediastinum and PleuraCassey Koi FarmNo ratings yet
- Pathology B - Gastrointestinal Tract (Esguerra, 2015)Document18 pagesPathology B - Gastrointestinal Tract (Esguerra, 2015)Ars MoriendiNo ratings yet
- Dse Pathogenesis/ Causes Diagnosis Complications TX Prognosis NotesDocument5 pagesDse Pathogenesis/ Causes Diagnosis Complications TX Prognosis NotesLuka Desabelle- JustoNo ratings yet
- ENT 1.2 Diseases of The Nose, Paranasal Sinuses, and Face PDFDocument18 pagesENT 1.2 Diseases of The Nose, Paranasal Sinuses, and Face PDFZazaNo ratings yet
- Gastrointestinal Tract PathologyDocument12 pagesGastrointestinal Tract PathologyTurinawe Bin ByensiNo ratings yet
- Pathophysiology of Respiratory System Feghiu I., Tacu L. Lutan VDocument55 pagesPathophysiology of Respiratory System Feghiu I., Tacu L. Lutan VLunguVictoriaNo ratings yet
- Hemoptysis - Case Presentation and DiscussionDocument52 pagesHemoptysis - Case Presentation and DiscussionPGHC100% (1)
- Systemic Pathology Study NotesDocument33 pagesSystemic Pathology Study NotesLaura BourqueNo ratings yet
- Pediatrics SamplexDocument6 pagesPediatrics SamplexThea SansonNo ratings yet
- Diseases of Blood Vessels Dr. Fe A. Bartolome, Maed, FpasmapDocument15 pagesDiseases of Blood Vessels Dr. Fe A. Bartolome, Maed, FpasmapJayesh MahajanNo ratings yet
- Headache History: Introduction - WIPPPDocument4 pagesHeadache History: Introduction - WIPPPAmjad_2020No ratings yet
- L6-PATHO-Neoplasia (Sept2821)Document12 pagesL6-PATHO-Neoplasia (Sept2821)Erald PaderangaNo ratings yet
- High Yield OSCE - PediaDocument1 pageHigh Yield OSCE - Pediarere choiNo ratings yet
- Pathophysiology of AgingDocument5 pagesPathophysiology of AgingRemelou Garchitorena AlfelorNo ratings yet
- Gastrointestinal Diseases Part1Document7 pagesGastrointestinal Diseases Part1sarguss14100% (1)
- EmfizemulDocument19 pagesEmfizemulMirela IoanaNo ratings yet
- Seatsallotement MedicalDocument26 pagesSeatsallotement MedicalSandeep BhatNo ratings yet
- ENDOCRINE PATHOLOGY WebpathDocument35 pagesENDOCRINE PATHOLOGY Webpathapi-3766657No ratings yet
- Surgical Anatomy of The Chest Wall, Pleura, and MediastinumDocument8 pagesSurgical Anatomy of The Chest Wall, Pleura, and MediastinumNooneNo ratings yet
- Small Intestine 01 PDFDocument9 pagesSmall Intestine 01 PDFfadoNo ratings yet
- Surgery QuestionsDocument19 pagesSurgery QuestionsdocaliNo ratings yet
- Test Bank On GI PathologyDocument14 pagesTest Bank On GI PathologySeff CausapinNo ratings yet
- Distal To Ligament of Treitz: CausesDocument8 pagesDistal To Ligament of Treitz: CausesKiara GovenderNo ratings yet
- Pathology of Digestive SystemDocument28 pagesPathology of Digestive SystemDianNursyifaRahmahNo ratings yet
- PRINCIPLES OF SURGERY (James R. Hupp Chapter 3 Notes) : 1. Develop A Surgical DiagnosisDocument5 pagesPRINCIPLES OF SURGERY (James R. Hupp Chapter 3 Notes) : 1. Develop A Surgical DiagnosisSonia LeeNo ratings yet
- Female Genital TractDocument5 pagesFemale Genital Tractsarguss14100% (1)
- Ch. 10 Skin, Hair and Nails - Bickley and BatesDocument2 pagesCh. 10 Skin, Hair and Nails - Bickley and BatesfufuNo ratings yet
- Anatomy of The Nose & Paranasal Air SinusesDocument4 pagesAnatomy of The Nose & Paranasal Air SinusesMusfique RashidNo ratings yet
- PATHO 4-3 Diseases of The EsophagusDocument7 pagesPATHO 4-3 Diseases of The EsophagusMiguel Cuevas DolotNo ratings yet
- UROLOGY 2020 (Doc BarcenasDocument33 pagesUROLOGY 2020 (Doc BarcenasJüdith Marie Reyes BauntoNo ratings yet
- Anat 4.3 GIT Histo - ZuluetaDocument8 pagesAnat 4.3 GIT Histo - Zuluetalovelots1234No ratings yet
- HerniaDocument5 pagesHerniasarguss14100% (5)
- IKD9 - Radiological Evaluation of Renal CystsDocument26 pagesIKD9 - Radiological Evaluation of Renal CystsRenal Association MauritiusNo ratings yet
- Molecular Diagnosis of Cystic FibrosisDocument25 pagesMolecular Diagnosis of Cystic Fibrosisyugen31No ratings yet
- Notes EntDocument43 pagesNotes Entadriana azmanNo ratings yet
- Ent Diseases of The Oral and Pharynx Dr. UyDocument7 pagesEnt Diseases of The Oral and Pharynx Dr. UyAileen EmyNo ratings yet
- Trusted Medical Answers-In Seconds.: Chest PainDocument22 pagesTrusted Medical Answers-In Seconds.: Chest PainntnquynhproNo ratings yet
- Neuro Written II TablesDocument10 pagesNeuro Written II TablesSolomon Seth SallforsNo ratings yet
- Robbins - 12 The HeartDocument5 pagesRobbins - 12 The HeartCM NajitoNo ratings yet
- Physiologic Monitoring of The Surgical PatientDocument56 pagesPhysiologic Monitoring of The Surgical PatientSeid Adem100% (2)
- Polycystic Kidney DiseaseDocument9 pagesPolycystic Kidney DiseaseCésar Aguilar ContrerasNo ratings yet
- IMCOMPILEDDocument16 pagesIMCOMPILEDFerdie MarcosNo ratings yet
- #Acute & Chronic Otitis MediaDocument3 pages#Acute & Chronic Otitis MediaameerabestNo ratings yet
- Mediastinum and Its ContentsDocument11 pagesMediastinum and Its ContentsPap YeeNo ratings yet
- PathologyDocument28 pagesPathologyninja-2001No ratings yet
- IM-Module B Summarized Notes (IBD)Document69 pagesIM-Module B Summarized Notes (IBD)DeepbluexNo ratings yet
- Head & Neck: Most CommonDocument6 pagesHead & Neck: Most CommonJüdith Marie Reyes BauntoNo ratings yet
- 14 Patho Head and Neck PathologyDocument10 pages14 Patho Head and Neck PathologyMartin TanNo ratings yet
- Peptic Ulcers: DR Yotham Phiri Mmed (Surg)Document46 pagesPeptic Ulcers: DR Yotham Phiri Mmed (Surg)Emmanuel MukukaNo ratings yet
- Respiratory Pathophysiology: B. Pimentel, M.D. University of Makati College of NursingDocument12 pagesRespiratory Pathophysiology: B. Pimentel, M.D. University of Makati College of NursingDoc JacqueNo ratings yet
- Chapter 10 - Diseases of Infancy and ChildhoodDocument17 pagesChapter 10 - Diseases of Infancy and ChildhoodAgnieszka Wisniewska100% (1)
- Physio Ob ReviewDocument368 pagesPhysio Ob ReviewMark LopezNo ratings yet
- Gyne 2.6 - Benign and Malignant Tumors of The Ovaries and Fallopian TubesDocument8 pagesGyne 2.6 - Benign and Malignant Tumors of The Ovaries and Fallopian TubesVon HippoNo ratings yet
- PleuraDocument6 pagesPleuraameerabest100% (1)
- Problem-based Approach to Gastroenterology and HepatologyFrom EverandProblem-based Approach to Gastroenterology and HepatologyJohn N. PlevrisNo ratings yet
- DTTB Monitoring Tool - Ver2Document5 pagesDTTB Monitoring Tool - Ver2Zandra Lyn AlundayNo ratings yet
- Orca Share Media1571859487287Document1 pageOrca Share Media1571859487287Zandra Lyn AlundayNo ratings yet
- Invitation Letter RCCEMT PDFDocument1 pageInvitation Letter RCCEMT PDFZandra Lyn AlundayNo ratings yet
- Final Checklist - HospitalDocument21 pagesFinal Checklist - HospitalZandra Lyn Alunday100% (1)
- Blue Book 2021Document82 pagesBlue Book 2021Tampungan AkunNo ratings yet
- Plan Testing FormDocument1 pagePlan Testing FormZandra Lyn AlundayNo ratings yet
- Course OutlineDocument1 pageCourse OutlineZandra Lyn AlundayNo ratings yet
- Hesitancy Monitoring Tool For Social MobilizationDocument6 pagesHesitancy Monitoring Tool For Social MobilizationZandra Lyn AlundayNo ratings yet
- AttendanceDocument1 pageAttendanceZandra Lyn AlundayNo ratings yet
- National SituationerDocument8 pagesNational SituationerZandra Lyn AlundayNo ratings yet
- SKM C360i-D22091315230 SVD Ebt-2 WordDocument11 pagesSKM C360i-D22091315230 SVD Ebt-2 WordZandra Lyn AlundayNo ratings yet
- Ao 2022 OmnibusDocument13 pagesAo 2022 OmnibusZandra Lyn AlundayNo ratings yet
- Intro To IcsDocument90 pagesIntro To IcsZandra Lyn AlundayNo ratings yet
- Pcap PathophysiologyDocument3 pagesPcap PathophysiologyZandra Lyn AlundayNo ratings yet
- Basic Incident Command System CourseDocument23 pagesBasic Incident Command System CourseZandra Lyn Alunday100% (1)
- Group PresentationDocument9 pagesGroup PresentationZandra Lyn AlundayNo ratings yet
- Emergency Operations Center Training CourseDocument2 pagesEmergency Operations Center Training CourseZandra Lyn AlundayNo ratings yet
- DAILY CENSUS 10-11 Mar 2017Document4 pagesDAILY CENSUS 10-11 Mar 2017Zandra Lyn AlundayNo ratings yet
- The NeckDocument2 pagesThe NeckZandra Lyn AlundayNo ratings yet
- Regurgitation and Aspiration: Learning ObjectivesDocument4 pagesRegurgitation and Aspiration: Learning ObjectivesGabriella Fritzie TanNo ratings yet
- Assignment 2023 24 DPS DurgapurDocument24 pagesAssignment 2023 24 DPS DurgapurSubham RoyNo ratings yet
- Murphy-2016-Microbes and Music - Assessing RiskDocument5 pagesMurphy-2016-Microbes and Music - Assessing RiskSarah MorrisNo ratings yet
- Section-I Mcqs and SeqsDocument3 pagesSection-I Mcqs and SeqsSadia NasirNo ratings yet
- 04 Respiration 2022 - AnswersDocument10 pages04 Respiration 2022 - Answersria khanNo ratings yet
- Unit 7 The Respiratory System: Learning OutcomesDocument9 pagesUnit 7 The Respiratory System: Learning OutcomesDeolita BadiangNo ratings yet
- Class 9 BiologyDocument7 pagesClass 9 BiologyGreatAkbar1No ratings yet
- Introduction To Microfluidics 2Nd Edition Patrick Tabeling Full ChapterDocument67 pagesIntroduction To Microfluidics 2Nd Edition Patrick Tabeling Full Chapterrobin.mccomb793100% (9)
- VTU Exam Question Paper With Solution of 21BE45 Biology For Engineers Oct-2023-Akshitha C ADocument26 pagesVTU Exam Question Paper With Solution of 21BE45 Biology For Engineers Oct-2023-Akshitha C Arockyv9964No ratings yet
- Respiratory Disorders 2.2Document74 pagesRespiratory Disorders 2.2Deenjane Nishi IgnacioNo ratings yet
- The Management of Near DrowningDocument8 pagesThe Management of Near DrowningneddiantiseptikaNo ratings yet
- CHAPTER 13: The Respiratory SystemDocument11 pagesCHAPTER 13: The Respiratory SystemAthena ChoiNo ratings yet
- IB HL Biology - Paper 3 - Condensed Answers To Past Paper QuestionsDocument9 pagesIB HL Biology - Paper 3 - Condensed Answers To Past Paper QuestionsIsabella María Rosales PorrasNo ratings yet
- Transport of Oxygen and Carbon Dioxide in Blood and Tissue FluidsDocument22 pagesTransport of Oxygen and Carbon Dioxide in Blood and Tissue FluidsmutialailaniNo ratings yet
- F211 OCR Biology 2011 January Mark SchemeDocument16 pagesF211 OCR Biology 2011 January Mark SchemeseongilNo ratings yet
- Worksheet Grade 8 Gas ExchangeDocument4 pagesWorksheet Grade 8 Gas ExchangeListya RahmawatiNo ratings yet
- Histology: Ust Faculty of Medicine and Surgery Class of 2016Document14 pagesHistology: Ust Faculty of Medicine and Surgery Class of 2016Ashley Beatriz PascualNo ratings yet
- HY PulmonaryDocument67 pagesHY PulmonaryJeniNo ratings yet
- December 2023 Current Affairs Magazine - MakeIASDocument73 pagesDecember 2023 Current Affairs Magazine - MakeIASHimanshu KesarwaniNo ratings yet
- RDS UptodateDocument14 pagesRDS UptodateYosef Dwi Cahyadi SalanNo ratings yet
- 8 Diseases of Infancy and ChildhoodDocument23 pages8 Diseases of Infancy and ChildhoodBalaji DNo ratings yet
- Cs Breast EngorgementDocument14 pagesCs Breast Engorgementamit85% (13)
- NFSC 471-Case StudyDocument8 pagesNFSC 471-Case Studyapi-242439244No ratings yet
- Reexpansion Pulmonary Edema : UpdateDocument6 pagesReexpansion Pulmonary Edema : UpdateWenny EudensiaNo ratings yet
- Functions of The Respiratory System: Inside-RestDocument7 pagesFunctions of The Respiratory System: Inside-RestRaven LacsonNo ratings yet
- Anatomy of The Respiratory SystemDocument4 pagesAnatomy of The Respiratory SystemMayden Grace GayatgayNo ratings yet
- Module 18 Respiratory FinalDocument15 pagesModule 18 Respiratory FinalJayR MendonesNo ratings yet
- MitochondriaDocument6 pagesMitochondriakevin smithNo ratings yet
- The Impact of Covid-19 Pandemic On Emotional and Mental Health of Senior High School Students in Isabela Sports Senior High SchoolDocument33 pagesThe Impact of Covid-19 Pandemic On Emotional and Mental Health of Senior High School Students in Isabela Sports Senior High SchoolMicha GuzmanNo ratings yet
- Histology MCQs Part 2Document54 pagesHistology MCQs Part 2RPh Krishna Chandra Jagrit71% (7)