Professional Documents
Culture Documents
Metoprolol (Lopressor, Toprol-XL) Considerations For Use : Mechanism of Action Dosing
Metoprolol (Lopressor, Toprol-XL) Considerations For Use : Mechanism of Action Dosing
Uploaded by
B PCopyright:
Available Formats
You might also like
- Drug Card MetoprololDocument3 pagesDrug Card Metoprololcelosia23100% (3)
- Aubrey Rose A. Vidon September 20, 2020 BSN 3Y1 - 2 Unit Task #4Document12 pagesAubrey Rose A. Vidon September 20, 2020 BSN 3Y1 - 2 Unit Task #4AriaNo ratings yet
- V11.5 (Browser) Release NotesDocument531 pagesV11.5 (Browser) Release NotesB PNo ratings yet
- V11.5 (EXE) Release NotesDocument393 pagesV11.5 (EXE) Release NotesB PNo ratings yet
- Cardiohelp Basic TrainingDocument35 pagesCardiohelp Basic TrainingAhmadNo ratings yet
- LabetalolDocument3 pagesLabetalolTri Purma Sari50% (2)
- AMA Form PDFDocument1 pageAMA Form PDFB PNo ratings yet
- Clinical Cases in AnesthesiaDocument539 pagesClinical Cases in AnesthesiaCamila Rodrigues Jaña100% (6)
- 2014-2016 YDS Bağlaç SorularıDocument6 pages2014-2016 YDS Bağlaç SorularıFatih Mehmet ŞenNo ratings yet
- RiteMED MetoprololDocument7 pagesRiteMED MetoprololAngie MandeoyaNo ratings yet
- Adult: PO HTN Initial: 50-100 Mg/day in Single or Divided Doses Increase SlowlyDocument3 pagesAdult: PO HTN Initial: 50-100 Mg/day in Single or Divided Doses Increase SlowlyJoanna Marie Lumbre BalbiranNo ratings yet
- 8 Drug StudyDocument19 pages8 Drug StudyLoyloy D ManNo ratings yet
- Drug Profile (Bisoprolol)Document16 pagesDrug Profile (Bisoprolol)Maryam KhushbakhatNo ratings yet
- Metoprolol PDFDocument3 pagesMetoprolol PDFCandy San DiegoNo ratings yet
- RamiprilDocument3 pagesRamiprilNovi YulianaNo ratings yet
- Metoprolol and AmiodaroneDocument5 pagesMetoprolol and AmiodaroneNolte BombayNo ratings yet
- Formulary 3rd Shifing AudsDocument81 pagesFormulary 3rd Shifing AudsSheila Lyn LacsonNo ratings yet
- Emergency MedsDocument24 pagesEmergency MedsNursyNurse100% (1)
- Generic Name: Albuterol Brand Names: Ventolin, Proventil, Accuneb, Vospire, ProairDocument9 pagesGeneric Name: Albuterol Brand Names: Ventolin, Proventil, Accuneb, Vospire, Proairwasiq AhmedNo ratings yet
- Drug Study Mother TDocument14 pagesDrug Study Mother TEuzelle Jeena ArandaNo ratings yet
- Drug StudyDocument11 pagesDrug StudyHennah Reblando100% (3)
- Generic Name: Brand Name: Apo-Metoprolol, Betaloc, Lopressor, Novo-Metoprolol, Nu-Drug ClassificationDocument4 pagesGeneric Name: Brand Name: Apo-Metoprolol, Betaloc, Lopressor, Novo-Metoprolol, Nu-Drug ClassificationKat ZNo ratings yet
- Bisoprolol Fumarate (Drug Study)Document2 pagesBisoprolol Fumarate (Drug Study)Franz.thenurse688871% (7)
- Drugs That Require Frequent Monitoring: Contraindicated In: Side Effects of ToxicityDocument9 pagesDrugs That Require Frequent Monitoring: Contraindicated In: Side Effects of ToxicityRhene GazmenNo ratings yet
- Pharmacology 3Document7 pagesPharmacology 3Maria Nichole JavarNo ratings yet
- Medication Worksheet: (You Will Need To Make Additional Copies of This Form)Document2 pagesMedication Worksheet: (You Will Need To Make Additional Copies of This Form)mcarter402100% (1)
- Downloaded From FILIPINO NURSES CENTRAL: Drugs That Require Frequent MonitoringDocument8 pagesDownloaded From FILIPINO NURSES CENTRAL: Drugs That Require Frequent MonitoringFilipino Nurses CentralNo ratings yet
- Adrenergic Blockers AtenololDocument3 pagesAdrenergic Blockers AtenololPoinsithia OrlandaNo ratings yet
- Generic: Acetazolamide Brand: DiamoxDocument33 pagesGeneric: Acetazolamide Brand: DiamoxAshley Topp100% (1)
- Drugs That Require Frequent MonitoringDocument14 pagesDrugs That Require Frequent MonitoringnurseoncervixNo ratings yet
- FDC - IrrationalDocument30 pagesFDC - Irrationaldivya lohithNo ratings yet
- Labetalol Hydro ChlorideDocument3 pagesLabetalol Hydro Chlorideapi-3797941100% (1)
- Pharmacology Drug StudyDocument6 pagesPharmacology Drug StudyShene Claire VigillaNo ratings yet
- Drug StudyDocument4 pagesDrug StudyTeanu Jose Gabrillo TamayoNo ratings yet
- Clinical Medications Worksheets: (Why Med Ordered) Contraindications/warnings/interactionsDocument3 pagesClinical Medications Worksheets: (Why Med Ordered) Contraindications/warnings/interactionsENo ratings yet
- Drug Study CCUDocument5 pagesDrug Study CCUKrishna Faith P. DelaraNo ratings yet
- EsmololDocument3 pagesEsmololTri Purma SariNo ratings yet
- Table 20.2-7 Medications For Treating Alcohol DependenceDocument1 pageTable 20.2-7 Medications For Treating Alcohol DependenceAbdualaziz AlmalkiNo ratings yet
- Adrenergic Blockers NebivololDocument2 pagesAdrenergic Blockers NebivololPoinsithia OrlandaNo ratings yet
- LisinoprilDocument3 pagesLisinoprilLIEZEL GRACE VELAYONo ratings yet
- MetoprololDocument3 pagesMetoprololapi-3797941100% (3)
- Medication CardsDocument5 pagesMedication CardsAndrew Harrison Lewis0% (1)
- AmiodaroneDocument2 pagesAmiodaroneanindiawNo ratings yet
- Heart Talk (Ii) in Pandemic Of: COVID-19Document27 pagesHeart Talk (Ii) in Pandemic Of: COVID-19Alles FirmansyahNo ratings yet
- Emergency Drugs KathDocument29 pagesEmergency Drugs Kathmajin655No ratings yet
- Drugs That Require Frequent MonitoringDocument22 pagesDrugs That Require Frequent MonitoringNader Smadi100% (7)
- Drug StudyDocument12 pagesDrug StudyAngeli A EstilloreNo ratings yet
- Drug StudyDocument9 pagesDrug StudyChristine PunsalanNo ratings yet
- Chapter 7 (Adrenergic Antagonists)Document64 pagesChapter 7 (Adrenergic Antagonists)Aneeza AhmadNo ratings yet
- Emergency DrugsDocument7 pagesEmergency DrugsMarie Angeline ManzanoNo ratings yet
- MECFS Clinician Coalition Treatment Recs V1Document9 pagesMECFS Clinician Coalition Treatment Recs V1Emilie ChateletNo ratings yet
- Coreg (Carvedilol) 6.25mgDocument3 pagesCoreg (Carvedilol) 6.25mgE100% (2)
- Drug Study On Emergency DrugsDocument15 pagesDrug Study On Emergency DrugsDennise Juayang100% (1)
- Atrial Fibrillation TDDocument6 pagesAtrial Fibrillation TDapi-594366475No ratings yet
- 1-Amiodarone (Cordarone, Pacerone) : Duration: 30-45 MinutesDocument3 pages1-Amiodarone (Cordarone, Pacerone) : Duration: 30-45 MinutesMahmmoud FuqahaNo ratings yet
- Drug StudyDocument9 pagesDrug StudyEzshkha OngueNo ratings yet
- Montra PharmacologyDocument19 pagesMontra PharmacologytressNo ratings yet
- Hemostan, Methergine CA Gluconate2Document4 pagesHemostan, Methergine CA Gluconate2Stacy MC PelitoNo ratings yet
- BenazeprilDocument2 pagesBenazeprilFeliciaDorghamNo ratings yet
- Obat Tugas Dokter PhathimhaDocument6 pagesObat Tugas Dokter PhathimhaMegan LewisNo ratings yet
- NeoblocDocument2 pagesNeoblocianecunar100% (2)
- Drug Study - Metropolol Tartrate (Neobloc)Document2 pagesDrug Study - Metropolol Tartrate (Neobloc)mikErlhNo ratings yet
- Critical Care Medications: Anti-Arrhythmics Study Guide: Critical Care EssentialsFrom EverandCritical Care Medications: Anti-Arrhythmics Study Guide: Critical Care EssentialsNo ratings yet
- Critical Care Medications: Vasopressors, Inotropes and Anti-Hypertensives Study Guide: Critical Care EssentialsFrom EverandCritical Care Medications: Vasopressors, Inotropes and Anti-Hypertensives Study Guide: Critical Care EssentialsNo ratings yet
- Medical Encyclopedia XXL: Prof. J.P. Schadé, M.D., Ph.D. D.Sc.hcFrom EverandMedical Encyclopedia XXL: Prof. J.P. Schadé, M.D., Ph.D. D.Sc.hcNo ratings yet
- Elements of The Ippe and Awv: IPPE - G0402 Initial AWV - G0438 Subsequent AWV - G0439 Information GatheringDocument1 pageElements of The Ippe and Awv: IPPE - G0402 Initial AWV - G0438 Subsequent AWV - G0439 Information GatheringB PNo ratings yet
- Analysis of Florida's New Law Regarding Pelvic ExaminationsDocument2 pagesAnalysis of Florida's New Law Regarding Pelvic ExaminationsB PNo ratings yet
- Bardmoor YMCA ScheduleDocument2 pagesBardmoor YMCA ScheduleB PNo ratings yet
- AMA Form Pdf236798479485y9340yrDocument1 pageAMA Form Pdf236798479485y9340yrB PNo ratings yet
- Against Medical Advice FormDocument1 pageAgainst Medical Advice FormB P100% (1)
- ARM Workshops Updated 4.30.2021Document4 pagesARM Workshops Updated 4.30.2021B PNo ratings yet
- BayCare - Doc and Coding - Atrial FibrillationDocument1 pageBayCare - Doc and Coding - Atrial FibrillationB PNo ratings yet
- 2021 Midyear - Final ICD-10-CM MappingsDocument247 pages2021 Midyear - Final ICD-10-CM MappingsB PNo ratings yet
- Echo Curriculum Delivery Tool Nov2016Document11 pagesEcho Curriculum Delivery Tool Nov2016Joseph BarkerNo ratings yet
- Clinical Indications, Treatment and Current PracticeDocument14 pagesClinical Indications, Treatment and Current PracticefadmayulianiNo ratings yet
- Nursing MnemonicsDocument11 pagesNursing MnemonicsMarco CalvaraNo ratings yet
- Concept Map f21 FinishedDocument5 pagesConcept Map f21 Finishedapi-601070065No ratings yet
- Healthy Bodies Slide3Document11 pagesHealthy Bodies Slide3Larissa RevillaNo ratings yet
- Classifying Electrocardiograph Waveforms Using Trained Deep Learning Neural Network Based On Wavelet RepresentationDocument9 pagesClassifying Electrocardiograph Waveforms Using Trained Deep Learning Neural Network Based On Wavelet RepresentationIAES IJAINo ratings yet
- GRP 3 Case Study - BurnsDocument7 pagesGRP 3 Case Study - BurnsToffee Trugillo100% (1)
- Exam #4 Study Blueprint 2019Document2 pagesExam #4 Study Blueprint 2019Stephanie DeeNo ratings yet
- Perioperative Medicine Managing Surgical Patients With Medical Problems by Chikwe, Joanna Walther, Axel Jones, PhilipDocument462 pagesPerioperative Medicine Managing Surgical Patients With Medical Problems by Chikwe, Joanna Walther, Axel Jones, PhilipIbrahim AlmohiniNo ratings yet
- Plan of Care For:: Nursing Diagnosis: Excess Fluid VolumeDocument3 pagesPlan of Care For:: Nursing Diagnosis: Excess Fluid VolumeKenji CadizNo ratings yet
- Laporan FaalDocument25 pagesLaporan FaalAgnes NathaniaNo ratings yet
- Physical Examination Form: Return by July 17ThDocument1 pagePhysical Examination Form: Return by July 17ThFlorian Ananias ByarugabaNo ratings yet
- Anesthesia Challenges in Patent Ductus ArteriosusDocument2 pagesAnesthesia Challenges in Patent Ductus ArteriosusChee LeongNo ratings yet
- ThrombosisDocument11 pagesThrombosisNobby Onist JuniorNo ratings yet
- ENZYME by DR JoeanDocument8 pagesENZYME by DR JoeanHenry Teepoh100% (1)
- Qworld USMLE Step1 Course 2019Document91 pagesQworld USMLE Step1 Course 2019Qworld50% (2)
- Clinical ElectrocardiographyDocument134 pagesClinical ElectrocardiographyHossain MuhammadNo ratings yet
- The Metaboreflex SynchronousDocument19 pagesThe Metaboreflex SynchronousDanai ZogaNo ratings yet
- Physrev 00043 2016Document57 pagesPhysrev 00043 2016Arie AnggaNo ratings yet
- Sinus Brad, Tach, PAC, PVCDocument17 pagesSinus Brad, Tach, PAC, PVCEdRobertArnadNo ratings yet
- Medical Surgical Nursing IIDocument9 pagesMedical Surgical Nursing IIAdarsh PatelNo ratings yet
- IntelliVue MX500 Patient Monitor TDS PDFDocument24 pagesIntelliVue MX500 Patient Monitor TDS PDFbenyvladNo ratings yet
- Nclex Questions FinalsDocument301 pagesNclex Questions Finalsticoystephanie100% (2)
- Monitoring Patient (Deskripsi) Fiky Niswati Yuslihah (P17250193025)Document3 pagesMonitoring Patient (Deskripsi) Fiky Niswati Yuslihah (P17250193025)Fiky NiswatiNo ratings yet
- Sample Cardiac Calcium Scoring ReportDocument3 pagesSample Cardiac Calcium Scoring ReportZyad DoskiNo ratings yet
- DBEABE614Document6 pagesDBEABE614bariNo ratings yet
- Vasoactive Agents in Shock.2Document8 pagesVasoactive Agents in Shock.2Linamaria LozanoNo ratings yet
Metoprolol (Lopressor, Toprol-XL) Considerations For Use : Mechanism of Action Dosing
Metoprolol (Lopressor, Toprol-XL) Considerations For Use : Mechanism of Action Dosing
Uploaded by
B POriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Metoprolol (Lopressor, Toprol-XL) Considerations For Use : Mechanism of Action Dosing
Metoprolol (Lopressor, Toprol-XL) Considerations For Use : Mechanism of Action Dosing
Uploaded by
B PCopyright:
Available Formats
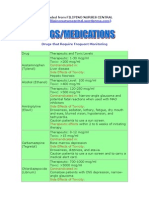
Metoprolol (Lopressor, Toprol-XL) Considerations for Use*
US/FDA Approved Indication: Heart Rate Control for Atrial Fibrillation
Black Box Warning* Abrupt cessation may exacerbate angina pectoris and MI.
Mechanism of Action Blocks binding of catecholamines to beta-1 receptors; Beta-1 selective
Dosing† Acute setting: 2.5 to 5 mg IV bolus over 2 minutes; may repeat every 5 minutes to a
maximum dose of 15 mg
Non-acute setting or maintenance:
Metoprolol tartrate (immediate-release): 25 to 100 mg PO twice daily
Metoprolol succinate (extended-release): 25 to 200 mg daily
Elderly: May need lower doses
Hepatic Impairment: May need lower doses
Renal Impairment: No dosage adjustment needed
Contraindications AV block
Bradycardia
cardiogenic shock
decompensated heart failure
sick sinus syndrome
pheochromocytoma
Major Side Effects hypotension, heart block, bradycardia, bronchospasm, HF
Dosage forms and Strengths PO:
25 mg, 50 mg, 100 mg immediate-release tablets (tartrate)
25 mg, 50 mg, 100 mg, 200 mg extended-release tablets (succinate)
IV: 5 mL ampules (1 mg/mL)
Special Notes Abrupt cessation my precipitate angina, MI, arrhythmias, or rebound HTN; discontinue
by tapering over 1-2 weeks.
Immediate-release form is metoprolol tartrate; extended-release form is metoprolol
succinate. When switching from immediate release to extended-release product, use
same total daily dose. The immediate and extended release products may not give
same clinical response on mg:mg basis; monitor response and side effects when
interchanging between metoprolol products.
Concomitant amiodarone, digoxin, disopyramide, or non-dihydropyridine calcium
channel blockers may increase the risk of bradycardia.
Monitor closely for HF exacerbation and hypotension when titrating dose.
Counseling Do not abruptly discontinue without physician’s advice.
Take with food or directly after eating.
Extended-release tablets may be broken in half, but do not chew or crush.
*Refer to prescribing information for more complete information.
†Dosages given in the table may differ from those recommended by the manufacturers.
Sources:
1. American College of Cardiology (ACC), American Heart Association (AHA), and the European Society of Cardiology (ESC). ACC/AHA/ESC 2006 Guidelines for the
Management of Patients With Atrial Fibrillation. Washington, DC: American College of Cardiology.
2. Heart Rhythm Society. AF360 Pocket Guide: Practical Rate and Rhythm Management of Atrial Fibrillation. 2010, Washington, DC: Heart Rhythm Society.
3. Tarascon Pocket Pharmacopoeia®2012.
You might also like
- Drug Card MetoprololDocument3 pagesDrug Card Metoprololcelosia23100% (3)
- Aubrey Rose A. Vidon September 20, 2020 BSN 3Y1 - 2 Unit Task #4Document12 pagesAubrey Rose A. Vidon September 20, 2020 BSN 3Y1 - 2 Unit Task #4AriaNo ratings yet
- V11.5 (Browser) Release NotesDocument531 pagesV11.5 (Browser) Release NotesB PNo ratings yet
- V11.5 (EXE) Release NotesDocument393 pagesV11.5 (EXE) Release NotesB PNo ratings yet
- Cardiohelp Basic TrainingDocument35 pagesCardiohelp Basic TrainingAhmadNo ratings yet
- LabetalolDocument3 pagesLabetalolTri Purma Sari50% (2)
- AMA Form PDFDocument1 pageAMA Form PDFB PNo ratings yet
- Clinical Cases in AnesthesiaDocument539 pagesClinical Cases in AnesthesiaCamila Rodrigues Jaña100% (6)
- 2014-2016 YDS Bağlaç SorularıDocument6 pages2014-2016 YDS Bağlaç SorularıFatih Mehmet ŞenNo ratings yet
- RiteMED MetoprololDocument7 pagesRiteMED MetoprololAngie MandeoyaNo ratings yet
- Adult: PO HTN Initial: 50-100 Mg/day in Single or Divided Doses Increase SlowlyDocument3 pagesAdult: PO HTN Initial: 50-100 Mg/day in Single or Divided Doses Increase SlowlyJoanna Marie Lumbre BalbiranNo ratings yet
- 8 Drug StudyDocument19 pages8 Drug StudyLoyloy D ManNo ratings yet
- Drug Profile (Bisoprolol)Document16 pagesDrug Profile (Bisoprolol)Maryam KhushbakhatNo ratings yet
- Metoprolol PDFDocument3 pagesMetoprolol PDFCandy San DiegoNo ratings yet
- RamiprilDocument3 pagesRamiprilNovi YulianaNo ratings yet
- Metoprolol and AmiodaroneDocument5 pagesMetoprolol and AmiodaroneNolte BombayNo ratings yet
- Formulary 3rd Shifing AudsDocument81 pagesFormulary 3rd Shifing AudsSheila Lyn LacsonNo ratings yet
- Emergency MedsDocument24 pagesEmergency MedsNursyNurse100% (1)
- Generic Name: Albuterol Brand Names: Ventolin, Proventil, Accuneb, Vospire, ProairDocument9 pagesGeneric Name: Albuterol Brand Names: Ventolin, Proventil, Accuneb, Vospire, Proairwasiq AhmedNo ratings yet
- Drug Study Mother TDocument14 pagesDrug Study Mother TEuzelle Jeena ArandaNo ratings yet
- Drug StudyDocument11 pagesDrug StudyHennah Reblando100% (3)
- Generic Name: Brand Name: Apo-Metoprolol, Betaloc, Lopressor, Novo-Metoprolol, Nu-Drug ClassificationDocument4 pagesGeneric Name: Brand Name: Apo-Metoprolol, Betaloc, Lopressor, Novo-Metoprolol, Nu-Drug ClassificationKat ZNo ratings yet
- Bisoprolol Fumarate (Drug Study)Document2 pagesBisoprolol Fumarate (Drug Study)Franz.thenurse688871% (7)
- Drugs That Require Frequent Monitoring: Contraindicated In: Side Effects of ToxicityDocument9 pagesDrugs That Require Frequent Monitoring: Contraindicated In: Side Effects of ToxicityRhene GazmenNo ratings yet
- Pharmacology 3Document7 pagesPharmacology 3Maria Nichole JavarNo ratings yet
- Medication Worksheet: (You Will Need To Make Additional Copies of This Form)Document2 pagesMedication Worksheet: (You Will Need To Make Additional Copies of This Form)mcarter402100% (1)
- Downloaded From FILIPINO NURSES CENTRAL: Drugs That Require Frequent MonitoringDocument8 pagesDownloaded From FILIPINO NURSES CENTRAL: Drugs That Require Frequent MonitoringFilipino Nurses CentralNo ratings yet
- Adrenergic Blockers AtenololDocument3 pagesAdrenergic Blockers AtenololPoinsithia OrlandaNo ratings yet
- Generic: Acetazolamide Brand: DiamoxDocument33 pagesGeneric: Acetazolamide Brand: DiamoxAshley Topp100% (1)
- Drugs That Require Frequent MonitoringDocument14 pagesDrugs That Require Frequent MonitoringnurseoncervixNo ratings yet
- FDC - IrrationalDocument30 pagesFDC - Irrationaldivya lohithNo ratings yet
- Labetalol Hydro ChlorideDocument3 pagesLabetalol Hydro Chlorideapi-3797941100% (1)
- Pharmacology Drug StudyDocument6 pagesPharmacology Drug StudyShene Claire VigillaNo ratings yet
- Drug StudyDocument4 pagesDrug StudyTeanu Jose Gabrillo TamayoNo ratings yet
- Clinical Medications Worksheets: (Why Med Ordered) Contraindications/warnings/interactionsDocument3 pagesClinical Medications Worksheets: (Why Med Ordered) Contraindications/warnings/interactionsENo ratings yet
- Drug Study CCUDocument5 pagesDrug Study CCUKrishna Faith P. DelaraNo ratings yet
- EsmololDocument3 pagesEsmololTri Purma SariNo ratings yet
- Table 20.2-7 Medications For Treating Alcohol DependenceDocument1 pageTable 20.2-7 Medications For Treating Alcohol DependenceAbdualaziz AlmalkiNo ratings yet
- Adrenergic Blockers NebivololDocument2 pagesAdrenergic Blockers NebivololPoinsithia OrlandaNo ratings yet
- LisinoprilDocument3 pagesLisinoprilLIEZEL GRACE VELAYONo ratings yet
- MetoprololDocument3 pagesMetoprololapi-3797941100% (3)
- Medication CardsDocument5 pagesMedication CardsAndrew Harrison Lewis0% (1)
- AmiodaroneDocument2 pagesAmiodaroneanindiawNo ratings yet
- Heart Talk (Ii) in Pandemic Of: COVID-19Document27 pagesHeart Talk (Ii) in Pandemic Of: COVID-19Alles FirmansyahNo ratings yet
- Emergency Drugs KathDocument29 pagesEmergency Drugs Kathmajin655No ratings yet
- Drugs That Require Frequent MonitoringDocument22 pagesDrugs That Require Frequent MonitoringNader Smadi100% (7)
- Drug StudyDocument12 pagesDrug StudyAngeli A EstilloreNo ratings yet
- Drug StudyDocument9 pagesDrug StudyChristine PunsalanNo ratings yet
- Chapter 7 (Adrenergic Antagonists)Document64 pagesChapter 7 (Adrenergic Antagonists)Aneeza AhmadNo ratings yet
- Emergency DrugsDocument7 pagesEmergency DrugsMarie Angeline ManzanoNo ratings yet
- MECFS Clinician Coalition Treatment Recs V1Document9 pagesMECFS Clinician Coalition Treatment Recs V1Emilie ChateletNo ratings yet
- Coreg (Carvedilol) 6.25mgDocument3 pagesCoreg (Carvedilol) 6.25mgE100% (2)
- Drug Study On Emergency DrugsDocument15 pagesDrug Study On Emergency DrugsDennise Juayang100% (1)
- Atrial Fibrillation TDDocument6 pagesAtrial Fibrillation TDapi-594366475No ratings yet
- 1-Amiodarone (Cordarone, Pacerone) : Duration: 30-45 MinutesDocument3 pages1-Amiodarone (Cordarone, Pacerone) : Duration: 30-45 MinutesMahmmoud FuqahaNo ratings yet
- Drug StudyDocument9 pagesDrug StudyEzshkha OngueNo ratings yet
- Montra PharmacologyDocument19 pagesMontra PharmacologytressNo ratings yet
- Hemostan, Methergine CA Gluconate2Document4 pagesHemostan, Methergine CA Gluconate2Stacy MC PelitoNo ratings yet
- BenazeprilDocument2 pagesBenazeprilFeliciaDorghamNo ratings yet
- Obat Tugas Dokter PhathimhaDocument6 pagesObat Tugas Dokter PhathimhaMegan LewisNo ratings yet
- NeoblocDocument2 pagesNeoblocianecunar100% (2)
- Drug Study - Metropolol Tartrate (Neobloc)Document2 pagesDrug Study - Metropolol Tartrate (Neobloc)mikErlhNo ratings yet
- Critical Care Medications: Anti-Arrhythmics Study Guide: Critical Care EssentialsFrom EverandCritical Care Medications: Anti-Arrhythmics Study Guide: Critical Care EssentialsNo ratings yet
- Critical Care Medications: Vasopressors, Inotropes and Anti-Hypertensives Study Guide: Critical Care EssentialsFrom EverandCritical Care Medications: Vasopressors, Inotropes and Anti-Hypertensives Study Guide: Critical Care EssentialsNo ratings yet
- Medical Encyclopedia XXL: Prof. J.P. Schadé, M.D., Ph.D. D.Sc.hcFrom EverandMedical Encyclopedia XXL: Prof. J.P. Schadé, M.D., Ph.D. D.Sc.hcNo ratings yet
- Elements of The Ippe and Awv: IPPE - G0402 Initial AWV - G0438 Subsequent AWV - G0439 Information GatheringDocument1 pageElements of The Ippe and Awv: IPPE - G0402 Initial AWV - G0438 Subsequent AWV - G0439 Information GatheringB PNo ratings yet
- Analysis of Florida's New Law Regarding Pelvic ExaminationsDocument2 pagesAnalysis of Florida's New Law Regarding Pelvic ExaminationsB PNo ratings yet
- Bardmoor YMCA ScheduleDocument2 pagesBardmoor YMCA ScheduleB PNo ratings yet
- AMA Form Pdf236798479485y9340yrDocument1 pageAMA Form Pdf236798479485y9340yrB PNo ratings yet
- Against Medical Advice FormDocument1 pageAgainst Medical Advice FormB P100% (1)
- ARM Workshops Updated 4.30.2021Document4 pagesARM Workshops Updated 4.30.2021B PNo ratings yet
- BayCare - Doc and Coding - Atrial FibrillationDocument1 pageBayCare - Doc and Coding - Atrial FibrillationB PNo ratings yet
- 2021 Midyear - Final ICD-10-CM MappingsDocument247 pages2021 Midyear - Final ICD-10-CM MappingsB PNo ratings yet
- Echo Curriculum Delivery Tool Nov2016Document11 pagesEcho Curriculum Delivery Tool Nov2016Joseph BarkerNo ratings yet
- Clinical Indications, Treatment and Current PracticeDocument14 pagesClinical Indications, Treatment and Current PracticefadmayulianiNo ratings yet
- Nursing MnemonicsDocument11 pagesNursing MnemonicsMarco CalvaraNo ratings yet
- Concept Map f21 FinishedDocument5 pagesConcept Map f21 Finishedapi-601070065No ratings yet
- Healthy Bodies Slide3Document11 pagesHealthy Bodies Slide3Larissa RevillaNo ratings yet
- Classifying Electrocardiograph Waveforms Using Trained Deep Learning Neural Network Based On Wavelet RepresentationDocument9 pagesClassifying Electrocardiograph Waveforms Using Trained Deep Learning Neural Network Based On Wavelet RepresentationIAES IJAINo ratings yet
- GRP 3 Case Study - BurnsDocument7 pagesGRP 3 Case Study - BurnsToffee Trugillo100% (1)
- Exam #4 Study Blueprint 2019Document2 pagesExam #4 Study Blueprint 2019Stephanie DeeNo ratings yet
- Perioperative Medicine Managing Surgical Patients With Medical Problems by Chikwe, Joanna Walther, Axel Jones, PhilipDocument462 pagesPerioperative Medicine Managing Surgical Patients With Medical Problems by Chikwe, Joanna Walther, Axel Jones, PhilipIbrahim AlmohiniNo ratings yet
- Plan of Care For:: Nursing Diagnosis: Excess Fluid VolumeDocument3 pagesPlan of Care For:: Nursing Diagnosis: Excess Fluid VolumeKenji CadizNo ratings yet
- Laporan FaalDocument25 pagesLaporan FaalAgnes NathaniaNo ratings yet
- Physical Examination Form: Return by July 17ThDocument1 pagePhysical Examination Form: Return by July 17ThFlorian Ananias ByarugabaNo ratings yet
- Anesthesia Challenges in Patent Ductus ArteriosusDocument2 pagesAnesthesia Challenges in Patent Ductus ArteriosusChee LeongNo ratings yet
- ThrombosisDocument11 pagesThrombosisNobby Onist JuniorNo ratings yet
- ENZYME by DR JoeanDocument8 pagesENZYME by DR JoeanHenry Teepoh100% (1)
- Qworld USMLE Step1 Course 2019Document91 pagesQworld USMLE Step1 Course 2019Qworld50% (2)
- Clinical ElectrocardiographyDocument134 pagesClinical ElectrocardiographyHossain MuhammadNo ratings yet
- The Metaboreflex SynchronousDocument19 pagesThe Metaboreflex SynchronousDanai ZogaNo ratings yet
- Physrev 00043 2016Document57 pagesPhysrev 00043 2016Arie AnggaNo ratings yet
- Sinus Brad, Tach, PAC, PVCDocument17 pagesSinus Brad, Tach, PAC, PVCEdRobertArnadNo ratings yet
- Medical Surgical Nursing IIDocument9 pagesMedical Surgical Nursing IIAdarsh PatelNo ratings yet
- IntelliVue MX500 Patient Monitor TDS PDFDocument24 pagesIntelliVue MX500 Patient Monitor TDS PDFbenyvladNo ratings yet
- Nclex Questions FinalsDocument301 pagesNclex Questions Finalsticoystephanie100% (2)
- Monitoring Patient (Deskripsi) Fiky Niswati Yuslihah (P17250193025)Document3 pagesMonitoring Patient (Deskripsi) Fiky Niswati Yuslihah (P17250193025)Fiky NiswatiNo ratings yet
- Sample Cardiac Calcium Scoring ReportDocument3 pagesSample Cardiac Calcium Scoring ReportZyad DoskiNo ratings yet
- DBEABE614Document6 pagesDBEABE614bariNo ratings yet
- Vasoactive Agents in Shock.2Document8 pagesVasoactive Agents in Shock.2Linamaria LozanoNo ratings yet