Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
8 viewsSoal Variasi Hal. 128
Soal Variasi Hal. 128
Uploaded by
Akhmad Hadi SantosoCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Surat PernyataanDocument1 pageSurat PernyataanAkhmad Hadi SantosoNo ratings yet
- Lembar Persetujuan Maju Dosen 2021 - TTDDocument14 pagesLembar Persetujuan Maju Dosen 2021 - TTDAkhmad Hadi SantosoNo ratings yet
- Penilaian Praktikum FDM Kelompok 2Document3 pagesPenilaian Praktikum FDM Kelompok 2Akhmad Hadi SantosoNo ratings yet
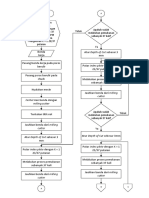
- Flowchart PP1 BubutDocument3 pagesFlowchart PP1 BubutAkhmad Hadi SantosoNo ratings yet
- Kritik Dan Saran Asisten - Akhmad Hadi .SDocument1 pageKritik Dan Saran Asisten - Akhmad Hadi .SAkhmad Hadi SantosoNo ratings yet
- Flowchart PP1 BubutDocument3 pagesFlowchart PP1 BubutAkhmad Hadi SantosoNo ratings yet
- Pentingnya Mental Health Di Masa Sekarang Bagi Generasi ZDocument2 pagesPentingnya Mental Health Di Masa Sekarang Bagi Generasi ZAkhmad Hadi SantosoNo ratings yet
- Flowchart PP2 MillingDocument2 pagesFlowchart PP2 MillingAkhmad Hadi SantosoNo ratings yet
- Poin Bahasan Tabel Pada Project 1 Pemrograman KomputerDocument1 pagePoin Bahasan Tabel Pada Project 1 Pemrograman KomputerAkhmad Hadi SantosoNo ratings yet
Soal Variasi Hal. 128
Soal Variasi Hal. 128
Uploaded by
Akhmad Hadi Santoso0 ratings0% found this document useful (0 votes)
8 views1 pageCopyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
0 ratings0% found this document useful (0 votes)
8 views1 pageSoal Variasi Hal. 128
Soal Variasi Hal. 128
Uploaded by
Akhmad Hadi SantosoCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
You are on page 1of 1
121
Pea TS
Pot}
Moe uw
FIGURE 3-33
‘Quality is related to the horizontal
distances on P-v and T-v diagrams.
Porth
FIGURE 3-34
The v value of a saturated
liquid~vapor mixture lies between
the v,and v, values at the specified
TorP.
Based on this equation, quality can be related to the horizontal distances
on a P-v or T-v diagram (Fig. 3-33). Ata given temperature or pressure, the
numerator of Eq. 3-5 is the distance between the actual state and the saturated
liquid state, and the denominator is the length of the entire horizontal line that
connects the saturated liquid and saturated vapor states. A state of 50 percent
quality lies in the middle of this horizontal Line.
The analysis given above can be repeated for internal energy and enthalpy
with the following results:
sig (kIkg) oo
Digg = By tty (kg) en
All the results are of the same format, and they can be summarized in a
single equation as
Jos = 44 ie
where y is V, u, or h. The subscript “avg” (for “average”) is usually dropped
for simplicity. The values of the average properties of the mixtures are
always between the values of the saturated liquid and the saturated vapor
properties (Fig. 3-34). That is,
Yang Me
Finally, all the saturated-mixture states are located under the saturation
curve, and to analyze saturated mixtures, all we need are saturated liquid
and saturated vapor data (Tables A~4 and A-5 in the case of water),
EXAMPLE 3-4 Pressure and Volume of a Saturated Mixture
A rigid tank contains 10 kg of water at 90°C. If 8 kg of the water is in the
liquid form and the rest is in the vapor form, determine (a) the pressure in
‘the tank and (6) the volume of the tank.
SOLUTION A rigid tank contains saturated mixture, The pressure and the
volume of the tank are to be determined
‘Analysis (a) The state of the saturated liquid-vapor mixture is shown in
Fig, 3-35. Since the two phases coexist in equilibrium, we have a saturated rix-
ture, and the pressure must be the saturation pressure at the given temperature:
P= Pyrgwc = T0183 KPa (Table A-4)
(2) At 90°C, we have v, = 0.001036 mig and v, = 2.3593 mig
(Table A~A). One way of finding the volume of the tank is to determine the
volume occupied by each phase and then add them:
V= Yt U, = my + mv,
(8 kg)(0.001036 m'Vkg) + (2-kg)(2.3593 mg)
473m?
Another way is to fitst determine the quality x, then the average specific
volume v, and finally the total volume:
im, _ Oke
m Tow °”
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Surat PernyataanDocument1 pageSurat PernyataanAkhmad Hadi SantosoNo ratings yet
- Lembar Persetujuan Maju Dosen 2021 - TTDDocument14 pagesLembar Persetujuan Maju Dosen 2021 - TTDAkhmad Hadi SantosoNo ratings yet
- Penilaian Praktikum FDM Kelompok 2Document3 pagesPenilaian Praktikum FDM Kelompok 2Akhmad Hadi SantosoNo ratings yet
- Flowchart PP1 BubutDocument3 pagesFlowchart PP1 BubutAkhmad Hadi SantosoNo ratings yet
- Kritik Dan Saran Asisten - Akhmad Hadi .SDocument1 pageKritik Dan Saran Asisten - Akhmad Hadi .SAkhmad Hadi SantosoNo ratings yet
- Flowchart PP1 BubutDocument3 pagesFlowchart PP1 BubutAkhmad Hadi SantosoNo ratings yet
- Pentingnya Mental Health Di Masa Sekarang Bagi Generasi ZDocument2 pagesPentingnya Mental Health Di Masa Sekarang Bagi Generasi ZAkhmad Hadi SantosoNo ratings yet
- Flowchart PP2 MillingDocument2 pagesFlowchart PP2 MillingAkhmad Hadi SantosoNo ratings yet
- Poin Bahasan Tabel Pada Project 1 Pemrograman KomputerDocument1 pagePoin Bahasan Tabel Pada Project 1 Pemrograman KomputerAkhmad Hadi SantosoNo ratings yet