Professional Documents
Culture Documents
ME 311 Thermo I Fall 2014 Homework 5 Solutions PDF
ME 311 Thermo I Fall 2014 Homework 5 Solutions PDF
Uploaded by
Illion IllionOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ME 311 Thermo I Fall 2014 Homework 5 Solutions PDF
ME 311 Thermo I Fall 2014 Homework 5 Solutions PDF
Uploaded by
Illion IllionCopyright:
Available Formats
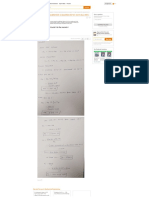
ME-311 – Thermodynamics 1 VerSteeg Fall 2014
Homework 5
1-69 Both a gage and a manometer are attached to a gas to measure its pressure. For a specified reading of gage
pressure, the difference between the fluid levels of the two arms of the manometer is to be determined for mercury and
water.
Properties The densities of water and mercury are given to be
water = 1000 kg/m3 and be Hg = 13,600 kg/m3.
Analysis The gage pressure is related to the vertical distance h between the
two fluid levels by
Pgage
Pgage g h
h
g
(a) For mercury,
Pgage
h
Hg g
80 kPa 1 kN/m 2 1000 kg/m s 2
0.60 m
m
(13,600 kg/m 3 )(9.81 m/s 2 ) 1 kPa
1 kN
er as
co
(b) For water,
eH w
Pgage 80 kPa 1 kN/m 2 1000 kg/m s 2
h 8.16 m
H 2O g (1000 kg/m 3 )(9.81 m/s 2 ) 1 kPa
o.
1 kN
rs e
ou urc
1-83 The gage pressure of air in a pressurized water tank is measured simultaneously by both a pressure gage and a
manometer. The differential height h of the mercury column is to be determined.
o
Assumptions The air pressure in the tank is uniform (i.e., its variation with elevation is negligible due to its low density), and
aC s
thus the pressure at the air-water interface is the same as the indicated gage pressure.
Properties We take the density of water to be w =1000 kg/m3. The specific gravities of oil and mercury are given to be 0.72
vi y re
and 13.6, respectively.
Analysis Starting with the pressure of air in the tank (point 1), and moving along the tube by adding (as we go down) or
subtracting (as we go u p) the gh terms until we reach the free surface of oil where the oil tube is exposed to the
ed d
atmosphere, and setting the result equal to Patm gives
P1 w ghw Hg ghHg oil ghoil Patm
ar stu
Rearranging,
P1 Patm oil ghoil Hg ghHg w ghw
is
or,
Th
P1,gage
SG oil hoil SG Hg hHg hw
w g
Substituting,
sh
80 kPa 1000 kg m/s 2
0.72 (0.75 m) 13.6 hHg 0.3 m
(1000 kg/m 3 )(9.81 m/s 2 ) 1 kPa. m 2
Solving for hHg gives hHg = 0.582 m. Therefore, the differential height of the mercury column must be 58.2 cm.
Discussion Double instrumentation like this allows one to verify the measurement of one of the instruments by the
measurement of another instrument.
Page 1 of 1
This study source was downloaded by 100000823667739 from CourseHero.com on 09-06-2021 23:25:55 GMT -05:00
https://www.coursehero.com/file/44171063/ME-311-Thermo-I-Fall-2014-Homework-5-Solutionspdf/
Powered by TCPDF (www.tcpdf.org)
You might also like
- PROBLEMS With SolutionDocument8 pagesPROBLEMS With Solutionbudoycarino0% (1)
- Quiz 3 Finals Employee Benefits PDFDocument4 pagesQuiz 3 Finals Employee Benefits PDFIllion IllionNo ratings yet
- Physics 2 Laboratory: School of Engineering and ArchitectureDocument27 pagesPhysics 2 Laboratory: School of Engineering and ArchitectureIllion IllionNo ratings yet
- HW1 9Document6 pagesHW1 9Eduardo VCNo ratings yet
- Fluid Mechanic AnswerDocument32 pagesFluid Mechanic AnswerIser88% (41)
- CH 3 Solutions 9th EdDocument150 pagesCH 3 Solutions 9th Edp-majidi91% (58)
- Mechanical Engineering Review Manual PDFDocument509 pagesMechanical Engineering Review Manual PDFKenneth SienaNo ratings yet
- Review Problems 1-85 A Hydraulic Lift Is Used To LiftDocument22 pagesReview Problems 1-85 A Hydraulic Lift Is Used To Liftpanalopee100% (5)
- CH 01Document9 pagesCH 01Mary Lorelyn EscoteNo ratings yet
- Assignment (Air Pollution)Document7 pagesAssignment (Air Pollution)Durga Prasad Murmu0% (1)
- Tarea de Termo 1Document4 pagesTarea de Termo 1Mileny Mileny Q PeñaNo ratings yet
- This Study Resource Was: Homework No.2 (MEE 340)Document7 pagesThis Study Resource Was: Homework No.2 (MEE 340)Kenneth SablayNo ratings yet
- 4HOMEWORKDocument6 pages4HOMEWORKBlue SkyNo ratings yet
- Fluid Statics ExamplesDocument14 pagesFluid Statics ExamplesJoy CloradoNo ratings yet
- Solution#1Document5 pagesSolution#1Exel Dua CincinNo ratings yet
- Solution 1330895172 PDFDocument1 pageSolution 1330895172 PDFAilsa AdzaniNo ratings yet
- Solution 1330895172 PDFDocument1 pageSolution 1330895172 PDFTheod S. VilaNo ratings yet
- Homework #2 Due Feb 6, 2008 Spring Semester 2008: ME 363 - Fluid MechanicsDocument6 pagesHomework #2 Due Feb 6, 2008 Spring Semester 2008: ME 363 - Fluid MechanicsMohannad NassarNo ratings yet
- CIVE1400 Fluid Mechanics Examples AnswerDocument32 pagesCIVE1400 Fluid Mechanics Examples Answerbhilacarlos10No ratings yet
- Fluids MechanicsModule FinalizeDocument86 pagesFluids MechanicsModule FinalizeRitsu TainakaNo ratings yet
- Assumptions 1 All The Liquids Are Incompressible. 2Document2 pagesAssumptions 1 All The Liquids Are Incompressible. 2Kaffi RockavankaNo ratings yet
- Tutorial - Lecture 2 Solutions-1Document8 pagesTutorial - Lecture 2 Solutions-1Bastián Olfos MárquezNo ratings yet
- Solution: First Sum Moments Clockwise About The Hinge A of The HandleDocument5 pagesSolution: First Sum Moments Clockwise About The Hinge A of The HandleUzziel De jesus OsorioNo ratings yet
- Thermodynamics 1: Gage Vac Vac AbsDocument2 pagesThermodynamics 1: Gage Vac Vac AbsItsMeRyanCNo ratings yet
- 3MANOEXMPLEDocument5 pages3MANOEXMPLEBlue SkyNo ratings yet
- Fluid Mechanics Examples and Answers: July 2016Document33 pagesFluid Mechanics Examples and Answers: July 2016bandulaNo ratings yet
- Homework 2Document4 pagesHomework 2Paul LeeNo ratings yet
- P P F F A A F A F A: Lifting of A Large Weight by A Small Force by The Application of Pascal's PrincipleDocument2 pagesP P F F A A F A F A: Lifting of A Large Weight by A Small Force by The Application of Pascal's PrincipleEhtisham RiazNo ratings yet
- Thermo 7e SM Chap01-1 ReduddcionDocument33 pagesThermo 7e SM Chap01-1 ReduddcionJonathanNo ratings yet
- Chapter-3 PPTDocument109 pagesChapter-3 PPTnunuNo ratings yet
- C3 SolutionsDocument2 pagesC3 SolutionsCharlie SumagaysayNo ratings yet
- Tutorial - Lecture 5 SolutionsDocument10 pagesTutorial - Lecture 5 SolutionsBastián Olfos MárquezNo ratings yet
- 11 GasesDocument17 pages11 Gasespuja ritongaNo ratings yet
- Taller Del Curso: Fundamentos de Mec Anica de FluidosDocument2 pagesTaller Del Curso: Fundamentos de Mec Anica de FluidosJuan Daniel CabreraNo ratings yet
- Ensc 388 - P4 - 51 PDFDocument1 pageEnsc 388 - P4 - 51 PDFOkky JayadiNo ratings yet
- MECHANICAL ENGINEER'S MANUAL (2018 New)Document196 pagesMECHANICAL ENGINEER'S MANUAL (2018 New)YURI G. MELLIZANo ratings yet
- Assignment1 Solutions - KNLDocument6 pagesAssignment1 Solutions - KNLsaraNo ratings yet
- Ensc 461 Tutorial, Week#6 - Refrigeration Cycle: High Pressure Side P 700kpaDocument6 pagesEnsc 461 Tutorial, Week#6 - Refrigeration Cycle: High Pressure Side P 700kpaArchie Gil DelamidaNo ratings yet
- 3Document10 pages3Ariel Carlos De LeonNo ratings yet
- CIVE1400 Fluid Mechanics Examples AnswerDocument20 pagesCIVE1400 Fluid Mechanics Examples AnswerddddddNo ratings yet
- Tutorial 3 - DMCF 2213 (Manometer)Document4 pagesTutorial 3 - DMCF 2213 (Manometer)shazwani zamriNo ratings yet
- Solucionario Guía 6 Termodinámica - Balance de Energía en Sistemas Cerrados 2-2022Document41 pagesSolucionario Guía 6 Termodinámica - Balance de Energía en Sistemas Cerrados 2-2022Fernanda MuñozNo ratings yet
- Thermodynamics An Engineering Approach 8Document1 pageThermodynamics An Engineering Approach 8Aracely PenaNo ratings yet
- Fluid 9ed Solution Manual PDFDocument919 pagesFluid 9ed Solution Manual PDFDermz GayosoNo ratings yet
- Flow Sheeting Process Engineering Calculations Assignment 2022Document10 pagesFlow Sheeting Process Engineering Calculations Assignment 2022TamirNo ratings yet
- Yuri Mechanical Engineers Manual PDFDocument196 pagesYuri Mechanical Engineers Manual PDFMike Rowen BanaresNo ratings yet
- Cie350 HW04 2009Document4 pagesCie350 HW04 2009謝政安No ratings yet
- Properties of Fluids PROBLEMSDocument12 pagesProperties of Fluids PROBLEMSJohn FerreNo ratings yet
- Sheet 2Document3 pagesSheet 2Amr OkashaNo ratings yet
- Basic Integration Problems With Answers PDFDocument158 pagesBasic Integration Problems With Answers PDFLily Antonette AgustinNo ratings yet
- I. FLUID MECHANICS I.1 Basic Concepts & Definitions: Fluid MechanicsDocument9 pagesI. FLUID MECHANICS I.1 Basic Concepts & Definitions: Fluid Mechanicsdprao196750% (2)
- Fluid 9ed Solution ManualDocument919 pagesFluid 9ed Solution ManualAmr f100% (1)
- FileDocument19 pagesFileRaymart CubidNo ratings yet
- Solution To Problem Set Fluid Mech PressureDocument6 pagesSolution To Problem Set Fluid Mech PressureMark Augusto V. AgusNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Transactions of the American Society of Civil Engineers, Vol. LXX, Dec. 1910 Locomotive Performance On Grades Of Various Lengths, Paper No. 1172From EverandTransactions of the American Society of Civil Engineers, Vol. LXX, Dec. 1910 Locomotive Performance On Grades Of Various Lengths, Paper No. 1172No ratings yet
- F Acadl 01Document12 pagesF Acadl 01Illion IllionNo ratings yet
- GIT Lecture 9 - Cybercrime Laws in The PhilippinesDocument137 pagesGIT Lecture 9 - Cybercrime Laws in The PhilippinesIllion IllionNo ratings yet
- Tsdchapter 13: Current Liabilities and ContingenciesDocument45 pagesTsdchapter 13: Current Liabilities and ContingenciesIllion IllionNo ratings yet
- Retained Earnings PDFDocument3 pagesRetained Earnings PDFIllion IllionNo ratings yet
- Test Bank Cost Accounting 14e by Carter Ch07 PDFDocument20 pagesTest Bank Cost Accounting 14e by Carter Ch07 PDFIllion IllionNo ratings yet
- Test Canvas: C1: FMGT123Document162 pagesTest Canvas: C1: FMGT123Illion IllionNo ratings yet
- This Study Resource Was: An Average Cost Per Unit For All JobsDocument7 pagesThis Study Resource Was: An Average Cost Per Unit For All JobsIllion IllionNo ratings yet
- This Study Resource Was: Process Costing - AverageDocument7 pagesThis Study Resource Was: Process Costing - AverageIllion IllionNo ratings yet
- MAS Lauderbach 1 PDFDocument210 pagesMAS Lauderbach 1 PDFIllion IllionNo ratings yet
- Final Deptals COST.1 PDFDocument5 pagesFinal Deptals COST.1 PDFIllion IllionNo ratings yet
- This Study Resource Was: Concerning Human Understanding and His Answer To The SelfDocument2 pagesThis Study Resource Was: Concerning Human Understanding and His Answer To The SelfIllion IllionNo ratings yet
- This Study Resource Was: Quiz 1 SolutionDocument2 pagesThis Study Resource Was: Quiz 1 SolutionIllion IllionNo ratings yet
- This Study Resource Was: Painter CorporationDocument3 pagesThis Study Resource Was: Painter CorporationIllion IllionNo ratings yet
- This Study Resource Was: Si Units Thermodynamics 6 EditionDocument9 pagesThis Study Resource Was: Si Units Thermodynamics 6 EditionIllion IllionNo ratings yet
- Chapter 5 - Product and Service Costing: Job-Order SystemDocument71 pagesChapter 5 - Product and Service Costing: Job-Order SystemIllion Illion0% (1)
- Course Introduction: Logic and Critical Thinking Course Code: Loct 1011 Course Crdit Hours: 3Document106 pagesCourse Introduction: Logic and Critical Thinking Course Code: Loct 1011 Course Crdit Hours: 3Illion IllionNo ratings yet
- LaquindanumDocument1 pageLaquindanumIllion IllionNo ratings yet
- Shareholdersx27 Equity Prac 1 PDF FreeDocument10 pagesShareholdersx27 Equity Prac 1 PDF FreeIllion IllionNo ratings yet
- Chapter 22: Retained Earnings (Dividends)Document12 pagesChapter 22: Retained Earnings (Dividends)Illion IllionNo ratings yet
- Question: 10. A Rigid Insulated Tank Is Separated Into Two Rooms by A Stiff PDocument2 pagesQuestion: 10. A Rigid Insulated Tank Is Separated Into Two Rooms by A Stiff PIllion IllionNo ratings yet
- This Study Resource Was: (Jefferson Corporation)Document8 pagesThis Study Resource Was: (Jefferson Corporation)Illion IllionNo ratings yet
- A Gas Exerts A Steady Pressure of 385 KPa On The T...Document1 pageA Gas Exerts A Steady Pressure of 385 KPa On The T...Illion Illion0% (1)
- 1 s2.0 S0950061813008076 MainDocument9 pages1 s2.0 S0950061813008076 MainAINA SAMNo ratings yet
- CokemakingTechnologies ComparisonDocument9 pagesCokemakingTechnologies Comparisonkselvan_1100% (1)
- Chemicals Zetag MSDS Magnasol - 2610G - CA - ENDocument6 pagesChemicals Zetag MSDS Magnasol - 2610G - CA - ENPromagEnviro.comNo ratings yet
- Antioxidant Potential of Black Tea (Camellia Sinensis L.) - A ReviewDocument5 pagesAntioxidant Potential of Black Tea (Camellia Sinensis L.) - A ReviewRatih Sukmarini SujendraNo ratings yet
- Evaluation of Palm Oil-Based Paracetamol SuppositoDocument8 pagesEvaluation of Palm Oil-Based Paracetamol SuppositoWidia TriNo ratings yet
- GCSE Chemistry Foundation Tier Topic Test 3Document31 pagesGCSE Chemistry Foundation Tier Topic Test 3Aryan AdhikariNo ratings yet
- World Journal of Pharmaceutical ResearchDocument14 pagesWorld Journal of Pharmaceutical ResearchDevanandDongreNo ratings yet
- Free Electron TheoryDocument44 pagesFree Electron TheoryAllen MarananNo ratings yet
- JVK Filter Elements enDocument16 pagesJVK Filter Elements enArun GuptaNo ratings yet
- Explaination of Primary & Secondary Stress With ExampleDocument4 pagesExplaination of Primary & Secondary Stress With ExampleAndrewNo ratings yet
- List of Packages As On 17.02.2018Document3 pagesList of Packages As On 17.02.2018mecon bhilaiNo ratings yet
- Mechanical Seals According To StandardsDocument6 pagesMechanical Seals According To StandardsSunit MishraNo ratings yet
- Tarea FotosíntesisDocument7 pagesTarea FotosíntesisAndy StevenNo ratings yet
- Impreglon 222M DataSheetDocument3 pagesImpreglon 222M DataSheetKyle S JonesNo ratings yet
- Jurnal InternasionalDocument9 pagesJurnal InternasionalalninditaNo ratings yet
- MSCCH 602Document229 pagesMSCCH 602hopeotaniel78No ratings yet
- Blank Sample PQR Form (SAW - Page 1) Procedure Qualification Record (PQR)Document2 pagesBlank Sample PQR Form (SAW - Page 1) Procedure Qualification Record (PQR)GMNo ratings yet
- Specific HeatDocument2 pagesSpecific HeatAina Beñasfre RafalesNo ratings yet
- Physicochemical Analysis of Municipal Water in Al Khums LibyaDocument4 pagesPhysicochemical Analysis of Municipal Water in Al Khums LibyaHaider AddewanyNo ratings yet
- SSC Junior Engineer Mechanical Recruitment Exam Guide 3rd Edition PDFDocument586 pagesSSC Junior Engineer Mechanical Recruitment Exam Guide 3rd Edition PDFBhavani Gujjari0% (1)
- Lubricant Viscocities 140hDocument8 pagesLubricant Viscocities 140hPablo Gaspar D'Agostini AmengualNo ratings yet
- Introduction To Safety in Chemical Process Industry PDFDocument136 pagesIntroduction To Safety in Chemical Process Industry PDFMireia MartíNo ratings yet
- Floor Cleaner Making ClassesDocument8 pagesFloor Cleaner Making ClassesMuhammad FaisalNo ratings yet
- Correlations StandingDocument14 pagesCorrelations StandingIngrid GarciaNo ratings yet
- Carbon Black - EncapsulationDocument10 pagesCarbon Black - EncapsulationWaltoy DinizNo ratings yet
- Pressure Vessel HandbookDocument494 pagesPressure Vessel HandbookmiguelmtzgroNo ratings yet
- B V P Vacuum Pump Company ProfileDocument10 pagesB V P Vacuum Pump Company ProfileravishankarNo ratings yet
- List of Tyre Pyrolysis Oil Companies in IndiaDocument2 pagesList of Tyre Pyrolysis Oil Companies in IndiaHaneesh ReddyNo ratings yet
- Phycobiliproteins (1987) PDFDocument225 pagesPhycobiliproteins (1987) PDFJackNo ratings yet