Professional Documents
Culture Documents
Use of Steam Table - Additional Problem
Use of Steam Table - Additional Problem
Uploaded by
JAIRUS RODAN CARI�OCopyright:
Available Formats
You might also like
- Vip1 120Document120 pagesVip1 120Paul AbonitaNo ratings yet
- Energy Balance CalculationDocument2 pagesEnergy Balance CalculationSzelee KuekNo ratings yet
- Inlet Entropy S Outlet Entropy S'Document9 pagesInlet Entropy S Outlet Entropy S'Kashan AslamNo ratings yet
- Tutorial 1: Basic Concept of ThermodynamicsDocument4 pagesTutorial 1: Basic Concept of ThermodynamicsKaka ZettyNo ratings yet
- Question 1 (15 Marks)Document5 pagesQuestion 1 (15 Marks)Farouk BassaNo ratings yet
- As 102 - Plates - Final Project - No AnsDocument5 pagesAs 102 - Plates - Final Project - No AnsHashirama SenjuNo ratings yet
- صادق سالم محمد Practicle Cycle and its CalculationDocument18 pagesصادق سالم محمد Practicle Cycle and its Calculationعبدالمحسن علي ENo ratings yet
- Thermo Problems (Final Exam 1)Document3 pagesThermo Problems (Final Exam 1)rii amosNo ratings yet
- MMAN2700ThermoProblemSheet5 - Pure SubstancesDocument3 pagesMMAN2700ThermoProblemSheet5 - Pure Substancesgrandw9524No ratings yet
- Assignment 8Document8 pagesAssignment 8Jihan MutiahNo ratings yet
- Air MixingDocument2 pagesAir MixingJom SanzNo ratings yet
- Thermochem - An Ice CalorimeterDocument7 pagesThermochem - An Ice CalorimeterJames CiapaNo ratings yet
- Tutorial 3Document2 pagesTutorial 3kaeshav manivannanNo ratings yet
- Chapter 6Document18 pagesChapter 6Xeen FortunyNo ratings yet
- ME-1100 Thermodynamics May - June 2022 - Trimester Tutorial - 3Document2 pagesME-1100 Thermodynamics May - June 2022 - Trimester Tutorial - 3Aiswarya Ramesh me21b011No ratings yet
- Thermo Cycle Problems With Solution 1Document12 pagesThermo Cycle Problems With Solution 1Maridil Joy IsidroNo ratings yet
- Mechanism of Thermoluminescence in Sm-And Eu-Doped Barium SulphateDocument10 pagesMechanism of Thermoluminescence in Sm-And Eu-Doped Barium Sulphatejanneth reyesNo ratings yet
- Charles Law ProblemsDocument2 pagesCharles Law ProblemsDanica OpolintoNo ratings yet
- Thermodynamics 03Document4 pagesThermodynamics 03Cullen MoralesNo ratings yet
- Properties of SteamDocument4 pagesProperties of SteamAndrew Grogan100% (1)
- Experiment 10-Spectrophotometric MethodsDocument6 pagesExperiment 10-Spectrophotometric MethodsMarc DiongcoNo ratings yet
- 1 - Energy and Energy BalancesDocument135 pages1 - Energy and Energy BalancesHabib Al-Aziz100% (2)
- Answer Problem Sheet-06 Me201 EntropyDocument5 pagesAnswer Problem Sheet-06 Me201 EntropyAtif IrshadNo ratings yet
- Properties of Pure Substances: CMT 458 - Noor Hafizah UyupDocument7 pagesProperties of Pure Substances: CMT 458 - Noor Hafizah UyupAnna WilsonNo ratings yet
- Introduction To Steam Tables and Mollier Diagram: 1. DefinitionDocument40 pagesIntroduction To Steam Tables and Mollier Diagram: 1. Definitionbikas_sahaNo ratings yet
- Thermodynamics 1 Chapter 07Document162 pagesThermodynamics 1 Chapter 07Devantharan NadesanNo ratings yet
- Cbse Test Paper-03 CLASS - XI CHEMISTRY (States of Matter: Gases and Liquids) Topic: - Ideal Gas EquationDocument1 pageCbse Test Paper-03 CLASS - XI CHEMISTRY (States of Matter: Gases and Liquids) Topic: - Ideal Gas Equationsatya176No ratings yet
- Calculator TechniquesDocument8 pagesCalculator TechniquesRafael DeocuarizaNo ratings yet
- Introductory Concepts and DefinitionsDocument3 pagesIntroductory Concepts and Definitionsisrael moizo dintsiNo ratings yet
- Continue Practice Exam Test Questions Part 2 of The SeriesDocument7 pagesContinue Practice Exam Test Questions Part 2 of The SeriesKenn Earl Bringino VillanuevaNo ratings yet
- Eat Transfer Coefficients For Submerged CoilsDocument13 pagesEat Transfer Coefficients For Submerged Coilsvitcon87100% (1)
- Practice Problems On EntropyDocument1 pagePractice Problems On EntropyNetra PujarNo ratings yet
- Themal Capacity WorksheetDocument4 pagesThemal Capacity WorksheetSukanya VedavyasaNo ratings yet
- Input: Ideal Gas Process Mass of Ideal Gas Velocity 1 Velocity 2 Pressure 1 and 2 - Temperature 1 Temperature 2Document5 pagesInput: Ideal Gas Process Mass of Ideal Gas Velocity 1 Velocity 2 Pressure 1 and 2 - Temperature 1 Temperature 2P SobremonteNo ratings yet
- 15me03 Thermodynamics Problems June2017Document19 pages15me03 Thermodynamics Problems June2017Praveen Vijay100% (1)
- Engineering Thermodynamics (Tutorial 1) PDFDocument4 pagesEngineering Thermodynamics (Tutorial 1) PDFSahil AcharyaNo ratings yet
- Exercises Chap 1&2Document3 pagesExercises Chap 1&2Nguyen Thuy Bao Ngoc B2107502No ratings yet
- Tharmal Science 2014 FDocument2 pagesTharmal Science 2014 FRajeshGuptaNo ratings yet
- Rankine Cycle Diagram: K KG KJ K KG KJ Kpa S Kpa S S XDocument16 pagesRankine Cycle Diagram: K KG KJ K KG KJ Kpa S Kpa S S Xanon_166336005No ratings yet
- Tutorial Sheet No. 1: UES011 Thermofluids (Thermodynamics)Document1 pageTutorial Sheet No. 1: UES011 Thermofluids (Thermodynamics)Vinay DograNo ratings yet
- EXAMDocument1 pageEXAMkelly evangelistaNo ratings yet
- Assignment JeeDocument3 pagesAssignment Jeevikas2504No ratings yet
- Compressible Gas CalculationDocument6 pagesCompressible Gas CalculationRafael RamonNo ratings yet
- Thermo 5th Chap07 P001Document25 pagesThermo 5th Chap07 P001Rodrigo Andre Zuniga JuarezNo ratings yet
- AssignmentDocument2 pagesAssignmentsilverstonerocky0No ratings yet
- ThermoDocument6 pagesThermoMLNDG boysNo ratings yet
- Chapter 1 Concepts Definition and Basic PrinciplesDocument8 pagesChapter 1 Concepts Definition and Basic PrinciplesEyron EyronNo ratings yet
- ATKDocument4 pagesATKAgung SuharmantoNo ratings yet
- Entropy ProbsDocument5 pagesEntropy ProbsFAzle RAbbyNo ratings yet
- Question 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT DsDocument6 pagesQuestion 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT Dsfivos_rgNo ratings yet
- Practice ProblemsDocument1 pagePractice ProblemsSanu SouravNo ratings yet
- Electron Charge To Mass Ratio E/m: J. Lukens, B. Reid, A. Tuggle PH 235-001, Group 4 18 January 2010Document7 pagesElectron Charge To Mass Ratio E/m: J. Lukens, B. Reid, A. Tuggle PH 235-001, Group 4 18 January 2010KhizarNo ratings yet
- Examples MENG580 Chapter 11 PDFDocument14 pagesExamples MENG580 Chapter 11 PDFYasser TarhiniNo ratings yet
- Examples On 2nd Law For A ProcessDocument13 pagesExamples On 2nd Law For A ProcessMaria SarwatNo ratings yet
- PropertiesDocument3 pagesPropertiesswap1983No ratings yet
- Tut Sheet 3 SolutionDocument7 pagesTut Sheet 3 SolutionDevkriti SharmaNo ratings yet
- Thermo Solutions - Part79 PDFDocument1 pageThermo Solutions - Part79 PDFLiz ArfinNo ratings yet
- Lec 14Document14 pagesLec 14Nilesh SinghNo ratings yet
- He Is There (Lifebreakthrough) (Key Of)Document4 pagesHe Is There (Lifebreakthrough) (Key Of)JAIRUS RODAN CARI�ONo ratings yet
- Participants' Insights Gained From Nasat Labs Virtual TourDocument4 pagesParticipants' Insights Gained From Nasat Labs Virtual TourJAIRUS RODAN CARI�ONo ratings yet
- TechnoDocument8 pagesTechnoJAIRUS RODAN CARI�ONo ratings yet
- Book1 ENGGMANDocument3 pagesBook1 ENGGMANJAIRUS RODAN CARI�ONo ratings yet
- EXPERIMENT 1: Determination of Temperature, PH, Turbidity and Conductivity Data SheetDocument3 pagesEXPERIMENT 1: Determination of Temperature, PH, Turbidity and Conductivity Data SheetJAIRUS RODAN CARI�ONo ratings yet
Use of Steam Table - Additional Problem
Use of Steam Table - Additional Problem
Uploaded by
JAIRUS RODAN CARI�OOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Use of Steam Table - Additional Problem
Use of Steam Table - Additional Problem
Uploaded by
JAIRUS RODAN CARI�OCopyright:
Available Formats
USE SECOND STEAM TABLE (NOT THE ONE FROM PERRY’S CHE HANDBOOK)
Determine the state of water and supply the missing properties (SV: specific volume, U: internal energy, H:
enthalpy and S: entropy).
P = 300 kPa, SV = 700 cm3/g
First, determine the state of the water (we cannot determine the missing properties of water unless we
know its state).
Step one: With the given pressure, determine the specific volume of the saturated liquid and saturated
vapor.
P, kPa SV (saturated liquid), cm3/g SV (saturated vapor), cm3/g
300 kPa 1.073 605.56
ANSWER: From the solution above, the property is greater than the specific volume of saturated vapor at
300 kPa. Therefore, the water is superheated steam.
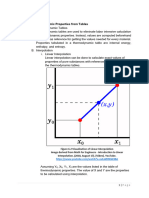
Step two: Determine the other missing properties:
Since it is superheated steam, we are going to find the missing properties basing from the pressure and the
given property (specific volume).
With a specific volume of 700 cm3/g, we are going to find values of specific volume under 300 kPa that make
700cm3/g in the middle.
From the steam table, 700 cm3/g is in between of 675.49 and 716.35. Use the other values under 675.49 and
716.35. Therefore, via interpolation, we are able to determine U, H and S.
SV, cm3/g U, kJ/kg H, kJ/kg S, kJ/kg-K
675.49 2610.8 2813.5 7.1990
716.35 2650.6 2865.5 7.3119
700 2634.6742 2844.6924 7.2667
Via interpolation (based from SV of 700), other values are determined.
You might also like
- Vip1 120Document120 pagesVip1 120Paul AbonitaNo ratings yet
- Energy Balance CalculationDocument2 pagesEnergy Balance CalculationSzelee KuekNo ratings yet
- Inlet Entropy S Outlet Entropy S'Document9 pagesInlet Entropy S Outlet Entropy S'Kashan AslamNo ratings yet
- Tutorial 1: Basic Concept of ThermodynamicsDocument4 pagesTutorial 1: Basic Concept of ThermodynamicsKaka ZettyNo ratings yet
- Question 1 (15 Marks)Document5 pagesQuestion 1 (15 Marks)Farouk BassaNo ratings yet
- As 102 - Plates - Final Project - No AnsDocument5 pagesAs 102 - Plates - Final Project - No AnsHashirama SenjuNo ratings yet
- صادق سالم محمد Practicle Cycle and its CalculationDocument18 pagesصادق سالم محمد Practicle Cycle and its Calculationعبدالمحسن علي ENo ratings yet
- Thermo Problems (Final Exam 1)Document3 pagesThermo Problems (Final Exam 1)rii amosNo ratings yet
- MMAN2700ThermoProblemSheet5 - Pure SubstancesDocument3 pagesMMAN2700ThermoProblemSheet5 - Pure Substancesgrandw9524No ratings yet
- Assignment 8Document8 pagesAssignment 8Jihan MutiahNo ratings yet
- Air MixingDocument2 pagesAir MixingJom SanzNo ratings yet
- Thermochem - An Ice CalorimeterDocument7 pagesThermochem - An Ice CalorimeterJames CiapaNo ratings yet
- Tutorial 3Document2 pagesTutorial 3kaeshav manivannanNo ratings yet
- Chapter 6Document18 pagesChapter 6Xeen FortunyNo ratings yet
- ME-1100 Thermodynamics May - June 2022 - Trimester Tutorial - 3Document2 pagesME-1100 Thermodynamics May - June 2022 - Trimester Tutorial - 3Aiswarya Ramesh me21b011No ratings yet
- Thermo Cycle Problems With Solution 1Document12 pagesThermo Cycle Problems With Solution 1Maridil Joy IsidroNo ratings yet
- Mechanism of Thermoluminescence in Sm-And Eu-Doped Barium SulphateDocument10 pagesMechanism of Thermoluminescence in Sm-And Eu-Doped Barium Sulphatejanneth reyesNo ratings yet
- Charles Law ProblemsDocument2 pagesCharles Law ProblemsDanica OpolintoNo ratings yet
- Thermodynamics 03Document4 pagesThermodynamics 03Cullen MoralesNo ratings yet
- Properties of SteamDocument4 pagesProperties of SteamAndrew Grogan100% (1)
- Experiment 10-Spectrophotometric MethodsDocument6 pagesExperiment 10-Spectrophotometric MethodsMarc DiongcoNo ratings yet
- 1 - Energy and Energy BalancesDocument135 pages1 - Energy and Energy BalancesHabib Al-Aziz100% (2)
- Answer Problem Sheet-06 Me201 EntropyDocument5 pagesAnswer Problem Sheet-06 Me201 EntropyAtif IrshadNo ratings yet
- Properties of Pure Substances: CMT 458 - Noor Hafizah UyupDocument7 pagesProperties of Pure Substances: CMT 458 - Noor Hafizah UyupAnna WilsonNo ratings yet
- Introduction To Steam Tables and Mollier Diagram: 1. DefinitionDocument40 pagesIntroduction To Steam Tables and Mollier Diagram: 1. Definitionbikas_sahaNo ratings yet
- Thermodynamics 1 Chapter 07Document162 pagesThermodynamics 1 Chapter 07Devantharan NadesanNo ratings yet
- Cbse Test Paper-03 CLASS - XI CHEMISTRY (States of Matter: Gases and Liquids) Topic: - Ideal Gas EquationDocument1 pageCbse Test Paper-03 CLASS - XI CHEMISTRY (States of Matter: Gases and Liquids) Topic: - Ideal Gas Equationsatya176No ratings yet
- Calculator TechniquesDocument8 pagesCalculator TechniquesRafael DeocuarizaNo ratings yet
- Introductory Concepts and DefinitionsDocument3 pagesIntroductory Concepts and Definitionsisrael moizo dintsiNo ratings yet
- Continue Practice Exam Test Questions Part 2 of The SeriesDocument7 pagesContinue Practice Exam Test Questions Part 2 of The SeriesKenn Earl Bringino VillanuevaNo ratings yet
- Eat Transfer Coefficients For Submerged CoilsDocument13 pagesEat Transfer Coefficients For Submerged Coilsvitcon87100% (1)
- Practice Problems On EntropyDocument1 pagePractice Problems On EntropyNetra PujarNo ratings yet
- Themal Capacity WorksheetDocument4 pagesThemal Capacity WorksheetSukanya VedavyasaNo ratings yet
- Input: Ideal Gas Process Mass of Ideal Gas Velocity 1 Velocity 2 Pressure 1 and 2 - Temperature 1 Temperature 2Document5 pagesInput: Ideal Gas Process Mass of Ideal Gas Velocity 1 Velocity 2 Pressure 1 and 2 - Temperature 1 Temperature 2P SobremonteNo ratings yet
- 15me03 Thermodynamics Problems June2017Document19 pages15me03 Thermodynamics Problems June2017Praveen Vijay100% (1)
- Engineering Thermodynamics (Tutorial 1) PDFDocument4 pagesEngineering Thermodynamics (Tutorial 1) PDFSahil AcharyaNo ratings yet
- Exercises Chap 1&2Document3 pagesExercises Chap 1&2Nguyen Thuy Bao Ngoc B2107502No ratings yet
- Tharmal Science 2014 FDocument2 pagesTharmal Science 2014 FRajeshGuptaNo ratings yet
- Rankine Cycle Diagram: K KG KJ K KG KJ Kpa S Kpa S S XDocument16 pagesRankine Cycle Diagram: K KG KJ K KG KJ Kpa S Kpa S S Xanon_166336005No ratings yet
- Tutorial Sheet No. 1: UES011 Thermofluids (Thermodynamics)Document1 pageTutorial Sheet No. 1: UES011 Thermofluids (Thermodynamics)Vinay DograNo ratings yet
- EXAMDocument1 pageEXAMkelly evangelistaNo ratings yet
- Assignment JeeDocument3 pagesAssignment Jeevikas2504No ratings yet
- Compressible Gas CalculationDocument6 pagesCompressible Gas CalculationRafael RamonNo ratings yet
- Thermo 5th Chap07 P001Document25 pagesThermo 5th Chap07 P001Rodrigo Andre Zuniga JuarezNo ratings yet
- AssignmentDocument2 pagesAssignmentsilverstonerocky0No ratings yet
- ThermoDocument6 pagesThermoMLNDG boysNo ratings yet
- Chapter 1 Concepts Definition and Basic PrinciplesDocument8 pagesChapter 1 Concepts Definition and Basic PrinciplesEyron EyronNo ratings yet
- ATKDocument4 pagesATKAgung SuharmantoNo ratings yet
- Entropy ProbsDocument5 pagesEntropy ProbsFAzle RAbbyNo ratings yet
- Question 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT DsDocument6 pagesQuestion 1. Liquid Water at 200 Kpa and 15: S S M S M T Q DT Dsfivos_rgNo ratings yet
- Practice ProblemsDocument1 pagePractice ProblemsSanu SouravNo ratings yet
- Electron Charge To Mass Ratio E/m: J. Lukens, B. Reid, A. Tuggle PH 235-001, Group 4 18 January 2010Document7 pagesElectron Charge To Mass Ratio E/m: J. Lukens, B. Reid, A. Tuggle PH 235-001, Group 4 18 January 2010KhizarNo ratings yet
- Examples MENG580 Chapter 11 PDFDocument14 pagesExamples MENG580 Chapter 11 PDFYasser TarhiniNo ratings yet
- Examples On 2nd Law For A ProcessDocument13 pagesExamples On 2nd Law For A ProcessMaria SarwatNo ratings yet
- PropertiesDocument3 pagesPropertiesswap1983No ratings yet
- Tut Sheet 3 SolutionDocument7 pagesTut Sheet 3 SolutionDevkriti SharmaNo ratings yet
- Thermo Solutions - Part79 PDFDocument1 pageThermo Solutions - Part79 PDFLiz ArfinNo ratings yet
- Lec 14Document14 pagesLec 14Nilesh SinghNo ratings yet
- He Is There (Lifebreakthrough) (Key Of)Document4 pagesHe Is There (Lifebreakthrough) (Key Of)JAIRUS RODAN CARI�ONo ratings yet
- Participants' Insights Gained From Nasat Labs Virtual TourDocument4 pagesParticipants' Insights Gained From Nasat Labs Virtual TourJAIRUS RODAN CARI�ONo ratings yet
- TechnoDocument8 pagesTechnoJAIRUS RODAN CARI�ONo ratings yet
- Book1 ENGGMANDocument3 pagesBook1 ENGGMANJAIRUS RODAN CARI�ONo ratings yet
- EXPERIMENT 1: Determination of Temperature, PH, Turbidity and Conductivity Data SheetDocument3 pagesEXPERIMENT 1: Determination of Temperature, PH, Turbidity and Conductivity Data SheetJAIRUS RODAN CARI�ONo ratings yet