Professional Documents
Culture Documents
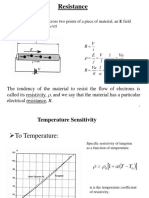
MSE201 Equation Sheet
MSE201 Equation Sheet
Uploaded by
JohnCopyright:
Available Formats
You might also like
- Lesson Plan: Science Grade 10Document2 pagesLesson Plan: Science Grade 10Phranxies Jean Blaya67% (3)
- Hukum Coulomb Dan Medan ListrikDocument66 pagesHukum Coulomb Dan Medan Listrikdavid purbaNo ratings yet
- Part - A (Physics)Document34 pagesPart - A (Physics)Deepak SharmaNo ratings yet
- Stability: Power System andDocument3 pagesStability: Power System andDeeksha ShreeNo ratings yet
- IV Smena, Zavrsni Ispit, Jul 2012Document2 pagesIV Smena, Zavrsni Ispit, Jul 2012citalacNo ratings yet
- Lecture 2 - EMDocument22 pagesLecture 2 - EMKybs nyhuNo ratings yet
- Spectroscopy: Microwave (Rotational) Infrared (Vibrational) Raman (Rotational & Vibrational) TextsDocument33 pagesSpectroscopy: Microwave (Rotational) Infrared (Vibrational) Raman (Rotational & Vibrational) TextsChloe KingNo ratings yet
- Analysis of Mechanism of Dew Point Measurement Using A Colpitts Oscillation CircuitDocument6 pagesAnalysis of Mechanism of Dew Point Measurement Using A Colpitts Oscillation Circuitmanish53970No ratings yet
- Lecture 9 09 11 22Document32 pagesLecture 9 09 11 22Alkit SharmaNo ratings yet
- EE 311 Final Exam SolutionsDocument15 pagesEE 311 Final Exam SolutionsamjadakramNo ratings yet
- Slides Lec 3ADocument17 pagesSlides Lec 3A5610Umar IqbalNo ratings yet
- Test - 6 SolutionsDocument4 pagesTest - 6 SolutionsashuisobaNo ratings yet
- Lecture 03a Coulombs Law 05102023 084456amDocument12 pagesLecture 03a Coulombs Law 05102023 084456amvacedok521No ratings yet
- 26 05 18 Morning PDFDocument35 pages26 05 18 Morning PDFApurbNo ratings yet
- 2024-JEE Main-6 - SolutionsDocument16 pages2024-JEE Main-6 - Solutionssinglaanush18No ratings yet
- Problem PR02.1Document22 pagesProblem PR02.1AliNo ratings yet
- Final Round 08 Version SDocument8 pagesFinal Round 08 Version Ssunmeetnaik08No ratings yet
- Aits 2122 FT V Jeem SolDocument19 pagesAits 2122 FT V Jeem Solchinmaya bakiNo ratings yet
- Oscillators & Applications LongstreetDocument4 pagesOscillators & Applications Longstreetnapoleon_velasc3617No ratings yet
- Accoustic EnclosureDocument20 pagesAccoustic EnclosureBhavani PrasadNo ratings yet
- Aipmt Neet Question Paper 2012 183Document36 pagesAipmt Neet Question Paper 2012 183ramNo ratings yet
- Fundamentals of AcousticsDocument18 pagesFundamentals of AcousticsEga Kartika AdhityaNo ratings yet
- CHM131 - Chapter 2 - Structure of Atom - PeriodicityDocument97 pagesCHM131 - Chapter 2 - Structure of Atom - PeriodicityLeo PietroNo ratings yet
- Overall Order of Irreversible Reactions From The Half-Life TDocument15 pagesOverall Order of Irreversible Reactions From The Half-Life Talice AnnabelleNo ratings yet
- Lec 2 SlidesDocument21 pagesLec 2 SlidesAbdelrahman EzzatNo ratings yet
- Sol JEEMain 11-Jan-MorningDocument16 pagesSol JEEMain 11-Jan-MorningRahul RajNo ratings yet
- Solutions of Exam# 1: K VR e Where K A Are Consts RDocument12 pagesSolutions of Exam# 1: K VR e Where K A Are Consts RSatyam KNo ratings yet
- Network Theory: Presented byDocument29 pagesNetwork Theory: Presented bySapata KumarNo ratings yet
- Chapter 2 - Structure of Atom PeridiocityDocument97 pagesChapter 2 - Structure of Atom PeridiocityAina AthirahNo ratings yet
- L5-Current and ResistanceDocument16 pagesL5-Current and ResistanceSyed Anas SohailNo ratings yet
- The Study of Modulation Schemes 変調方式に関する研究Document20 pagesThe Study of Modulation Schemes 変調方式に関する研究khkamalNo ratings yet
- EEE209 Online2Document28 pagesEEE209 Online2MeowNo ratings yet
- Lec 2 Ch2 Coulomb Law 2020 (1)Document50 pagesLec 2 Ch2 Coulomb Law 2020 (1)Abdo AskrNo ratings yet
- LT10 - ResistanceDocument12 pagesLT10 - ResistanceAbhishek Kumar (M20EE051)No ratings yet
- From Unimap, Malaysia Louisiana State U HK City U Eastern Washington UDocument31 pagesFrom Unimap, Malaysia Louisiana State U HK City U Eastern Washington UKingLokeshNo ratings yet
- Quantizing Radiation: Michael Fowler, 5/4/06Document8 pagesQuantizing Radiation: Michael Fowler, 5/4/06Katie RobbinsNo ratings yet
- Eeng 455 LectureDocument174 pagesEeng 455 Lectureezekielmuriithi34No ratings yet
- Mock Test-4 Physics P2 SolutionDocument3 pagesMock Test-4 Physics P2 Solutiongk128074No ratings yet
- SMB 1 Xi Chem Mod14Document11 pagesSMB 1 Xi Chem Mod14Aniruddha MondalNo ratings yet
- Aits 2122 FT Vii Jeea Paper 1 Sol PDFDocument12 pagesAits 2122 FT Vii Jeea Paper 1 Sol PDFSantanu SahaNo ratings yet
- Physics 20 July 2021 (SHIFT - 1) Question With SolutionDocument17 pagesPhysics 20 July 2021 (SHIFT - 1) Question With SolutionMehul MayankNo ratings yet
- Physics, Biology: ChemistryDocument36 pagesPhysics, Biology: ChemistryYASH JAINNo ratings yet
- M.SC - Physics - 2019Document23 pagesM.SC - Physics - 2019Pitambar RoyNo ratings yet
- Aits 1819 FT II Jeem SolDocument15 pagesAits 1819 FT II Jeem SolScribd nowNo ratings yet
- Molecular KineticsDocument6 pagesMolecular Kineticssonia.morell.obNo ratings yet
- (C-4 A-1) SolutionDocument4 pages(C-4 A-1) SolutionSachin DedhiaNo ratings yet
- PT - 09Document7 pagesPT - 09261217raghavsharmaNo ratings yet
- Lu 1993Document5 pagesLu 1993Vijay HuseNo ratings yet
- Lec 2 Coulomb LawDocument45 pagesLec 2 Coulomb LawalmkaaasedNo ratings yet
- EC Test-7 SolDocument39 pagesEC Test-7 SolGanesh M SurangeNo ratings yet
- JEE Main 2021 17 March Shift 1 PhysicsDocument13 pagesJEE Main 2021 17 March Shift 1 PhysicsAditya Raj SinghNo ratings yet
- Electronic Configuration: Prepared By: A. HarrisDocument42 pagesElectronic Configuration: Prepared By: A. HarrisRoshane RoachNo ratings yet
- Inorganic Chemistry I: Prof. A D L I M, M.SCDocument28 pagesInorganic Chemistry I: Prof. A D L I M, M.SCFiTri Yani SyarbiniNo ratings yet
- Physics Work Sheet-SS JCT - AIATS-02 - SolDocument3 pagesPhysics Work Sheet-SS JCT - AIATS-02 - Solhari kroviNo ratings yet
- 651 IntroNotes1Document36 pages651 IntroNotes1gowrimanohar1975No ratings yet
- Chemistry QuestionsDocument2 pagesChemistry QuestionsSudeep NayakNo ratings yet
- Part C - Physics: JEE-MAIN-2015-CMP-10Document21 pagesPart C - Physics: JEE-MAIN-2015-CMP-10Praveen MaramNo ratings yet
- Dwnload Full Elements of Chemical Reaction Engineering 4th Edition Fogler Solutions Manual PDFDocument14 pagesDwnload Full Elements of Chemical Reaction Engineering 4th Edition Fogler Solutions Manual PDFgauntreprovalaxdjx100% (15)
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Exercises in Electronics: Operational Amplifier CircuitsFrom EverandExercises in Electronics: Operational Amplifier CircuitsRating: 3 out of 5 stars3/5 (1)
- Feynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterFrom EverandFeynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterNo ratings yet
- FB ABE Group ReviewerDocument16 pagesFB ABE Group ReviewerGlaiza Moratilla LantacaNo ratings yet
- What IS Inorganic ChemistryDocument2 pagesWhat IS Inorganic ChemistryRoja ReddyNo ratings yet
- Skid Design and AnalysisDocument3 pagesSkid Design and AnalysisdgkmurtiNo ratings yet
- Laws of Motion Class IXDocument2 pagesLaws of Motion Class IXSantoshPathakNo ratings yet
- Design Optimization of Composite Submarine Pressure HullDocument4 pagesDesign Optimization of Composite Submarine Pressure HullInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- MCQ - Ece - Ipc 416 - YaunDocument3 pagesMCQ - Ece - Ipc 416 - YaunYaun, Aslie Jane S.No ratings yet
- Methods For Analyzing Power System Small Signal Stability: Provided by Memorial University Research RepositoryDocument114 pagesMethods For Analyzing Power System Small Signal Stability: Provided by Memorial University Research RepositoryPooja kNo ratings yet
- 7500-Rn Rev Edit 2011Document4 pages7500-Rn Rev Edit 2011cristian perla martinezNo ratings yet
- Experiment 2: Transient Response of A Second-Order SystemDocument12 pagesExperiment 2: Transient Response of A Second-Order SystemReza KühnNo ratings yet
- Physics Iup Itb Bab 4 - 5Document15 pagesPhysics Iup Itb Bab 4 - 5Emmyr FaiqNo ratings yet
- 18mat411 MAT-1Document4 pages18mat411 MAT-1M.A rajaNo ratings yet
- 1999 - Experimental Investigation of The Acoustical Characteristics of University ClassroomsDocument10 pages1999 - Experimental Investigation of The Acoustical Characteristics of University ClassroomsArquitetogeek PontocomNo ratings yet
- Genesis Series 2" & 3" Steel Meters: Smith Meter PD MeterDocument6 pagesGenesis Series 2" & 3" Steel Meters: Smith Meter PD MeterEnder ZenginobuzNo ratings yet
- HK222 CLC Sample 3 2Document5 pagesHK222 CLC Sample 3 2Tuấn Khang TừNo ratings yet
- New Integrated-Optical Mach-Zehnder ModulatorDocument7 pagesNew Integrated-Optical Mach-Zehnder Modulatorพลวัต โพธิ์รุ้งNo ratings yet
- TS-ELEC-04 - Specification of Electric Cables - R1Document23 pagesTS-ELEC-04 - Specification of Electric Cables - R1SUSOVAN BISWASNo ratings yet
- Pembahasan Tes Bahasa Inggris 1Document13 pagesPembahasan Tes Bahasa Inggris 1Aripin SastranegaraNo ratings yet
- Chapter 5 Section 1: The Mcgraw-Hill Companies, Inc. Permission Required For Presentation or DisplayDocument285 pagesChapter 5 Section 1: The Mcgraw-Hill Companies, Inc. Permission Required For Presentation or DisplayPriyanka ChauahnNo ratings yet
- QUIZ 1 Attempt ReviewDocument1 pageQUIZ 1 Attempt ReviewMaevin WooNo ratings yet
- Comparing Concrete On The Basis of The Bond Developed With Reinforcing SteelDocument4 pagesComparing Concrete On The Basis of The Bond Developed With Reinforcing SteelEvert RiveraNo ratings yet
- Sieve Analysis of Fine and Course AggregatesDocument13 pagesSieve Analysis of Fine and Course AggregatesPablo Gomes73% (30)
- Presbyopia Correcting Iols For Phakic & Pseudophakic Eyes: Eps Technology: A Special Concept With Multiple ApplicationsDocument8 pagesPresbyopia Correcting Iols For Phakic & Pseudophakic Eyes: Eps Technology: A Special Concept With Multiple ApplicationsAbhijit PatilNo ratings yet
- Testing Machine Testindo RevisiDocument4 pagesTesting Machine Testindo RevisiRayhan AlfazaNo ratings yet
- MCP-15-10-11th (PQRS) Code-BDocument20 pagesMCP-15-10-11th (PQRS) Code-BRaju SinghNo ratings yet
- ĐỀ HSG ANH LỚP 9 năm 2021 - 802Document2 pagesĐỀ HSG ANH LỚP 9 năm 2021 - 802Hồ Ngọc TrAnhNo ratings yet
- La Concepcion College, Inc.: Evaluation SheetDocument1 pageLa Concepcion College, Inc.: Evaluation SheetMark MarcosNo ratings yet
- Ultramid® A3HG5 en SI - Product DatasheetDocument2 pagesUltramid® A3HG5 en SI - Product Datasheetshahin_723No ratings yet
- Fundamentals of Computational Fluid Dynamics The Finite Volume Method (Clovis R. Maliska)Document436 pagesFundamentals of Computational Fluid Dynamics The Finite Volume Method (Clovis R. Maliska)reinaldoburconNo ratings yet
- Centrifuge Model Testing of Soils: A Literature ReviewDocument47 pagesCentrifuge Model Testing of Soils: A Literature ReviewPaul LUKNo ratings yet
MSE201 Equation Sheet
MSE201 Equation Sheet
Uploaded by
JohnOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MSE201 Equation Sheet
MSE201 Equation Sheet
Uploaded by
JohnCopyright:
Available Formats
MSE 201
Exam Equation Sheet
To receive full or partial credit on numerical problems, you must show your calculations in step-by-
step fashion. Units must be shown when applicable and all plots must have labeled axes. Be
sure that you read and answer all parts of each question.
Constants, equations and figures are provided on the last pages of the exam.
It will help to review these pages before beginning the test.
Equations and Useful Information
NA = 6.02 x 1023 mole-1 k = 8.62 x 10-5 eV/atom-K K = oC + 273
R = 1.987 cal/K-mole = 8.314 J/K-mole 109 nm = 106 m = 102 cm = 1 m
1GPa = 103MPa = 109Pa=109N/m2 1 J/m2 = 1 N/m Area of a circle = R2
4 3
109 nm = 106 m = 102 cm = 1 m Volume of sphere R 1 Kg = 1000 g = 106 mg
3
4R 4R
ao ao 2R 2 Qv / kT
3 2 Nv Ne
[uvw] <uvw> (hkl) {hkl} electron charge = 1.6 x 10-19 C
NA
N
A = (l-lo)/lo = l/lo = Load/Ao
2 x y
r=1/2 y y= y/2E
z z
2 F l lo l
E Ur y y
2 2E
y
Ao l0 lo
Ao Ad lf lo Ao Af
%CW 100 %EL 100 %RA 100
Ao lf Ao
1/ 2
a
m o
KIc Y a
t
n'( Ac AA )
RSS = cos ( ) cos ( )
VC N A
You might also like
- Lesson Plan: Science Grade 10Document2 pagesLesson Plan: Science Grade 10Phranxies Jean Blaya67% (3)
- Hukum Coulomb Dan Medan ListrikDocument66 pagesHukum Coulomb Dan Medan Listrikdavid purbaNo ratings yet
- Part - A (Physics)Document34 pagesPart - A (Physics)Deepak SharmaNo ratings yet
- Stability: Power System andDocument3 pagesStability: Power System andDeeksha ShreeNo ratings yet
- IV Smena, Zavrsni Ispit, Jul 2012Document2 pagesIV Smena, Zavrsni Ispit, Jul 2012citalacNo ratings yet
- Lecture 2 - EMDocument22 pagesLecture 2 - EMKybs nyhuNo ratings yet
- Spectroscopy: Microwave (Rotational) Infrared (Vibrational) Raman (Rotational & Vibrational) TextsDocument33 pagesSpectroscopy: Microwave (Rotational) Infrared (Vibrational) Raman (Rotational & Vibrational) TextsChloe KingNo ratings yet
- Analysis of Mechanism of Dew Point Measurement Using A Colpitts Oscillation CircuitDocument6 pagesAnalysis of Mechanism of Dew Point Measurement Using A Colpitts Oscillation Circuitmanish53970No ratings yet
- Lecture 9 09 11 22Document32 pagesLecture 9 09 11 22Alkit SharmaNo ratings yet
- EE 311 Final Exam SolutionsDocument15 pagesEE 311 Final Exam SolutionsamjadakramNo ratings yet
- Slides Lec 3ADocument17 pagesSlides Lec 3A5610Umar IqbalNo ratings yet
- Test - 6 SolutionsDocument4 pagesTest - 6 SolutionsashuisobaNo ratings yet
- Lecture 03a Coulombs Law 05102023 084456amDocument12 pagesLecture 03a Coulombs Law 05102023 084456amvacedok521No ratings yet
- 26 05 18 Morning PDFDocument35 pages26 05 18 Morning PDFApurbNo ratings yet
- 2024-JEE Main-6 - SolutionsDocument16 pages2024-JEE Main-6 - Solutionssinglaanush18No ratings yet
- Problem PR02.1Document22 pagesProblem PR02.1AliNo ratings yet
- Final Round 08 Version SDocument8 pagesFinal Round 08 Version Ssunmeetnaik08No ratings yet
- Aits 2122 FT V Jeem SolDocument19 pagesAits 2122 FT V Jeem Solchinmaya bakiNo ratings yet
- Oscillators & Applications LongstreetDocument4 pagesOscillators & Applications Longstreetnapoleon_velasc3617No ratings yet
- Accoustic EnclosureDocument20 pagesAccoustic EnclosureBhavani PrasadNo ratings yet
- Aipmt Neet Question Paper 2012 183Document36 pagesAipmt Neet Question Paper 2012 183ramNo ratings yet
- Fundamentals of AcousticsDocument18 pagesFundamentals of AcousticsEga Kartika AdhityaNo ratings yet
- CHM131 - Chapter 2 - Structure of Atom - PeriodicityDocument97 pagesCHM131 - Chapter 2 - Structure of Atom - PeriodicityLeo PietroNo ratings yet
- Overall Order of Irreversible Reactions From The Half-Life TDocument15 pagesOverall Order of Irreversible Reactions From The Half-Life Talice AnnabelleNo ratings yet
- Lec 2 SlidesDocument21 pagesLec 2 SlidesAbdelrahman EzzatNo ratings yet
- Sol JEEMain 11-Jan-MorningDocument16 pagesSol JEEMain 11-Jan-MorningRahul RajNo ratings yet
- Solutions of Exam# 1: K VR e Where K A Are Consts RDocument12 pagesSolutions of Exam# 1: K VR e Where K A Are Consts RSatyam KNo ratings yet
- Network Theory: Presented byDocument29 pagesNetwork Theory: Presented bySapata KumarNo ratings yet
- Chapter 2 - Structure of Atom PeridiocityDocument97 pagesChapter 2 - Structure of Atom PeridiocityAina AthirahNo ratings yet
- L5-Current and ResistanceDocument16 pagesL5-Current and ResistanceSyed Anas SohailNo ratings yet
- The Study of Modulation Schemes 変調方式に関する研究Document20 pagesThe Study of Modulation Schemes 変調方式に関する研究khkamalNo ratings yet
- EEE209 Online2Document28 pagesEEE209 Online2MeowNo ratings yet
- Lec 2 Ch2 Coulomb Law 2020 (1)Document50 pagesLec 2 Ch2 Coulomb Law 2020 (1)Abdo AskrNo ratings yet
- LT10 - ResistanceDocument12 pagesLT10 - ResistanceAbhishek Kumar (M20EE051)No ratings yet
- From Unimap, Malaysia Louisiana State U HK City U Eastern Washington UDocument31 pagesFrom Unimap, Malaysia Louisiana State U HK City U Eastern Washington UKingLokeshNo ratings yet
- Quantizing Radiation: Michael Fowler, 5/4/06Document8 pagesQuantizing Radiation: Michael Fowler, 5/4/06Katie RobbinsNo ratings yet
- Eeng 455 LectureDocument174 pagesEeng 455 Lectureezekielmuriithi34No ratings yet
- Mock Test-4 Physics P2 SolutionDocument3 pagesMock Test-4 Physics P2 Solutiongk128074No ratings yet
- SMB 1 Xi Chem Mod14Document11 pagesSMB 1 Xi Chem Mod14Aniruddha MondalNo ratings yet
- Aits 2122 FT Vii Jeea Paper 1 Sol PDFDocument12 pagesAits 2122 FT Vii Jeea Paper 1 Sol PDFSantanu SahaNo ratings yet
- Physics 20 July 2021 (SHIFT - 1) Question With SolutionDocument17 pagesPhysics 20 July 2021 (SHIFT - 1) Question With SolutionMehul MayankNo ratings yet
- Physics, Biology: ChemistryDocument36 pagesPhysics, Biology: ChemistryYASH JAINNo ratings yet
- M.SC - Physics - 2019Document23 pagesM.SC - Physics - 2019Pitambar RoyNo ratings yet
- Aits 1819 FT II Jeem SolDocument15 pagesAits 1819 FT II Jeem SolScribd nowNo ratings yet
- Molecular KineticsDocument6 pagesMolecular Kineticssonia.morell.obNo ratings yet
- (C-4 A-1) SolutionDocument4 pages(C-4 A-1) SolutionSachin DedhiaNo ratings yet
- PT - 09Document7 pagesPT - 09261217raghavsharmaNo ratings yet
- Lu 1993Document5 pagesLu 1993Vijay HuseNo ratings yet
- Lec 2 Coulomb LawDocument45 pagesLec 2 Coulomb LawalmkaaasedNo ratings yet
- EC Test-7 SolDocument39 pagesEC Test-7 SolGanesh M SurangeNo ratings yet
- JEE Main 2021 17 March Shift 1 PhysicsDocument13 pagesJEE Main 2021 17 March Shift 1 PhysicsAditya Raj SinghNo ratings yet
- Electronic Configuration: Prepared By: A. HarrisDocument42 pagesElectronic Configuration: Prepared By: A. HarrisRoshane RoachNo ratings yet
- Inorganic Chemistry I: Prof. A D L I M, M.SCDocument28 pagesInorganic Chemistry I: Prof. A D L I M, M.SCFiTri Yani SyarbiniNo ratings yet
- Physics Work Sheet-SS JCT - AIATS-02 - SolDocument3 pagesPhysics Work Sheet-SS JCT - AIATS-02 - Solhari kroviNo ratings yet
- 651 IntroNotes1Document36 pages651 IntroNotes1gowrimanohar1975No ratings yet
- Chemistry QuestionsDocument2 pagesChemistry QuestionsSudeep NayakNo ratings yet
- Part C - Physics: JEE-MAIN-2015-CMP-10Document21 pagesPart C - Physics: JEE-MAIN-2015-CMP-10Praveen MaramNo ratings yet
- Dwnload Full Elements of Chemical Reaction Engineering 4th Edition Fogler Solutions Manual PDFDocument14 pagesDwnload Full Elements of Chemical Reaction Engineering 4th Edition Fogler Solutions Manual PDFgauntreprovalaxdjx100% (15)
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Exercises in Electronics: Operational Amplifier CircuitsFrom EverandExercises in Electronics: Operational Amplifier CircuitsRating: 3 out of 5 stars3/5 (1)
- Feynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterFrom EverandFeynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterNo ratings yet
- FB ABE Group ReviewerDocument16 pagesFB ABE Group ReviewerGlaiza Moratilla LantacaNo ratings yet
- What IS Inorganic ChemistryDocument2 pagesWhat IS Inorganic ChemistryRoja ReddyNo ratings yet
- Skid Design and AnalysisDocument3 pagesSkid Design and AnalysisdgkmurtiNo ratings yet
- Laws of Motion Class IXDocument2 pagesLaws of Motion Class IXSantoshPathakNo ratings yet
- Design Optimization of Composite Submarine Pressure HullDocument4 pagesDesign Optimization of Composite Submarine Pressure HullInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- MCQ - Ece - Ipc 416 - YaunDocument3 pagesMCQ - Ece - Ipc 416 - YaunYaun, Aslie Jane S.No ratings yet
- Methods For Analyzing Power System Small Signal Stability: Provided by Memorial University Research RepositoryDocument114 pagesMethods For Analyzing Power System Small Signal Stability: Provided by Memorial University Research RepositoryPooja kNo ratings yet
- 7500-Rn Rev Edit 2011Document4 pages7500-Rn Rev Edit 2011cristian perla martinezNo ratings yet
- Experiment 2: Transient Response of A Second-Order SystemDocument12 pagesExperiment 2: Transient Response of A Second-Order SystemReza KühnNo ratings yet
- Physics Iup Itb Bab 4 - 5Document15 pagesPhysics Iup Itb Bab 4 - 5Emmyr FaiqNo ratings yet
- 18mat411 MAT-1Document4 pages18mat411 MAT-1M.A rajaNo ratings yet
- 1999 - Experimental Investigation of The Acoustical Characteristics of University ClassroomsDocument10 pages1999 - Experimental Investigation of The Acoustical Characteristics of University ClassroomsArquitetogeek PontocomNo ratings yet
- Genesis Series 2" & 3" Steel Meters: Smith Meter PD MeterDocument6 pagesGenesis Series 2" & 3" Steel Meters: Smith Meter PD MeterEnder ZenginobuzNo ratings yet
- HK222 CLC Sample 3 2Document5 pagesHK222 CLC Sample 3 2Tuấn Khang TừNo ratings yet
- New Integrated-Optical Mach-Zehnder ModulatorDocument7 pagesNew Integrated-Optical Mach-Zehnder Modulatorพลวัต โพธิ์รุ้งNo ratings yet
- TS-ELEC-04 - Specification of Electric Cables - R1Document23 pagesTS-ELEC-04 - Specification of Electric Cables - R1SUSOVAN BISWASNo ratings yet
- Pembahasan Tes Bahasa Inggris 1Document13 pagesPembahasan Tes Bahasa Inggris 1Aripin SastranegaraNo ratings yet
- Chapter 5 Section 1: The Mcgraw-Hill Companies, Inc. Permission Required For Presentation or DisplayDocument285 pagesChapter 5 Section 1: The Mcgraw-Hill Companies, Inc. Permission Required For Presentation or DisplayPriyanka ChauahnNo ratings yet
- QUIZ 1 Attempt ReviewDocument1 pageQUIZ 1 Attempt ReviewMaevin WooNo ratings yet
- Comparing Concrete On The Basis of The Bond Developed With Reinforcing SteelDocument4 pagesComparing Concrete On The Basis of The Bond Developed With Reinforcing SteelEvert RiveraNo ratings yet
- Sieve Analysis of Fine and Course AggregatesDocument13 pagesSieve Analysis of Fine and Course AggregatesPablo Gomes73% (30)
- Presbyopia Correcting Iols For Phakic & Pseudophakic Eyes: Eps Technology: A Special Concept With Multiple ApplicationsDocument8 pagesPresbyopia Correcting Iols For Phakic & Pseudophakic Eyes: Eps Technology: A Special Concept With Multiple ApplicationsAbhijit PatilNo ratings yet
- Testing Machine Testindo RevisiDocument4 pagesTesting Machine Testindo RevisiRayhan AlfazaNo ratings yet
- MCP-15-10-11th (PQRS) Code-BDocument20 pagesMCP-15-10-11th (PQRS) Code-BRaju SinghNo ratings yet
- ĐỀ HSG ANH LỚP 9 năm 2021 - 802Document2 pagesĐỀ HSG ANH LỚP 9 năm 2021 - 802Hồ Ngọc TrAnhNo ratings yet
- La Concepcion College, Inc.: Evaluation SheetDocument1 pageLa Concepcion College, Inc.: Evaluation SheetMark MarcosNo ratings yet
- Ultramid® A3HG5 en SI - Product DatasheetDocument2 pagesUltramid® A3HG5 en SI - Product Datasheetshahin_723No ratings yet
- Fundamentals of Computational Fluid Dynamics The Finite Volume Method (Clovis R. Maliska)Document436 pagesFundamentals of Computational Fluid Dynamics The Finite Volume Method (Clovis R. Maliska)reinaldoburconNo ratings yet
- Centrifuge Model Testing of Soils: A Literature ReviewDocument47 pagesCentrifuge Model Testing of Soils: A Literature ReviewPaul LUKNo ratings yet