Professional Documents
Culture Documents
(Coturnix Coturnix Japonica) by Cloacal Examination: Identification of Sex of Day-Old Quail
(Coturnix Coturnix Japonica) by Cloacal Examination: Identification of Sex of Day-Old Quail
Uploaded by
AlanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
(Coturnix Coturnix Japonica) by Cloacal Examination: Identification of Sex of Day-Old Quail
(Coturnix Coturnix Japonica) by Cloacal Examination: Identification of Sex of Day-Old Quail
Uploaded by
AlanCopyright:
Available Formats
GROWTH AND REPRODUCTION OF QUAIL 469
1961. Evaluation of Coturnix (Japanese quail) Yamashina, Y., 1961. Quail breeding in Japan. J.
as a pilot animal for poultry. Poultry Sci. 40: Bombay Nat. Hist. Soc, 58: 216-222 (Abstract
651-657. No. 732, A.B.A., 3 1 : 124, 1963).
Identification of Sex of Day-Old Quail {Coturnix
coturnix japonica) by Cloacal Examination1
KAZUTAKA HOMMA,2 THOMAS D. SIOPES, WILBOR O. WILSON
AND LARRY Z. MCFARLAND
Department of Poultry Husbandry and Anatomy, University of California, Davis
(Received for publication September 16, 1965)
E ARLY determination of the sex of
Japanese quail (Coturnix coturnix
time and expense may be achieved by sex-
ing day-old birds.
Two methods of sexing day-old chickens
japonica) is desirable when these birds are
raised for experimental purposes. Sexual have been used. One is the use of color
dimorphism does not become obvious until marker genes for autosexing. The other
the contour feathers emerge when the quail method involves examination of the cloaca
are 2 to 3 weeks of age. The birds usually or vent. A preliminary trial of this latter
are sexed by the difference in color of the method for sexing day-old quail has been
breast feathers, the male having brownish- reported in Japan as part of a general text-
red feathers and the female character- book of chick sexing (Maeta, 19S0).
istically having tan (gray) feathers with The present paper describes a practical
black speckles. method of sexing day-old quail by cloacal
In some mutant stocks, color of the examination and gives data relative to vari-
feathers remains identical in both sexes ations in their genital structures and sex
even after sexual maturity, e.g., the "ghost ratio. Although day-old coturnix quail
bar mutant" and other types of albinos. In weigh less than 8 g. and the diameter of the
these stocks, identification of the sex is pos- cloacal opening is less than 3 mm., the ac-
sible by observing the development of the curacy of sexing quail by cloacal examina-
cloacal gland after the birds were 4 weeks tion is comparable to or better than that
of age. This gland is located in the dorsal reported for day-old chickens.
wall of the cloaca and becomes hypertro-
MATERIALS AND METHODS
phic in sexually active males.
The above two methods are not satisfac- 1. Equipment—Initially we used for ex-
tory for experiments where one sex is pre- amination either a binocular dissecting mi-
ferred, and a very early initiation of experi- croscope (American Optical to 20 X) or a
mental procedure is desired. A saving in Luxolamp with 2 X magnification and uti-
lizing a 22 watt ring-shaped fluorescent
Supported in part by USPHS research grant light. After a little practice we no longer
NB 04171 from the National Institute of Neuro- needed magnification for sexing "typical"
logical Diseases and Blindness.
2
Present address: Department of Animal
males or females. By adjusting the direc-
Physiology, Faculty of Agriculture, Nagoya Uni- tion of illumination, the reflection of light
versity, Nagoya, Japan. from the mucosal surface or from the sur-
470 K. HOMMA, T. D. SIOPES, W. O. WILSON AND L. Z. MCFARLAND
Male Davis for a number of generations. Day-
old quail were transferred from the hatch-
ery to the laboratory of the poultry farm
and subjected to sexing as soon as possible.
Each quail was examined independently by
two members of the staff; the sex later was
verified by direct postmortem examination
of the gonads.
3. Handling of the day-old quail—To
facilitate examination, the head or neck of
each quail was held between the index and
middle fingers of the left hand, with the
head of the bird down. The abdominal wall
was pressed gently with the thumb of the
Female left hand to evert the cloaca and to cause
defecation. The surface of the vent was
cleaned of urine and feces with a piece of
soft tissue paper. The regions to be ob-
served then were manipulated with the
index finger and the thumb of the right
hand to obtain the optimum view.
t' fe^c',^— FO * ^ ^ 5 5 f r " i
RESULTS
1. Morphological basis of sexing—Fig-
*; * „K • '-
ure 1 illustrates the slightly everted cloacae
of typical male and female day-old quail.
In the male, a heart-shaped fold or protu-
c d berance is evident in the midventral area of
the cloaca; it is characterized by a deep
median fissure on its dorsal surface. In the
'-da-por04 female, the fold usually is flat and does not
protrude as in the male. In most females
the fold has a slight median fissure on its
dorsal margin (Fig. 1, c and d).
In addition, both sexes usually have a
e f small genital process located medially on
FIG. 1. Everted cloacae of typical and atypical the ventral cloacal margin (Fig. 1, a). This
day-old quail. Illustrations are oriented with ven-
tral side down. (P) Protuberance, (G) Genital
structure is equivalent to the genital pro-
process, (FO) Genital fold. cess of the chicken, in which it serves as an
important criterion for sexing (Masui and
face of the magnifying glass was mini- Hashimoto, 1933; Canfield, 1940, 1941).
mized. 2. Sexing by protuberance and by geni-
2. Materials—The birds used were from tal process—In the first series of experi-
several lines of coturnix that had been ments we compared the relative merits of
maintained in the Poultry Husbandry De- using either the genital process or the pro-
partment of the University of California at tuberance as a criterion for sexing the
SEXING DAY-OLD QUAIL 471
TABLE 1.—Comparison of accuracy of sexing day-old cottirnix based on examination
of the genital process or the protuberance
Exp. No. No. of quail Accuray (%) Area Examined Source of Quail
1 45 66.6 Genital Process U.C.D. 908
2 59 64.4 Genital Process U.C.D. 908
3 52 51.9 Genital Process U.C.D. 908
4 26 73.2 Genital Process U.C.D. 908XOklahoma
Total 182 Av. 62.2
5 36 91.7 Protuberance U.C.D. 908XOkIahoma
6 59 94.9 Protuberance U.C.D. 908XOklahoma
7 62 93.5 Protuberance U.C.D. 908 and 903
Total 157 Av. 93.0
quail. We usually found that neither the Sexing as few as 100 quail generally pro-
shape nor color of the genital process was vided sufficient experience to attain an ac-
important in sexing this species. Only curacy of 92%. Higher levels of accuracy
males with an exceptionally large cylindri- required additional practice and a special
cal genital process could be sexed accurate- technique. (See Section 4 below). Figure 2
ly. Sexing based on protuberal morphology illustrates the improvement brought about
was found to be more accurate. The accu- by experience in sexing a large number of
racy of the two methods is compared in quail.
Table 1. 4. Special aids for sexing of atypical
3. Typical and atypical genital struc- quail—The use of Evans blue (0.25%, sa-
tures—In the course of sexing the quail, a line solution) is helpful in sexing atypical
considerable percentage of atypical quail quail. It is applied to the back of the fold
was found. Of the first 845 birds examined, or protuberance with a fine tipped pipette;
137 (16.2%) were atypical and 83.8% typ- the excess dye then was removed by wiping
ical. The main variations in this group in- with a tissue. In males the deep median
cluded small protuberances in the male, fissure fills with the dye solution making a
and either a very swollen genital fold or a fine but distinct blue line. In females, the
flat fold with deep median fissure in the fe- median fissure is limited to the dorsal mar-
male. The accuracy of sexing the atypical gin of the genital fold, and hence the dye
quail was 78.1%; this contrasted with 99%
for the typical quail. If atypical quail were
sexed by guess only, the expected accuracy
should be 50%. Therefore, the combined
accuracy of sexing quail expected of begin-
ners should approach 92%. (If one assumes
84% typical with 100% accuracy and 16
atypical with 50% accuracy.)
The most difficult atypical cases we ex-
perienced were females with male-like geni-
tal structures. Fortunately, birds of this
type seldom occurred. Only nine out of 845 400 800 1200 I60O 2000 2400
quail examined (1.07%) were of this type. Number of birds Inspected
Therefore, the standard of accuracy should FIG. 2. Improvement of accuracy in sexing of
be set at 99% rather than 100%. quail with experience.
472 K. HOMMA, T. D. SIOPES, W. 0. WILSON AND L. Z. MCFAELAND
solution does not form a blue line. This a smooth margin (Fig. 1, f), which made
method is especially effective in identifying sexing easy. As a result, accuracy of sexing
atypical males. was 98.3% in 6 separate experiments in-
Phloxine B, a fluorescent dye, was used volving 235 quail.
in one trial and the birds were inspected 6. Secondary sex ratio—The total num-
under ultraviolet light. However, the ber of quail chicks examined was 2,453.
fluorescent dye gave no special advantage Based on postmortem gonadal examination,
over Evans blue. 1,288 (52.51) were males and 1,165
5. Effects of age, body size, and breed (47.49) were females. Deviation from an
on sexing—The growth of quail is very expected 50-50 distribution of sexes was
rapid. Development of the genital struc- statistically significant at the 2.5% level.
tures is approximately proportional to the This sex ratio was similar to that reported
general growth of the birds during the first by Godfrey et al. (1955) for the chicken.
few days after hatch. When the birds were
SUMMARY
kept for 1 week under a stimulatory light-
ing regimen and with access to ample food A method for determining sex of day-old
and water, sexing was accomplished more quail is described. The method based on
easily than with day-old quail. When quail the morphology of the genital protuberance
were kept in the dark, or on a restricted of the male, had an accuracy of 99%.
diet for 3 days after hatch, sexing was very The size and shape of the genital fold or
difficult. An accuracy of 83.1% or 64/77 protuberance was different in quail of
was obtained sexing fasted quail. Small or U.C.D. line 908 than in quail of the Okla-
weak quail were not easy to sex. If sexing homa line. The accuracy of the determina-
were done soon after hatching on selected tion was influenced by genetic and environ-
vigorous quail, errors due to environmental mental factors.
and age factors would be less. Similar re- The secondary (post-hatch) sex ratio of
sults in sexing weak day-old chickens were 2,453 quail was 52.51 males.
reported by Gibbs (193S).
REFERENCES
The genital structures of quail differ Canfield, T. H., 1940. Sex determination of day-
markedly among various genetic lines. old chicks. Poultry Sci. 19: 235-238.
Males of U.C.D. line 908 were identified Canfield, T. H., 1941. Sex determination of day-
more easily than were the females of the old chicks. II. Type determination. Poultry
same line. The reverse was true with birds Sci. 20:327-328.
Gibbs, C. S., 1935. A Guide to Sexing Chicks.
of the "Ghost bar" strain obtained by Orange Judd Publishing Co., New York.
crossing the Oklahoma line, contributed by Godfrey, G. F., C. C. Brunson and B. L. Good-
Dr. J. C. Gilbreath, with U.C.D. line 908 man, 1955. Secondary and tertiary sex ratio in
and their descendents. In these quail the the domestic fowl. Poultry Sci. 34: 27-29.
male protuberance was much smaller in Maeta, I., 1950. Chick Sexing Readers, 68-73.
Japanese Poultry Publishing Co., Nagoya, Ja-
size than that of males of any other line, pan.
making the males difficult to sex. However, Masui, K., and J. Hashimoto, 1933. Sexing Baby
all females of this line had a flat fold with Chicks, Journal Print Co., Vancouver, Canada.
AUGUST 3-10. SEVENTH INTERNATIONAL CONGRESS OF NUTRITION,
HAMBURG, GERMANY
You might also like
- Trio Reading 2 Unit 1 Answer KeyDocument15 pagesTrio Reading 2 Unit 1 Answer KeyMo Notie67% (3)
- MCQ - Vernacular ArchitectureDocument3 pagesMCQ - Vernacular ArchitectureMatilda Correya0% (1)
- Web It CertDocument1 pageWeb It CertGuna SeelanNo ratings yet
- Hatchlings of The Deep-Sea Octopus Graneledone Boreopacifica Are The Largest and Most Advanced KnownDocument3 pagesHatchlings of The Deep-Sea Octopus Graneledone Boreopacifica Are The Largest and Most Advanced KnownCarlos MeirellesNo ratings yet
- Allen Press and American Society of Ichthyologists and Herpetologists (ASIH) Are Collaborating With JSTOR To Digitize, CopeiaDocument4 pagesAllen Press and American Society of Ichthyologists and Herpetologists (ASIH) Are Collaborating With JSTOR To Digitize, CopeiawilllNo ratings yet
- Conner 1980Document18 pagesConner 1980Diary ThalitaNo ratings yet
- Somatosensory Determinants of Lordosis in Female Rats: Behavioral Definition of The Estrogen EffectDocument12 pagesSomatosensory Determinants of Lordosis in Female Rats: Behavioral Definition of The Estrogen EffectHümay ÜnalNo ratings yet
- Animal System Worksheet 1Document2 pagesAnimal System Worksheet 1sanchezjhamy.1No ratings yet
- Sachs Quail 69Document19 pagesSachs Quail 69shrutiNo ratings yet
- Ergatoid Reproductives in Nasutifermes Columbicus Ptera Term I D Ae)Document11 pagesErgatoid Reproductives in Nasutifermes Columbicus Ptera Term I D Ae)Johana CaezNo ratings yet
- Canvetj00317 0015Document8 pagesCanvetj00317 0015nofanvetNo ratings yet
- Ultrasonographic Appearance and Histopathological Findings of The Genital Tract in Healthy Nulliparous Female RabbitsDocument10 pagesUltrasonographic Appearance and Histopathological Findings of The Genital Tract in Healthy Nulliparous Female RabbitsAkın SeverNo ratings yet
- Vince 1964Document2 pagesVince 1964chimera01No ratings yet
- Evidence For The Theory of Evolution - Station BigDocument10 pagesEvidence For The Theory of Evolution - Station BigshusuishigakiNo ratings yet
- Bhattacharya1970 Sexed TenebrioDocument1 pageBhattacharya1970 Sexed TenebrioLuciana BarbozaNo ratings yet
- Bio 110 Revised Ex 9 Animal Tissues Anatomy of The Cane Toad (Credits To The Owner)Document6 pagesBio 110 Revised Ex 9 Animal Tissues Anatomy of The Cane Toad (Credits To The Owner)Jasmin CervantesNo ratings yet
- Rather, Periods: Mus Musculus: Induction TerritoryDocument3 pagesRather, Periods: Mus Musculus: Induction TerritoryGabriel Rubio LiraNo ratings yet
- Spermatophores of Some North American Scorpion S (Arachnida, Scorpiones)Document14 pagesSpermatophores of Some North American Scorpion S (Arachnida, Scorpiones)Alfredo PerettiNo ratings yet
- 1979 - Francke - Observations On The Reproductive Biology and Life History of Mega Corm Us Gertschi Diaz Scorpiones Chactidae MegacorminaeDocument8 pages1979 - Francke - Observations On The Reproductive Biology and Life History of Mega Corm Us Gertschi Diaz Scorpiones Chactidae MegacorminaeadriendrixNo ratings yet
- Ciclo Estral-Paper2Document6 pagesCiclo Estral-Paper2Monica MarconiNo ratings yet
- Atresia Ani With Scrotal Anomaly in A GoatDocument1 pageAtresia Ani With Scrotal Anomaly in A GoatAskep geaNo ratings yet
- Breeding AND Molt Schedules of The Rufous-Collared Sparrow in Coastal Per6Document20 pagesBreeding AND Molt Schedules of The Rufous-Collared Sparrow in Coastal Per6Daniel ReyesNo ratings yet
- EvoDevo Milestones 1-12Document24 pagesEvoDevo Milestones 1-12Giovana Brito De LimaNo ratings yet
- Science 2 - 5 Mark QuestionsDocument30 pagesScience 2 - 5 Mark QuestionsRohit BhosleNo ratings yet
- Jnnpsyc00297 0079Document8 pagesJnnpsyc00297 0079EccoNo ratings yet
- Gravity As The SoleDocument1 pageGravity As The SolelevitsaiNo ratings yet
- Woodburne 1960Document7 pagesWoodburne 1960nimaelhajjiNo ratings yet
- Review of Prostate Anatomy and Embryology and The Etiology of Benign Prostatic Hyperplasia PDFDocument10 pagesReview of Prostate Anatomy and Embryology and The Etiology of Benign Prostatic Hyperplasia PDFAlejandro GuerreroNo ratings yet
- Anatomical and Histological Structure of Reproductive Organs in Male Flying Squirrel Hylopetes Lepidus (Horsfield, 1822)Document6 pagesAnatomical and Histological Structure of Reproductive Organs in Male Flying Squirrel Hylopetes Lepidus (Horsfield, 1822)sarah khoerun nisaNo ratings yet
- Some Observations On The Habits and Placentation of Tatu NovemcinctumDocument110 pagesSome Observations On The Habits and Placentation of Tatu NovemcinctumcerradointernetNo ratings yet
- Biofluorescence in Nocturnal Crepuscular MammalsDocument6 pagesBiofluorescence in Nocturnal Crepuscular MammalsTanyut HuidromNo ratings yet
- Description of Reproductive System of Indian Water Scorpion, Laccotrephes Maculatus Fabr. (Hemiptera, Heteroptera: Nepidae)Document13 pagesDescription of Reproductive System of Indian Water Scorpion, Laccotrephes Maculatus Fabr. (Hemiptera, Heteroptera: Nepidae)Kanhiya MahourNo ratings yet
- Sexual Dimorphism and Life History of Keichousaurus Hui (Reptilia: Sauropterygia)Document9 pagesSexual Dimorphism and Life History of Keichousaurus Hui (Reptilia: Sauropterygia)Adnana CebucNo ratings yet
- Baskin 2001Document9 pagesBaskin 2001deddyoskarNo ratings yet
- A Rarest Case of Post-Partum Uterine Prolapsed in Doe RabbitDocument2 pagesA Rarest Case of Post-Partum Uterine Prolapsed in Doe RabbitReissa YuniaNo ratings yet
- The Biology of Flesh Fly, Boettcherisca Peregrina (Robineau-Desvoidy, 1830) (Diptera: Sarcophagidae)Document8 pagesThe Biology of Flesh Fly, Boettcherisca Peregrina (Robineau-Desvoidy, 1830) (Diptera: Sarcophagidae)nazmirNo ratings yet
- 1962 A Behavoral Sink SecureDocument23 pages1962 A Behavoral Sink SecureJIL JOSUE JIMENEZ AVALOSNo ratings yet
- American Association For The Advancement of Science ScienceDocument4 pagesAmerican Association For The Advancement of Science ScienceharisankarhsNo ratings yet
- Cockroaches Encyc Anim Behav 2010Document7 pagesCockroaches Encyc Anim Behav 2010JibrinNo ratings yet
- The Epididymis Re-Visited: A Personal View: Experience and HistoryDocument6 pagesThe Epididymis Re-Visited: A Personal View: Experience and HistoryAhmad SolihinNo ratings yet
- Moriyama 2018Document8 pagesMoriyama 2018Gabriel BuonoNo ratings yet
- Walter-Trillmich1994 Article FemaleAggressionAndMalePeace-kDocument8 pagesWalter-Trillmich1994 Article FemaleAggressionAndMalePeace-kIgor LériasNo ratings yet
- Incidence of The Bell'Clapper Deformity in An Autopsy SeriesDocument3 pagesIncidence of The Bell'Clapper Deformity in An Autopsy SeriesOttofianus Hewick KalangiNo ratings yet
- Bivalves and CephalopodsDocument27 pagesBivalves and CephalopodsChotu shendeNo ratings yet
- Neuro 2Document5 pagesNeuro 2Ivan RisnaNo ratings yet
- A Twisting Story How A Single Gene Twists A SnailDocument8 pagesA Twisting Story How A Single Gene Twists A SnailGabriel BuonoNo ratings yet
- (24537837 - Folia Veterinaria) The Use of Ultrasonography in Diagnostic Imaging of ReptilesDocument7 pages(24537837 - Folia Veterinaria) The Use of Ultrasonography in Diagnostic Imaging of ReptilesKmii TspNo ratings yet
- Ghysen 2005Document7 pagesGhysen 2005Araceli Enríquez OvandoNo ratings yet
- Ultrasonographic Anatomy of Reproductive Female LeDocument13 pagesUltrasonographic Anatomy of Reproductive Female LeLaily IlmiNo ratings yet
- SCH Miss Eur 1966Document3 pagesSCH Miss Eur 1966LilisNo ratings yet
- Gender-Related Differences in The Morphology of The Lacrimal GlandDocument6 pagesGender-Related Differences in The Morphology of The Lacrimal GlandpedrojakubiakNo ratings yet
- Leach 1940Document15 pagesLeach 1940J.D. NobleNo ratings yet
- Leach 1940Document15 pagesLeach 1940J.D. NobleNo ratings yet
- Growth Guinea-Pig: Biphasic CycleDocument4 pagesGrowth Guinea-Pig: Biphasic CycleEveAriNo ratings yet
- Parasitology - Parastrongylus CantonensisDocument20 pagesParasitology - Parastrongylus CantonensisNicole ManogNo ratings yet
- Ciclos Ovulatorios Cortos Despues Efecto Macho 2006Document13 pagesCiclos Ovulatorios Cortos Despues Efecto Macho 2006DantiNo ratings yet
- Ent Gordon Barbero 2009 Mpala Dung Beetles 2Document30 pagesEnt Gordon Barbero 2009 Mpala Dung Beetles 2Prix GellNo ratings yet
- Alvarez 2003 Microscopic Anatomy of Settled Cypris Larvae of Octolasmis Californiana Cirripedia LepadomorphaDocument7 pagesAlvarez 2003 Microscopic Anatomy of Settled Cypris Larvae of Octolasmis Californiana Cirripedia LepadomorphaDaniel Mateo Rangel ReséndezNo ratings yet
- Morphological Characterization of The Female Prostate (Skene's Gland or Paraurethral Gland)Document5 pagesMorphological Characterization of The Female Prostate (Skene's Gland or Paraurethral Gland)jeihNo ratings yet
- J Bbagrm 2009 02 002Document7 pagesJ Bbagrm 2009 02 002Raymundo Lopez NNo ratings yet
- Lee 1975Document11 pagesLee 1975RichardDavidNo ratings yet
- Gurdon 1986Document32 pagesGurdon 1986Nicolle BuenoNo ratings yet
- Source of The Nile Avalon Hill Play AidDocument1 pageSource of The Nile Avalon Hill Play AidIdejuDNRNo ratings yet
- State of Tennessee Department of TransportationDocument24 pagesState of Tennessee Department of TransportationFOX 17 NewsNo ratings yet
- Student Time Table For 5th To 10th June-2023: Room No. For OfflineDocument2 pagesStudent Time Table For 5th To 10th June-2023: Room No. For OfflineNaitik AdityaNo ratings yet
- Assess 311 WEEK1Document8 pagesAssess 311 WEEK1cloe reginaldoNo ratings yet
- Lge 2010sr Stakeholder CommunicationDocument6 pagesLge 2010sr Stakeholder CommunicationAnh Khoa PhạmNo ratings yet
- Who - Global Report Measles N Rubela 2015 PDFDocument56 pagesWho - Global Report Measles N Rubela 2015 PDFAbongky AbroeryNo ratings yet
- Aavani AvittamDocument2 pagesAavani AvittamUmashakti PeethNo ratings yet
- The Importance of Early Childhood EducationDocument4 pagesThe Importance of Early Childhood EducationImam Dwi MaryantoNo ratings yet
- Michael King - PresentationDocument14 pagesMichael King - PresentationArk GroupNo ratings yet
- Esl / Efl Resources: ImperativesDocument2 pagesEsl / Efl Resources: ImperativesMario Canela100% (1)
- Facing Faces PDFDocument16 pagesFacing Faces PDFNayyararNo ratings yet
- John Deere Tractor Service Manual JD S Tm4336Document14 pagesJohn Deere Tractor Service Manual JD S Tm4336oscarNo ratings yet
- Conserve For The BetterDocument8 pagesConserve For The BetterMayRoseLazoNo ratings yet
- Lesson 2B Implementation of Nursing Care of The Older Adult Psychosocial Care of Older AdultDocument6 pagesLesson 2B Implementation of Nursing Care of The Older Adult Psychosocial Care of Older Adultczeremar chanNo ratings yet
- 7DSM21019 - 22003 - PPH Building No 7 - 02Document5 pages7DSM21019 - 22003 - PPH Building No 7 - 02FARHANAH AFIQAH RAZMANNo ratings yet
- Tolerances: Drawing Third Angle Projection REV MillimetersDocument12 pagesTolerances: Drawing Third Angle Projection REV MillimetersAaron JuarezNo ratings yet
- 1 EF1A - HDT - Money - To - Bitcoins - CSP20B1B PDFDocument15 pages1 EF1A - HDT - Money - To - Bitcoins - CSP20B1B PDFMohit KumarNo ratings yet
- Gina Endaya vs. Ernesto VillaosDocument6 pagesGina Endaya vs. Ernesto VillaosMuffinNo ratings yet
- SAP User Manual PDFDocument55 pagesSAP User Manual PDFmadesuendaNo ratings yet
- Primary Sources of ShariahDocument15 pagesPrimary Sources of ShariahAeina FaiiziNo ratings yet
- User Guide For Stock Overview (HANA and non-HANA) : SymptomDocument2 pagesUser Guide For Stock Overview (HANA and non-HANA) : SymptomAshok MohanNo ratings yet
- 3d Panel SystemDocument25 pages3d Panel Systemprobir100% (3)
- CEO Monetization Playbook - VvimpDocument28 pagesCEO Monetization Playbook - VvimpvkandulaNo ratings yet
- Design BriefDocument216 pagesDesign BriefGia Vinh Bui TranNo ratings yet
- Hepatology MRCP1Document87 pagesHepatology MRCP1Raouf Ra'fat SolimanNo ratings yet
- Mathematics: Stage 9 Paper 1Document230 pagesMathematics: Stage 9 Paper 1Cambridge International Manager0% (2)
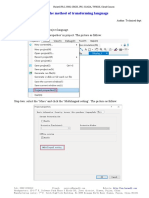
- Haiwell-Guidance For Transforming LanguageDocument5 pagesHaiwell-Guidance For Transforming LanguageMosfet AutomationNo ratings yet