Professional Documents
Culture Documents
AIDSFree - Product Sheets ERIS Infographic
AIDSFree - Product Sheets ERIS Infographic
Uploaded by
Tinsae GetachewOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
AIDSFree - Product Sheets ERIS Infographic
AIDSFree - Product Sheets ERIS Infographic
Uploaded by
Tinsae GetachewCopyright:
Available Formats
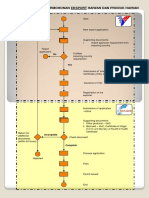
Electronic Regulatory Information System
The Ethiopian Food and Drug Administration (EFDA) oversees the market authorization and import permit approval
for both medical and food products for a wide variety of vendors from multiple countries using the Electronic Regula-
tory Information System (eRIS).
The eRIS is an umbrella system comprised of three component sub-systems which work together:
• i-License, which allows entities • i-Register, which is used to • i-Import, which is used to
to apply for a certificate of manage the market authorization manage the import process
competency to register and process where an applicant seeks for medical products, once they
import products into Ethiopia. to register a medical product in are registered in Ethiopia.
Ethiopia for later import.
Import Apply for Certificate of Review
Applicant EFDA
Competency License application
Receive Certificate of Award Certificate of
Competency License Certificate of Competency License
Competency License
Use Certificate of Competency
License to apply to register Review
a product for market application
authorization for import
Receive market
authorization approval Approve registration
Market Authorization
Approval
Utilize market authorization
approval to apply to
import product
Generate
automatic
import
permit
certificate
Import products Approve import
Import Permit
Certificate
Port Officer
Utilize i-Import to clear
imported items at port
Fully online, both applicants and EFDA use eRIS to manage the licensing, registration, and import application process
using this shared portal. This has dramatically increased processing efficiency and transparency and facilitated one
unbroken chain of information – from application to port.
You might also like
- IATA TIDS GoLite Comparison - CanadaDocument6 pagesIATA TIDS GoLite Comparison - CanadadrsinghNo ratings yet
- Music Sync License PDFDocument3 pagesMusic Sync License PDFKahmishKhan100% (1)
- Certificate of Solo ParentDocument3 pagesCertificate of Solo ParentJessica Cindy100% (2)
- Export Module ProcessDocument28 pagesExport Module ProcessparasbarbhayaNo ratings yet
- Advance AuthorisationDocument25 pagesAdvance AuthorisationGuneesh ChawlaNo ratings yet
- Guideline On Application of Licenses 2nd Edition - July 2023Document12 pagesGuideline On Application of Licenses 2nd Edition - July 2023bkmyataiNo ratings yet
- Organizational Context AnalysisDocument7 pagesOrganizational Context AnalysisAlayou TeferaNo ratings yet
- Bengal Industries: ExpansionDocument8 pagesBengal Industries: ExpansionArpit GoelNo ratings yet
- IMPEXSystem Importers User ManualDocument21 pagesIMPEXSystem Importers User ManualAdebiyi IsmailNo ratings yet
- Carta Alir Proses Permohonan Eksport Haiwan Dan Produk HaiwanDocument1 pageCarta Alir Proses Permohonan Eksport Haiwan Dan Produk HaiwanSean YongNo ratings yet
- Dangerous Drug Act and Regu OnlineDocument51 pagesDangerous Drug Act and Regu Onlineabdullah2020No ratings yet
- Permission Required To Secure An Import Clearance For A DroneDocument3 pagesPermission Required To Secure An Import Clearance For A DroneSubrata MukherjeeNo ratings yet
- PEZA Citizens CharterDocument159 pagesPEZA Citizens CharterLeah Marie Sernal100% (1)
- Manual CEROL-Manufacturing (ENG-2.3) - LPPOMDocument73 pagesManual CEROL-Manufacturing (ENG-2.3) - LPPOMChairul AbdiNo ratings yet
- MIDA - GD SPM ManufacturerDocument3 pagesMIDA - GD SPM ManufacturerFekre RaziNo ratings yet
- Sub Section VIDocument9 pagesSub Section VIdvnambNo ratings yet
- Improving Regional Trade Processes and Procedures: India Country StudyDocument18 pagesImproving Regional Trade Processes and Procedures: India Country Studyjayhanuman12378No ratings yet
- SIJILAT Petroluem License RequirmentsDocument2 pagesSIJILAT Petroluem License Requirmentsnaqi3154No ratings yet
- Gem StructureDocument7 pagesGem StructureRohit TendersNo ratings yet
- SGS PCA Kuwait Dat88asheet A4 EN 17 V1Document7 pagesSGS PCA Kuwait Dat88asheet A4 EN 17 V1Skander HamadiNo ratings yet
- PEZA Charter2016Document158 pagesPEZA Charter2016mynet_peterNo ratings yet
- Customer Import Export ProcedureDocument12 pagesCustomer Import Export ProcedureMahesh DahifaleNo ratings yet
- The Hong Kong Certification Centre LTDDocument1 pageThe Hong Kong Certification Centre LTDkamuthan553No ratings yet
- Evidencia 8 Presentation "Steps To Export"Document5 pagesEvidencia 8 Presentation "Steps To Export"Eliana patricia Betancur canoNo ratings yet
- SOP Export and Import - Kenya Dairy BoardDocument15 pagesSOP Export and Import - Kenya Dairy BoardRachel GateiNo ratings yet
- IAF MD 4 Computer Assisted Auditing Techniques CAAT Issue 1 2008Document7 pagesIAF MD 4 Computer Assisted Auditing Techniques CAAT Issue 1 2008carlosprieto36No ratings yet
- Advance Authorization SchemeDocument7 pagesAdvance Authorization SchemeJohn AnandNo ratings yet
- PEZA Citizen's Charter PDFDocument154 pagesPEZA Citizen's Charter PDFRomer LesondatoNo ratings yet
- Application For Import Permit: Process FlowDocument4 pagesApplication For Import Permit: Process FlowAaron ValdezNo ratings yet
- Registration Formalities For Exports: Unit 1Document29 pagesRegistration Formalities For Exports: Unit 1iampavi91100% (1)
- LTO Requirements Brand New VehicleDocument4 pagesLTO Requirements Brand New VehicleAleli SantosNo ratings yet
- Service CatalogDocument29 pagesService CatalogAbdulaziz AlmutiriNo ratings yet
- Philippine Economic Zone AuthorityDocument161 pagesPhilippine Economic Zone AuthorityZetsky AlbolerasNo ratings yet
- VW - tb.00!07!08 VIN Number Plate or Safety Certification Label Requires ReplacementDocument4 pagesVW - tb.00!07!08 VIN Number Plate or Safety Certification Label Requires ReplacementSlobodanNo ratings yet
- Export Promotion Council: A Indepth Look Into International BusinessDocument14 pagesExport Promotion Council: A Indepth Look Into International BusinessBhavith KNo ratings yet
- EGCA Registration ProcessDocument58 pagesEGCA Registration ProcessAbxysNo ratings yet
- Materi Sosialisasi Vendor Registrasi Melalui Web Aplikasi Ivendor For User Dan Vendor NF - Update V2 - WatermarkDocument65 pagesMateri Sosialisasi Vendor Registrasi Melalui Web Aplikasi Ivendor For User Dan Vendor NF - Update V2 - WatermarkAffanNo ratings yet
- 3 MAISchemeDocument23 pages3 MAISchemeSohail SheikhNo ratings yet
- Intercommerce Network Services - Compress PDFDocument54 pagesIntercommerce Network Services - Compress PDFJessica GutierrezNo ratings yet
- Overview of New OTC Application Process 6-23-15Document1 pageOverview of New OTC Application Process 6-23-15dragonboyy3kNo ratings yet
- Inspection Management System (IMS) - Vendor: RDMP Jo BalikpapanDocument10 pagesInspection Management System (IMS) - Vendor: RDMP Jo BalikpapanIRC RDMPNo ratings yet
- Serviceguide 2022 EngDocument88 pagesServiceguide 2022 Engatif.mNo ratings yet
- Export Promotion Schemes and Incentives: By: Prof. Kalyan deDocument62 pagesExport Promotion Schemes and Incentives: By: Prof. Kalyan devneet_shrmNo ratings yet
- IATF Rules 5th Edition Sanctioned Interpretations November 2018Document7 pagesIATF Rules 5th Edition Sanctioned Interpretations November 2018Desmond MahadeoNo ratings yet
- eGCA User Manual - Pilot Registration ProcessDocument53 pageseGCA User Manual - Pilot Registration Processnaveen thomasNo ratings yet
- Foreign Trading SystemDocument13 pagesForeign Trading Systemswetha100% (1)
- Legal Update - Regulation On The Import Activties - YLC LawDocument4 pagesLegal Update - Regulation On The Import Activties - YLC LawTyfun PrasthikaNo ratings yet
- REC CE and GOV Profile 2023 ADocument1 pageREC CE and GOV Profile 2023 Asahr.ceNo ratings yet
- Crmpos 2Document20 pagesCrmpos 2Candidoskie BerdinNo ratings yet
- How Do I Apply For A Permit? (Basic) : 1. Apply From Import ConditionsDocument6 pagesHow Do I Apply For A Permit? (Basic) : 1. Apply From Import ConditionsCatalina LoyolaNo ratings yet
- IEC CODE DetailsDocument4 pagesIEC CODE DetailsAmandeep Singh BediNo ratings yet
- CAP FRM RFC Request For Certificate of Conformity English LatestDocument1 pageCAP FRM RFC Request For Certificate of Conformity English LatestAmeen BishryNo ratings yet
- #6 UST Training Oct2022 QA SystemDocument121 pages#6 UST Training Oct2022 QA Systemtl xNo ratings yet
- EPCG License RequirementsDocument14 pagesEPCG License RequirementsAmit AshishNo ratings yet
- Types of Licenses for Import ExportDocument16 pagesTypes of Licenses for Import Export13 Pragnesh HiwaraleNo ratings yet
- 7.guideline For Application For Provisional Eligibility CertificateDocument14 pages7.guideline For Application For Provisional Eligibility CertificateKumar SrinivasanNo ratings yet
- Vendor RegistrationDocument31 pagesVendor RegistrationShailesh kumarNo ratings yet
- Slide-Esap - PA3Document25 pagesSlide-Esap - PA3Muhammad Dafan NurhamzahNo ratings yet
- A Typical Procurement Process For High Valued ItemsDocument1 pageA Typical Procurement Process For High Valued Itemskarthik rNo ratings yet
- A Step-by-Step Guide For Operator Data Submissions and The Remote Review of Documents - Final Rev.0Document11 pagesA Step-by-Step Guide For Operator Data Submissions and The Remote Review of Documents - Final Rev.0KhaledMazenNo ratings yet
- Telecommunication Equipment Certification Process at MOTC (Ministry of Telecommunication and Communication)Document5 pagesTelecommunication Equipment Certification Process at MOTC (Ministry of Telecommunication and Communication)Ahmad WiramdaniNo ratings yet
- Anylogistix Ple Software Licensing Agreement: 1. DefinitionsDocument8 pagesAnylogistix Ple Software Licensing Agreement: 1. DefinitionsAtul SudhakaranNo ratings yet
- Sellers Accreditation Form 2023Document1 pageSellers Accreditation Form 2023zernaelmarie6No ratings yet
- Class Note On Contract LawDocument11 pagesClass Note On Contract LawMustafizur Rahman Deputy ManagerNo ratings yet
- Mutants & Masterminds 3e - Power Profile - Morphing PowersDocument6 pagesMutants & Masterminds 3e - Power Profile - Morphing PowersMichael MorganNo ratings yet
- Driver's License BillDocument2 pagesDriver's License BillSteven DoyleNo ratings yet
- Map Update Information Guide: / Norteamérica Guía Informativa de Actualización Del MapaDocument12 pagesMap Update Information Guide: / Norteamérica Guía Informativa de Actualización Del Mapaalberto cortesNo ratings yet
- Remixer Contract TemplateDocument4 pagesRemixer Contract Templatedamien.schuhlerNo ratings yet
- SportsFest Parent Consent FinalDocument3 pagesSportsFest Parent Consent FinalMark Peejay ResiduoNo ratings yet
- Important Notes:: Professional Regulation Commission Action Sheet For AuthenticationDocument1 pageImportant Notes:: Professional Regulation Commission Action Sheet For AuthenticationRoma Mae AtienzaNo ratings yet
- EHS-F-12 Lifting Operations PermitDocument2 pagesEHS-F-12 Lifting Operations PermitIbrahim EsmatNo ratings yet
- Alex Latimer Gordon Latimer Patrick LatimerDocument18 pagesAlex Latimer Gordon Latimer Patrick LatimerPriya KNo ratings yet
- Long Island N.V. - Gaming Curacao LicenseDocument2 pagesLong Island N.V. - Gaming Curacao LicenseIrenilda Vieira Dos SantosNo ratings yet
- Mechanical Permit ChecklistDocument1 pageMechanical Permit ChecklistThe MatrixNo ratings yet
- Honda ANF100Document8 pagesHonda ANF100cjamalangNo ratings yet
- LLRDocument1 pageLLRMd ShahulNo ratings yet
- 8dio Adagio Violins ManualDocument21 pages8dio Adagio Violins ManualGabriel EnachiNo ratings yet
- Good Life - Burna Boy X Afrobeat X Dancehall Type Beat 2020 (Prod by Cobby Dollar X Aymix X Pincho Beats) - BasicMP3 - ContractDocument5 pagesGood Life - Burna Boy X Afrobeat X Dancehall Type Beat 2020 (Prod by Cobby Dollar X Aymix X Pincho Beats) - BasicMP3 - ContractMax BarrowNo ratings yet
- Multi - The New GirlDocument16 pagesMulti - The New GirlMutiara A.RNo ratings yet
- BPOM SKI LicenseDocument5 pagesBPOM SKI LicenseYohan AlamsyahNo ratings yet
- License Agreement RA PanelsDocument1 pageLicense Agreement RA PanelsFlorin KiritescuNo ratings yet
- DL - Interim - Document - (JAZZY - GREEN - DL) .PDF (2) (1) (4) - 1Document1 pageDL - Interim - Document - (JAZZY - GREEN - DL) .PDF (2) (1) (4) - 1shawntaeadams04No ratings yet
- Document ChecklistDocument2 pagesDocument Checklistbilijoabucejo.acmhNo ratings yet
- Severance ManualDocument19 pagesSeverance ManualCepoi Cosmin IonutNo ratings yet
- Let S Count LegsDocument12 pagesLet S Count LegsShakira HeNo ratings yet
- Sunil Behera DL LicenceDocument1 pageSunil Behera DL LicenceSUNIL KUMAR PRADHANNo ratings yet
- Flatmates Episode 43 - Language PointDocument3 pagesFlatmates Episode 43 - Language PointWillem van AmerongenNo ratings yet
- Civil-Structural-Permit BACKDocument2 pagesCivil-Structural-Permit BACKAndrea Mae Sanchez100% (1)
- License Agreement Free SampleDocument3 pagesLicense Agreement Free Sampleapi-235666177No ratings yet