Professional Documents
Culture Documents
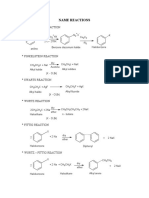
Alcohols Phenols and Ethers
Alcohols Phenols and Ethers
Uploaded by
Subath KumarOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alcohols Phenols and Ethers
Alcohols Phenols and Ethers
Uploaded by
Subath KumarCopyright:
Available Formats
Alcohols, Phenols and Ethers Part - 1
Func�onal Group- OH Structure
Suffix to be used - ol
Homologous series-
142 pm
:O: 96 pm
Methanol (CH3OH) H
Ethanol (C2H5OH) Alcohol C 108.90 H
Propanol (C3H7OH) H
Butanol (C4H9OH) H
Pentanol (C5H11OH) and so on Methanol
Classifica�on Nomenclature
C2H5OH Compound Common name IUPAC name
Monohydric alcohols CH3-OH Methyl alcohol Methanol
CH3-CH2-CH2-OH n-Propyl alcohol Propan-1-ol
Compounds Compounds
containing containing CH3-CH-CH3 Isopropyl alcohol Propan-2-ol
OH
C -OH bond C -OH bond
sp3 sp2 CH3-CH2-CH2-CH2-OH n-Butyl alcohol Butan-1-ol
CH3-CH2-CH2-CH2-OH sec-Butyl alcohol Butan-2-ol
* Primary, secondary * Vinylic alcohol CH3-CH-CH2-OH Isobutyl alcohol 2-Methylpropan-1-ol
CH3
and ter�ary alcohols CH3
* Allylic alcohols CH3-C-OH tert-Butyl alcohol 2-Methylpropan-2-ol
CH3
* Benzylic alcohols HO-H2C-CH2-OH Ethylene glycol Ethane-1,2-diol
CH2-CH-CH2 Glycerol Propane -1,2,3-triol
CH2OH CH2OH OH OH OH
CH2OH CHOH OH
OH CH3
CH2OH
Dihydric alcohols Trihydric alcohols Cyclohexanol 2-Methylcyclopentanol
Preparatory Methods Physical Proper�es
R-CH=CH2 H2O/H⁺
1. Boiling Point-
Markownikoff addi�on H2O 2 * Higher than other organic compounds having
equal molecular masses
R-CH=CH2 B2H6 * The boiling point decreases with an increase
in branching in alipha�c carbon chains
An� markownikoff addi�on H2O2/H2O
2. Solubility-
* The hydroxyl group in alcohol is involved in the
R-C-Cl N2/H2
forma�on of intermolecular hydrogen bonding
O Na + C2H5OH * The solubility of alcohol in water decreases
R-OH with the increase in the size of the alkyl group
H2/Pd
RCHO
LiAlH4
R-COOH i) LiAlH4
R-COO-R’ ii) H2O H O
H
CH3 - CH2 - CH2 - O: H O
:
For the prepara�on of -
10 alcohol – HCHO
H H
i) RMgX
20 alcohol – RCHO O
30 alcohol – R-C-R ii) H2O/H⁺ H H
O
Reac�ons involving cleavage of O-H bond Reac�ons involving cleavage of C–O bond
Reac�on with Metals
2R-O-H + 2Na 2R-O-Na+H2 ROH + HX → R-X + H2O
Sodium Reac�on with
alkoxide hydrogen halides
CH3 CH3
6 CH3-C-OH + 2Al ( )
2 CH3-C-O Al+3H2 Acidity of Alcohols
RCH2OH
Cu
CH3 3
RCHO
CH3 573K
tert-Butyl alcohol Aluminium
tert-butoxide Cu
R-CH-R’ R-C-R’
R R Dehydrogena�on OH
573K
O
R CH2OH > CHOH >> R C- OH
R R CH3 CH3
CH3-C=CH2
Primary Secondary Ter�ary Cu
R-C-OH
Chemical CH3
573K
Proper�es
Alcohols: Weaker acids than water
Acidity H OH
RCH2OH
Oxida�on
R-O: + H-O:-H R-O-H + :OH
: :
: :
: :
of Alcohols R-C=O R-C=O
Base Acid Conjugate Conjugate Aldehyde Carboxylic
CrO3
acid
RCH2OH
acid base
RCHO
Oxida�on
PCC
Esterifica�on CH3-CH=CH-CH2OH CH3-CH=CH-CHO
CrO3
O H⁺ O R-CH-R’ R-C-R’
R-C-OH + H-O-R R-C-O-R’ + H-O-H OH O
⥮
Esterifica�on Sec- alcohol Ketone
Acid Alcohol Ester
Reac�on with phosphorus trihalides
Some Commercially Important Alcohols
3CH3-CH2-CH2-OH + PCl3 → 3CH3-CH2-CH2-Cl + H3PO3
Dehydra�on
Methanol
H2SO4
Prepara�on C2H5OH CH2=CH2+H2O
443K
ZnO-Cr2O3
CO + 2H2 CH3OH OH
200-300 atm 85%H3PO4
573-673 K CH3CHCH3 CH3-CH=CH2+H2O
440K
Proper�es
CH3 CH2
20%H3PO4
* Methanol is a colourless liquid and boils CH3-C-OH CH3-C-CH3+H2O
at 337 K 358K
CH3
* It is highly poisonous in nature
The rela�ve ease of dehydra�on of alcohols follows the
Ethanol following order:
Ter�ary > Secondary > Primary
Prepara�on
Invertase
C12H22O11 + H2O C6H12O6 + C6H12O6
Glucose Fructose
Zymase
C6H12O6 2C2H5OH + 2CO2
Proper�es
* Ethanol is a colourless liquid with boiling point 351 K
* It is used as a solvent in paint industry and in the prepara�on of a number of carbon compounds
Alcohols, Phenols and Ethers Part - 2
Structure
It is a white crystalline compound that has a
dis�nc�ve odour 1090
H
OH Phenol
:
O
:
136 pm
Classifica�on Nomenclature
OH OH
CH3
OH CH3 CH3 CH3
OH
Monohydric Phenol
OH
OH
OH
Phenol o-Cresol m-Cresol p-Cresol
OH Phenol 2-Methylphenol 3-Methylphenol 4-Methylphenol
Dihydric Phenol OH
OH OH
OH OH
OH
OH
OH
OH Catechol
Benzene-1,2-diol
Hydroquinone or quinol
Benzene-1,4-diol
Trihydric Phenol
Physical Proper�es Prepara�on
- +
1. Boiling Point- OH O Na OH From
* Phenols have higher boiling points in +NaOH
623 K HCl Haloarenes
300 atm
comparison to other organic compounds
having equal molecular masses
From
R R
-
O Na
+
OH Benzenesul-
H O
R-O-H-O-H-O-H-O- R O R Oleum (i) NaOH
phonic acid
H R H (ii) H⁺
H H
H-O-H-O-H-O- O O
From
Phenols + -
NH2 N2Cl OH Diazonium
NaNO2 H 2O salts
+N2+HCl
+HCl Warm
Aniline Benzene diazonium
2. Solubility- chloride
* The hydroxyl groups are responsible for the
solubility of phenol in water CH3 CH3 From
* The solubility of phenol decreases with the CH3-CH CH3-C-O-O-H OH Cumene
O2 H⁺
increase in the size of the aryl group +CH3COCH3
H2 O
Cumene Cumene
hydroperoxide
Chemical Proper�es
Electrophilic aroma�c subs�tu�on Reimer-Tiemann Reac�on
OH O Na⁺ O Na⁺ OH
1) Nitra�on CHCl2 NaOH CHO H⁺ CHO
CHCl3 + aq NaOH
OH OH OH
Dilute HNO3 NO2 Intermediate Salicylaldehyde
+
NO2 Reac�on of Phenol with zinc dust
o-Nitrophenol p-Nitrophenol
OH
2) Halogena�on
+ Zn + ZnO
OH OH OH
Br2 in CS2 Br
+
273 K
Br Oxida�on
Minor Major
OH O
Kolbe’s reac�on
Na2Cr2O7
OH ONa OH H2SO4
NaOH (i) CO2 COOH
O
(iI) H⁺
benzoquinne
2-Hydroxybenzoic acid
(salicylic acid)
Alcohols, Phenols and Ethers Part - 3
sp3 hybridised
Structure
141 pm
:O:
H H
Ether C 111.7 0
C
H H
H H
Methoxymethane
Classifica�on Nomenclature
Compound Common name IUPAC name
CH3OCH3 Dimethyl ether Methoxymethane
CH3 CH2 - O - CH2 CH3 O
diethyl ether tetrahydrofuran C2H5OC2H5 Diethyl ether Ethoxyethane
CH3OCH2CH2CH3 Methyl n-propyl ether 1-Methoxypropane
Symmetrical Ethers
C6H5OCH3 Methyl phenyl ether Methoxybenzene
(Anisole) (Anisole)
C6H5OCH2CH3 Ethyl phenyl ether Ehoxybenzene
(Phenetole)
C6H5O(CH2)6-CH3 Heptyl phenyl ether 1-Phenoxyheptane
O CH3
CH3O-CH-CH3 Methyl isopropyl ether 2-Methoxypropane
CH3
C6H5-O-CH2-CH2-CH-CH3 Phenyl isopentyl ether 3-Methylbutoxybenzene
CH3
methyl phenyl ether CH3-O-CH2-CH2-OCH3 1,2-Dimethoxyethane
Unsymmetrical Ethers H3C CH3
OC2H5 2-Ethoxy-
-1,1-dimethylcyclohexane
Prepara�on Physical Proper�es
1. Miscibility
1. By dehydra�on of alcohol
Miscibility with water resembles those of
H2SO4 alcohols of the same molecular mass
CH2=CH2
443 K
CH3CH2OH
H2SO4
C2H5OC2H5 R
413 K :O: H H
O
: :
2. Williamson synthesis R
R-X+R’-ONa⁺ R-O-R’+Na X
: :
: :
2. Boiling Points
* Lower than alcohols
- + * This is due to the presence of hydrogen
:OH :O Na :O-R
:
bonding in alcohols which is absent in
ethers
R-X
+ NaOH
+
:OR +OR +OR OR OR
:
- -
↔ ↔ ↔ ↔
-
R-O-R + HX → RX + R-OH Electrophilic subs�tu�on
R-OH+HX → R-X + H2O Cleavage of C–O bond in ethers
OCH3 OCH3 OCH3
Br2 in Br
+
O-R OH Ethanoic acid
Chemical Br
+H-X +R-X Proper�es p - Bromoanisole o - Bromoanisole
(major) (minor)
Halogena�on
Friedel-Cra�s reac�on
Nitra�on
OCH3 OCH3 OCH3
CH3
Anhyd. AlCl3
+CH3Cl +
CS2
OCH3 OCH3 OCH3
H2SO4 NO2 CH3
HNO3
+ Methyl 2- methoxy- 4- methoxy-
chloride toluene toluene
NO2 (Minor) (Major)
2- Nitroanisole 4- Nitroanisole
(Minor) (Major) OCH3 OCH3 OCH3
COCH3
Anhyd. AlCl3
+CH3COCl +
COCH3
Ethanoyl 2- methoxy- 4- methoxy-
chloride acetophenone acetophenone
(Minor) (Major)
You might also like
- Functional Group Transformation NotebookDocument78 pagesFunctional Group Transformation NotebookchedhedNo ratings yet
- Atomic Structure Cheat SheetDocument39 pagesAtomic Structure Cheat Sheetmark Zukerberg50% (2)
- Worksheet-Nernst Equation PDFDocument4 pagesWorksheet-Nernst Equation PDFLedd SleddNo ratings yet
- PMR Spectroscopy: Solved Problems Volume : IIFrom EverandPMR Spectroscopy: Solved Problems Volume : IIRating: 5 out of 5 stars5/5 (3)
- Solomon's Chapter 15 SolutionDocument34 pagesSolomon's Chapter 15 SolutionRobert0% (2)
- 11.alcohol, Phenol & Ethers Colour BookletDocument84 pages11.alcohol, Phenol & Ethers Colour BookletVishal Malik100% (1)
- Pdf-Haloalkanes and HaloarenesDocument159 pagesPdf-Haloalkanes and HaloarenesOmkar Singh Shekhawat100% (2)
- NCERT Solutions Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic AcidsDocument41 pagesNCERT Solutions Class 12 Chemistry Chapter 12 Aldehydes, Ketones and Carboxylic AcidsVidyakulNo ratings yet
- Revision Notes For Class 12 CBSE Chemistry, Amines - TopperlearningDocument11 pagesRevision Notes For Class 12 CBSE Chemistry, Amines - TopperlearningRishabh BhandariNo ratings yet
- Aldehydes Ketones Carboxylic AcidsDocument119 pagesAldehydes Ketones Carboxylic AcidsKashvi KhandelwalNo ratings yet
- CBSE Class 12 Alcohol Phenol and Ether Study NotesDocument378 pagesCBSE Class 12 Alcohol Phenol and Ether Study NotesV T PRIYANKANo ratings yet
- Organic Net PyqDocument537 pagesOrganic Net Pyqpranjal jangid100% (1)
- Photosynthesis Short ConceptsDocument51 pagesPhotosynthesis Short Conceptsauguste noe100% (2)
- CBSE Class 12 Haloalkanes and Haloarenes Study NotesDocument355 pagesCBSE Class 12 Haloalkanes and Haloarenes Study NotesDharaneesh S.k.100% (1)
- Class 12th Biology Short Revision NotesDocument3 pagesClass 12th Biology Short Revision NotesVishesh Chandra100% (1)
- Chemistry Chapter 11 Alcohol, Phenol and EtherDocument32 pagesChemistry Chapter 11 Alcohol, Phenol and EtherVidyakulNo ratings yet
- Aldehydes Ketones and Carboxylic AcidsDocument37 pagesAldehydes Ketones and Carboxylic Acidsssheeladevi84100% (1)
- Organic Chemistry - Class 12th - Practice MCQsDocument22 pagesOrganic Chemistry - Class 12th - Practice MCQsLiza DahiyaNo ratings yet
- Coordination Compounds Board 1 Shot PDFDocument25 pagesCoordination Compounds Board 1 Shot PDFGaurav67% (6)
- Atomic Structure Short Notes 7 PageDocument7 pagesAtomic Structure Short Notes 7 PageSubhajit GoraiNo ratings yet
- Chemical KineticsDocument60 pagesChemical KineticsThe Rock75% (4)
- Organic Chemistry Chapter 8Document41 pagesOrganic Chemistry Chapter 8채종희No ratings yet
- Aldehydes, Ketones and Carboxylic Acids NotesDocument74 pagesAldehydes, Ketones and Carboxylic Acids Notessamay gujratiNo ratings yet
- Chap 01 Some Basic Principles of Organic ChemistryDocument13 pagesChap 01 Some Basic Principles of Organic ChemistryParth JainNo ratings yet
- General Organic ChemistryDocument10 pagesGeneral Organic ChemistryRiddhi Chatterjee100% (2)
- Previous Years Board Question of Alkyl and Aryl Halide PDFDocument13 pagesPrevious Years Board Question of Alkyl and Aryl Halide PDFKomal TripathiNo ratings yet
- Revision Notes For Class 12 CBSE Chemistry, Aldehydes, Ketones and Carboxylic Acids - TopperlearningDocument15 pagesRevision Notes For Class 12 CBSE Chemistry, Aldehydes, Ketones and Carboxylic Acids - TopperlearningRishabh Bhandari100% (1)
- Alcohols, Phenols and Ethers NotesDocument8 pagesAlcohols, Phenols and Ethers Notesmajji satishNo ratings yet
- Problems On Named ReactionsDocument103 pagesProblems On Named ReactionsBapu ThoratNo ratings yet
- PRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNDocument3 pagesPRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNABD 17No ratings yet
- Reaction Reactants Products Conditions Mechanism Other: AlkanesDocument3 pagesReaction Reactants Products Conditions Mechanism Other: AlkanesInzamam A HaqueNo ratings yet
- Learn Periodic Table in HindiDocument21 pagesLearn Periodic Table in HindiSiobhan Reed67% (3)
- Class 12 Chapter 12 Aldehydes, Ketones and Carboxylic AcidsDocument114 pagesClass 12 Chapter 12 Aldehydes, Ketones and Carboxylic AcidsPratyush KumarNo ratings yet
- Alcohols WsDocument5 pagesAlcohols WsVedanta DesikNo ratings yet
- Important Questions For CBSE Class 12 Chemistry The P-Block ElementsDocument41 pagesImportant Questions For CBSE Class 12 Chemistry The P-Block ElementsyndtfndtgndNo ratings yet
- Revision Notes For Class 12 CBSE Chemistry, Alcohols, Phenols and Ethers - TopperlearningDocument10 pagesRevision Notes For Class 12 CBSE Chemistry, Alcohols, Phenols and Ethers - TopperlearningRishabh Bhandari67% (3)
- Medica Wing Mind Map For Biology NeetDocument80 pagesMedica Wing Mind Map For Biology NeetFaizan AliNo ratings yet
- Electrochemistry Class 12 NotesDocument53 pagesElectrochemistry Class 12 NotesGirish Arora0% (1)
- Name Reactions: Sandmeyer'S ReactionDocument9 pagesName Reactions: Sandmeyer'S ReactionSai Krishnan100% (1)
- Solution: STR Ycl AssesDocument40 pagesSolution: STR Ycl AssesArka DeyNo ratings yet
- Organic Comp - Distinguish TestsDocument1 pageOrganic Comp - Distinguish TestsKatniss TathagataNo ratings yet
- Alcohols, Phenols and EthersDocument28 pagesAlcohols, Phenols and EthersDnyanesh Shinde100% (1)
- NitrogenDocument58 pagesNitrogenPriyansh Mishra100% (2)
- 12 Chemistry Notes ch11 Alcohols Phenols and EthersDocument8 pages12 Chemistry Notes ch11 Alcohols Phenols and Ethersmv7602456No ratings yet
- Electrophilic Addition of Alkenes NotesDocument17 pagesElectrophilic Addition of Alkenes NotesAnanda Vijayasarathy100% (1)
- Physics XII Concept Maps PDFDocument15 pagesPhysics XII Concept Maps PDFcbsegirlsaipmt100% (1)
- CH 6 - Organic ReactionsDocument18 pagesCH 6 - Organic Reactionskevincai96No ratings yet
- Stereoisomerism Exercise PDFDocument51 pagesStereoisomerism Exercise PDFGOURISH AGRAWAL100% (3)
- Hydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEDocument25 pagesHydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEanaswara santhoshNo ratings yet
- Inorganic Memory Chart PDFDocument22 pagesInorganic Memory Chart PDFUtkarsh GuptaNo ratings yet
- Revision Notes Organic ChemistryDocument30 pagesRevision Notes Organic ChemistryAtharav Porwal100% (1)
- WWW - Crackjee.xyz: Organic ChemistryDocument9 pagesWWW - Crackjee.xyz: Organic ChemistryRau100% (1)
- Gujcet Sample Paper For Biology - EnglishDocument171 pagesGujcet Sample Paper For Biology - EnglishDharav Solanki100% (3)
- Goc and Isomerism Notes - PMD - 1 PDFDocument46 pagesGoc and Isomerism Notes - PMD - 1 PDFrutvik bhoraniyaNo ratings yet
- 100 Organic Reagentspptx - 230327 - 085539 PDFDocument15 pages100 Organic Reagentspptx - 230327 - 085539 PDFHeera MeenaNo ratings yet
- As Chemistry Organic MindmapDocument1 pageAs Chemistry Organic MindmapDương Thị Ngọc HiềnNo ratings yet
- SN1, SN2, E1, E2Document39 pagesSN1, SN2, E1, E2Dian AnggrainiNo ratings yet
- Alcohol Phenol and Ethers NCEDocument50 pagesAlcohol Phenol and Ethers NCENurhikmah NurhikmahNo ratings yet
- 11.alcohol, Phenol & Ethers Colour Booklet PDFDocument59 pages11.alcohol, Phenol & Ethers Colour Booklet PDFMridu BhandariNo ratings yet
- Alkoholi: OH C C OH OH CDocument53 pagesAlkoholi: OH C C OH OH CElvir MNo ratings yet
- Org. Chem. (Chapter 8)Document25 pagesOrg. Chem. (Chapter 8)Jia LinNo ratings yet
- 11 - Alcohol Ethers Thiols Wks KeyDocument5 pages11 - Alcohol Ethers Thiols Wks KeyMaria Aira Mendoza100% (1)
- Course Planner: Class-Xii Vijeta (01jpa)Document2 pagesCourse Planner: Class-Xii Vijeta (01jpa)Pratyush Jain100% (1)
- Wurtz ReactionDocument2 pagesWurtz ReactionAnush KhillareNo ratings yet
- Competency Exam in Organic ChemistryDocument4 pagesCompetency Exam in Organic ChemistryRaymond Yabut100% (1)
- Mass SpectraDocument18 pagesMass SpectraMoustafa ElsadanyNo ratings yet
- A Textbook of Organic Chemistry (1923) - Sudborough PDFDocument935 pagesA Textbook of Organic Chemistry (1923) - Sudborough PDFbabithyNo ratings yet
- Organic Chemistry Revision Questions-1Document3 pagesOrganic Chemistry Revision Questions-1jatin2006gamil.comNo ratings yet
- Unit 11 Alcohols Ethers Thiols UST Template 1Document31 pagesUnit 11 Alcohols Ethers Thiols UST Template 1Daniel BalubalNo ratings yet
- IR Spectrum TableDocument22 pagesIR Spectrum TableJanno MallariNo ratings yet
- Reactions P-Hydroxybenzyl Alcohol Derivatives and Their Methyl Ethers With Molecular Chlorine'Document6 pagesReactions P-Hydroxybenzyl Alcohol Derivatives and Their Methyl Ethers With Molecular Chlorine'Sandipan SahaNo ratings yet
- Organic Chemistry Test-1 On Total Syllabus: Single CorrectDocument5 pagesOrganic Chemistry Test-1 On Total Syllabus: Single CorrectVanshaj GuptaNo ratings yet
- Practice - Organic Nomenclature: Directions: Write Your Answers To The Following Questions in The Space Provided. UseDocument8 pagesPractice - Organic Nomenclature: Directions: Write Your Answers To The Following Questions in The Space Provided. UseKeshav GuptaNo ratings yet
- Families of Carbon CompoundsDocument39 pagesFamilies of Carbon CompoundsAlexNo ratings yet
- Wade Organic Chemistry A II Bottom 415 1091Document216 pagesWade Organic Chemistry A II Bottom 415 1091溫緯中No ratings yet
- DP Unit 10 & 20. Organic ChemistryDocument18 pagesDP Unit 10 & 20. Organic ChemistrydeaNo ratings yet
- Vihasibio Sciences PVT LTD, - Product ListDocument703 pagesVihasibio Sciences PVT LTD, - Product ListvihasifinechemNo ratings yet
- An Overview of The Synthetic Routes To The BestDocument55 pagesAn Overview of The Synthetic Routes To The BestAntônio Neto MachadoNo ratings yet
- Chemistry AlcoholsDocument44 pagesChemistry AlcoholsSayan Kumar KhanNo ratings yet
- Catalysis of The Epoxy-Carboxyl Reaction: Technical ArticlesDocument9 pagesCatalysis of The Epoxy-Carboxyl Reaction: Technical ArticlesAdhvik PuriNo ratings yet
- Alcohols, Phenols and EthersDocument3 pagesAlcohols, Phenols and EthersCJ's Music GalleryNo ratings yet
- Cargo Comp - ChartDocument2 pagesCargo Comp - ChartNihat DğnNo ratings yet
- GocDocument108 pagesGocAtul VermaNo ratings yet
- DCC CouplingDocument16 pagesDCC CouplingWookyoung LeeNo ratings yet
- FIITJEE Medical Test Series 20-22 - Revised 21st January 2022Document1 pageFIITJEE Medical Test Series 20-22 - Revised 21st January 2022rithvikNo ratings yet
- Chemistry Class 10 Chapter 11Document14 pagesChemistry Class 10 Chapter 11Rahim BakhshNo ratings yet
- Ethers and Epoxides Thiols and SulfidesDocument39 pagesEthers and Epoxides Thiols and SulfidesNguyệt BìnhNo ratings yet
- CPP - Alcohol Ether PhenolDocument12 pagesCPP - Alcohol Ether PhenoldivyanshjoshidpsjkpNo ratings yet
- IChO 2009 Prep Prob PracticalDocument15 pagesIChO 2009 Prep Prob PracticalRSLNo ratings yet
- Exer 2Document21 pagesExer 2Kate TaguiamNo ratings yet