Professional Documents
Culture Documents
A Substance Undergoing Reduction 2. A Substance Undergoing Oxidation. 3. A Reducing Agent. 4. An Oxidizing Agent
A Substance Undergoing Reduction 2. A Substance Undergoing Oxidation. 3. A Reducing Agent. 4. An Oxidizing Agent
Uploaded by
Elisa Paulo0 ratings0% found this document useful (0 votes)
10 views2 pagesThis document is a chemistry exercise on oxidation-reduction (redox) reactions. It contains questions that ask students to identify substances undergoing oxidation or reduction in redox reactions, which elements are more readily oxidized in pairs, and whether chemical equations represent redox reactions or non-redox reactions while identifying the oxidized and reduced elements. The exercise is intended to test a student's understanding of key concepts related to redox reactions.
Original Description:
Original Title
Copy-of-EXERCISE-NO.-3
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document is a chemistry exercise on oxidation-reduction (redox) reactions. It contains questions that ask students to identify substances undergoing oxidation or reduction in redox reactions, which elements are more readily oxidized in pairs, and whether chemical equations represent redox reactions or non-redox reactions while identifying the oxidized and reduced elements. The exercise is intended to test a student's understanding of key concepts related to redox reactions.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
10 views2 pagesA Substance Undergoing Reduction 2. A Substance Undergoing Oxidation. 3. A Reducing Agent. 4. An Oxidizing Agent
A Substance Undergoing Reduction 2. A Substance Undergoing Oxidation. 3. A Reducing Agent. 4. An Oxidizing Agent
Uploaded by
Elisa PauloThis document is a chemistry exercise on oxidation-reduction (redox) reactions. It contains questions that ask students to identify substances undergoing oxidation or reduction in redox reactions, which elements are more readily oxidized in pairs, and whether chemical equations represent redox reactions or non-redox reactions while identifying the oxidized and reduced elements. The exercise is intended to test a student's understanding of key concepts related to redox reactions.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
CHEM 01
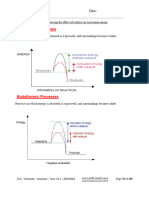
EXERCISE NO. 3
OXIDATION-REDUCTION REACTIONS (REDOX)
NAME: Alava, Christopher Aldrin R. RATING:
SECTION / DAY & TIME: CEIT 08-101A / Tuesday; 8:02AM DATE: 10-19-2021
I. Tell whether the substance gains electrons or loses electrons in a redox reaction.
1. A substance undergoing reduction
2. A substance undergoing oxidation.
3. A reducing agent.
4. An oxidizing agent.
II. Which is more readily oxidized in each pair?
1. Mg and Cl____________________________
2. K and I_________________________
3. Na and N____________________________
4. F and Ca_______________________
III. Identify if the following reactions as redox or non redox, Which elements are oxidized? Which
elements are reduced?
1.N2(g) + 3H2(g) --> 2NH3
2. AlCl3(s) + 3H2O(l) --> Al(OH)3(s) + 3HCL(aq)(g)
3. NaOH(aq) + HCl(aq) --> NaCl(aq) + Cu(s)
4. Zn(s) + CuCl2(aq) --> ZnCl2(aq) + Cu(s)
5. Fe2O3(s) + 2Al(s) --> 2Fe(s) + Al2O3(s)
Readox Non-Redox Oxidized Reduced
You might also like
- 2ND Term S2 Chemistry... - 2Document44 pages2ND Term S2 Chemistry... - 2Adelowo Daniel100% (2)
- General Chemistry 2 Online: Oxidation-Reduction Activity SeriesDocument12 pagesGeneral Chemistry 2 Online: Oxidation-Reduction Activity SeriesirfanNo ratings yet
- Redox Reaction PDFDocument12 pagesRedox Reaction PDFErsan ResurreccionNo ratings yet
- TEST 2 Topic 3Document3 pagesTEST 2 Topic 3Ashutosh SharmaNo ratings yet
- Redox Reactions NotesDocument30 pagesRedox Reactions NotesLil' NyehNo ratings yet
- (Week 5) - Module 5-Gen - Chem. 2Document14 pages(Week 5) - Module 5-Gen - Chem. 2Diana Joy Ancheta CldheiNo ratings yet
- General Chemistryt 2 Week 5Document7 pagesGeneral Chemistryt 2 Week 5Charles David NavidadNo ratings yet
- STEM Gen Chem 2 Q3 M3Document29 pagesSTEM Gen Chem 2 Q3 M3Roland AgraNo ratings yet
- Advance Chem Q4module1.Oxidation-Reduction - ReactionDocument13 pagesAdvance Chem Q4module1.Oxidation-Reduction - ReactionAmelita TupazNo ratings yet
- Redox Quiz 5Document2 pagesRedox Quiz 5Azain CardenasNo ratings yet
- Unit 11 - Redox and Electrochem NotesDocument28 pagesUnit 11 - Redox and Electrochem NotesIbsa BekamaNo ratings yet
- Final Report 3Document10 pagesFinal Report 3TOBIRAMA SenkuNo ratings yet
- Laporan Anor Unit 3Document63 pagesLaporan Anor Unit 3Nur hasanah NanaNo ratings yet
- STEM Gen Chem 2 Q4 M2Document20 pagesSTEM Gen Chem 2 Q4 M2Roland AgraNo ratings yet
- 1314 Lab - Single Replacement Lab As Inquiry BasedDocument2 pages1314 Lab - Single Replacement Lab As Inquiry Basedapi-239433858No ratings yet
- Class10 PT1Document2 pagesClass10 PT1Sonica SainiNo ratings yet
- Oxidation Reduction Reactions Redox Reactions Notes 2011Document20 pagesOxidation Reduction Reactions Redox Reactions Notes 2011Poovaraahan RaghuveeranNo ratings yet
- Science Class X NotesDocument134 pagesScience Class X NotesSanyam SinghNo ratings yet
- Learning Activity Sheet General Chemistry 2 (Q4 - Lessons 5 and 6) Oxidation - Reduction ReactionDocument12 pagesLearning Activity Sheet General Chemistry 2 (Q4 - Lessons 5 and 6) Oxidation - Reduction ReactionJeffrey YumangNo ratings yet
- Redox: Oxidation-Reduction Reactions: Pre-Lab DiscDocument12 pagesRedox: Oxidation-Reduction Reactions: Pre-Lab DiscKaren LazoNo ratings yet
- Chemistry 9th CH 7Document21 pagesChemistry 9th CH 7Faheem UllahNo ratings yet
- General Biology: Peralta III Edwin C. STEM-GalileoDocument5 pagesGeneral Biology: Peralta III Edwin C. STEM-GalileoSavage CabbageNo ratings yet
- 12 Modul of Pahang For RedoxDocument37 pages12 Modul of Pahang For RedoxkhayranizamNo ratings yet
- Oxidation NumbersDocument2 pagesOxidation Numberskent_tam6119No ratings yet
- Oxidation States (Oxidation Numbers)Document2 pagesOxidation States (Oxidation Numbers)kent_tam6119No ratings yet
- Oxidation-Reduction ReactionDocument20 pagesOxidation-Reduction ReactionFitria Ramadhani PuspitasariNo ratings yet
- POGIL Oxidation and Reduction-S-1Document6 pagesPOGIL Oxidation and Reduction-S-1demyeets64No ratings yet
- Electrochemistry Part 1Document10 pagesElectrochemistry Part 1Shofwa AnnisaaNo ratings yet
- Note 28 Oct 2023Document21 pagesNote 28 Oct 2023Jihan Abou GhaddaraNo ratings yet
- Oxidizing and Reducing AgentDocument9 pagesOxidizing and Reducing Agentabuzar khalidNo ratings yet
- Revsion Shit NiggasDocument15 pagesRevsion Shit NiggasAhmed KhanNo ratings yet
- Redox Reactions - Shobhit NirwanDocument12 pagesRedox Reactions - Shobhit NirwanAadarsh PandeyNo ratings yet
- Chemistry 12th 15 - 02 - 2024 - 135626Document1 pageChemistry 12th 15 - 02 - 2024 - 135626alihassan4100387No ratings yet
- Test Icse ChemistryDocument4 pagesTest Icse Chemistryvishudhanandchoudhary9056No ratings yet
- Module 3 Assingment 1 AssessmentDocument5 pagesModule 3 Assingment 1 Assessmentapi-516602929No ratings yet
- AP Chemistry - Oxidation Numbers PracticeDocument2 pagesAP Chemistry - Oxidation Numbers Practicemartialartsgrl21No ratings yet
- Oxidation - Reduction Reactions and Titrations: Che 401: Analytical ChemistryDocument16 pagesOxidation - Reduction Reactions and Titrations: Che 401: Analytical ChemistryScrappy WellNo ratings yet
- CH 13Document2 pagesCH 13usmanrathore784No ratings yet
- 1 - REDOX Unit Exam STUDENT Studyguide 2015 - 8Document12 pages1 - REDOX Unit Exam STUDENT Studyguide 2015 - 8AYESHA NAAZNo ratings yet
- Year 11 2019 Chemistry QuizDocument9 pagesYear 11 2019 Chemistry Quizuyenkhuu06No ratings yet
- Activity 7 - Types of Chemical ReactionsDocument3 pagesActivity 7 - Types of Chemical Reactionss.saligumba.joseferdinandmarcosjayNo ratings yet
- 9.1 Oxidation and ReductionDocument50 pages9.1 Oxidation and Reductionzaraagarwal06No ratings yet
- Full SorularDocument127 pagesFull Sorularezgigeyik02No ratings yet
- Q4-Worksheet - Week 6Document8 pagesQ4-Worksheet - Week 6Gian EvangelistaNo ratings yet
- 09 Redox ReactionsDocument1 page09 Redox ReactionsNkemzi Elias NzetengenleNo ratings yet
- Balancing of Chemical ReactionDocument17 pagesBalancing of Chemical ReactionDaisyNo ratings yet
- Corrosion Inhibition Ability of Shorea Robusta (Sakhu) Leaves)Document4 pagesCorrosion Inhibition Ability of Shorea Robusta (Sakhu) Leaves)Dr. Syed Khalid HasanNo ratings yet
- 9th Class Panjab Board Chemistry Full BookDocument25 pages9th Class Panjab Board Chemistry Full Bookayeshafurqan319No ratings yet
- Daily Question Set For ChemistryDocument29 pagesDaily Question Set For ChemistryMichael KwokNo ratings yet
- Redox Application FinalDocument65 pagesRedox Application FinalHemanth HegdeNo ratings yet
- Account For The Trends Observed in All Properties in Table 1 in Terms of Structure and Bonding. AnswerDocument4 pagesAccount For The Trends Observed in All Properties in Table 1 in Terms of Structure and Bonding. AnswerZeneisha Horrise WilliamsNo ratings yet
- Eportfolio Standard 3Document4 pagesEportfolio Standard 3api-384664466No ratings yet
- CBSE Class 10 Chemistry - Chemical Reactions and Equations ConceptsDocument4 pagesCBSE Class 10 Chemistry - Chemical Reactions and Equations ConceptsFredrick RodriguesNo ratings yet
- IB Chemistry SL - Chapter 3 Review QuestionsDocument4 pagesIB Chemistry SL - Chapter 3 Review Questionsshawnfi jusrmk100% (1)
- Science Support Material 1Document207 pagesScience Support Material 1yajurv Trivedi officialNo ratings yet
- Electrochemical and Electrocatalytic Reactions of Carbon DioxideFrom EverandElectrochemical and Electrocatalytic Reactions of Carbon DioxideB.P. SullivanRating: 5 out of 5 stars5/5 (1)
- Organic Reaction Mechanisms 1982: An annual survey covering the literature dated December 1981 through November 1982From EverandOrganic Reaction Mechanisms 1982: An annual survey covering the literature dated December 1981 through November 1982A. C. KnipeNo ratings yet
- Entrepreneur Q2 M18Document15 pagesEntrepreneur Q2 M18Elisa PauloNo ratings yet
- Chemistry 1 - 11 - Q1 - M8Document14 pagesChemistry 1 - 11 - Q1 - M8Elisa PauloNo ratings yet
- PARENT Consent GraduationDocument1 pagePARENT Consent GraduationElisa PauloNo ratings yet
- Technology and Livelihood Education: Electronic Products Assembly and ServicingDocument12 pagesTechnology and Livelihood Education: Electronic Products Assembly and ServicingElisa PauloNo ratings yet