Professional Documents
Culture Documents
Intracranial Infections Clinical and Imaging Characteristics
Intracranial Infections Clinical and Imaging Characteristics
Uploaded by
Riyan NuelOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Intracranial Infections Clinical and Imaging Characteristics
Intracranial Infections Clinical and Imaging Characteristics
Uploaded by
Riyan NuelCopyright:
Available Formats
Acta Radiologica
ISSN: 0284-1851 (Print) 1600-0455 (Online) Journal homepage: https://www.tandfonline.com/loi/iard20
Intracranial Infections: Clinical and Imaging
Characteristics
B. R. Foerster, M. M. Thurnher, P. N. Malani, M. Petrou, F. Carets-Zumelzu &
P. C. Sundgren
To cite this article: B. R. Foerster, M. M. Thurnher, P. N. Malani, M. Petrou, F. Carets-Zumelzu
& P. C. Sundgren (2007) Intracranial Infections: Clinical and Imaging Characteristics, Acta
Radiologica, 48:8, 875-893
To link to this article: https://doi.org/10.1080/02841850701477728
Published online: 04 Aug 2009.
Submit your article to this journal
Article views: 2946
View related articles
Citing articles: 1 View citing articles
Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=iard20
REVIEW ARTICLE ACTA RADIOLOGICA
Intracranial Infections: Clinical and Imaging Characteristics
B. R. FOERSTER, M. M. THURNHER, P. N. MALANI, M. PETROU, F. CARETS-ZUMELZU & P. C. SUNDGREN
Department of Radiology, and Divisions of Infectious Diseases and Geriatric Medicine, Department of Internal
Medicine, University of Michigan Medical Center, Ann Arbor, Michigan, USA; Department of Radiology,
Neuroradiology, Medical University Vienna, Austria; Veterans Affairs Ann Arbor Healthcare System, Ann Arbor,
Michigan, USA; Geriatric Research Education and Clinical Center (GRECC), Ann Arbor, Michigan, USA
Foerster BR, Thurnher MM, Malani PN, Petrou M, Carets-Zumelzu F, Sundgren PC.
Intracranial infections: clinical and imaging characteristics. Acta Radiol 2007;48:875–
893.
The radiologist plays a crucial role in identifying and narrowing the differential diagnosis
of intracranial infections. A thorough understanding of the intracranial compartment
anatomy and characteristic imaging findings of specific pathogens, as well incorporation
of the clinical information, is essential to establish correct diagnosis. Specific types of
infections have certain propensities for different anatomical regions within the brain. In
addition, the imaging findings must be placed in the context of the clinical setting,
particularly in immunocompromised and human immunodeficiency virus (HIV)-positive
patients. This paper describes and depicts infections within the different compartments of
the brain. Pathology-proven infectious cases are presented in both immunocompetent
and immunocompromised patients, with a discussion of the characteristic findings of
each pathogen. Magnetic resonance spectroscopy (MRS) characteristics for several
infections are also discussed.
Key words: Infection; intracranial infection; meningitis; MR spectroscopy; neuroradiol-
ogy
Pia C. Sundgren, Department of Radiology, Division of Neuroradiology, University of
Michigan, Room B2A209D, 1500 E Medical Center Drive, Ann Arbor, MI 48109-0030,
USA (tel. +1 734 763 3253, fax. +1 734 764 2412, e-mail. sundgren@umich.edu)
Accepted for publication April 21, 2007
Intracranial infections include a wide range of Imaging modalities
different processes, each with unique clinical char-
acteristics. Many intracranial infections progress Computed tomography (CT) and magnetic reso-
rapidly and result in significant morbidity and nance imaging (MRI) are the two primary imaging
mortality if appropriate therapies are not initiated modalities used in the setting of suspected central
promptly. Clinical presentations of intracranial nervous system infection. While CT is widely
infection vary significantly. Common manifestations available and very useful for rapid assessment of
include altered mental status, seizures, as well as hydrocephalus, mass lesions, hemorrhage, or acute
more subtle focal deficits, such as cranial nerve brain edema prior to lumbar puncture, MRI is often
palsies (10). In each case, the radiologist plays a vital required to detect more subtle findings. MRI is
role in the diagnostic workup of these infections. By more sensitive, especially for cerebral spinal fluid
interfacing with the clinicians who care for these (CSF) involvement, leptomeningitis, empyema, ven-
patients, the radiologist can help direct appropriate triculitis, vasculitis, and infarctions (58, 61).
testing and treatment, ultimately decreasing the However, MRI is not as widely available and can
morbidity associated with these infections. be logistically challenging to obtain in the acutely ill
In this review, we will discuss several important patient.
intracranial infections. We will briefly describe the
salient clinical features of specific infections and Computed tomography (CT)
offer guidance related to the utility of imaging The standard pre- and post-contrast-enhanced CT
modalities in particular settings. protocol of the head includes 5-mm-slice axial
DOI 10.1080/02841850701477728 # 2007 Taylor & Francis
876 B. R. Foerster et al.
images though the entire brain, including brain, soft become thickened, and an inflammatory exudate
tissue, and bone windows. While contrast can aid in can cover the brain, particularly in the dependent
the detection of small lesions and leptomeningeal regions, such as the basal cisterns.
enhancement, it is not required to exclude findings CT imaging is generally obtained prior to lumbar
that may preclude lumbar puncture, such as a focal puncture to exclude a mass lesion or other signs of
mass. In many types of infection, CT findings can be elevated intracranial pressure. In addition to helping
nonspecific or even normal, especially in early stages exclude other diagnoses, such a subarachnoid
(21). hemorrhage, CT can also identify complications
from meningitis, as discussed below (58). Non-
contrast CT and MR imaging can be normal in
Magnetic resonance imaging (MRI)
early cases of meningitis (14). Administration of
Conventional gadolinium-enhanced MRI of the
contrast may be helpful to detect diffuse meningeal
brain should include: 1) axial and sagittal pre- and
enhancement, with MRI being more sensitive than
post-contrast T1-weighted images; 2) coronal post-
CT (66); meningeal enhancement is not, however,
contrast T1-weighted images; 3) axial T2-weighted
specific to the diagnosis of infectious meningitis and
and fluid-attenuated inversion recovery (FLAIR)-
can be seen in other diagnoses, such as leptome-
weighted images; and 4) diffusion-weighted imaging
ningeal carcinomatosis (58). FLAIR MR imaging
(DWI). MR spectroscopy (MRS) has also been
can demonstrate high signal in the subarachnoid
shown to be useful in the evaluation of infection,
spaces, which reflects high protein content in the
since brain abscesses and certain pathogens are
CSF (14, 30, 56). High signal in the subarachnoid
characterized by specific resonances that are not
space is also nonspecific and can be seen with
present in uninfected tissue. Table 1 summarizes MR leptomeningeal carcinomatosis and subarachnoid
spectroscopy findings found in several pathogens. hemorrhage. Fig. 1 demonstrates the CT and MRI
meningeal and subarachnoid findings that can be
seen in bacterial meningitis.
Anatomic compartments After excluding a mass lesion, the most important
role of neuroimaging is to identify potential
Bacterial meningitis complications of meningitis, such as infarction,
Meningitis, or inflammation of the meninges, is hydrocephalus, ventriculitis, brain empyema, and
among the most serious and morbid of all infec- venous sinus thrombosis. Communicating hydro-
tions. A high index of suspicion, followed by rapid cephalus is a common complication, with the
diagnosis and treatment are essential to prevent inflammatory debris obstructing the flow and
severe sequelae and death. Diagnosis requires an reabsorption of CSF (58). Pyogenic ventriculitis is
abnormal number of white blood cells (WBC) in the a very severe complication of meningitis. Imaging
CSF. Classic clinical characteristics include head- findings in this setting include periventricular high
ache and neck stiffness, followed by mental status FLAIR signal, ependymal enhancement, ventricular
changes. Microbiologic culture results from blood debris, and fluid–fluid levels in the ventricles (11,
or CSF remain the gold standard for diagnosis, 22). MRI is the method of choice for the detection
although this requires several days to complete. of venous thrombosis secondary to meningitis, with
In terms of basic pathophysiology, bacteria lodge a high signal intensity seen on spin-echo sequences
in the venous sinuses, creating inflammatory in the venous sinuses, reflecting thrombus forma-
changes that interfere with CSF drainage and tion. The subsequent venous thrombosis can lead to
potentially causing hydrocephalus. Early in infec- infarctions that do not conform to well-defined
tion, the pia and arachnoid matter become con- arterial territories and have accompanying hemor-
gested and hyperemic. Later, the leptomeninges rhage (39).
Table 1. Infectious MR spectroscopy findings
Infection Findings
HSV Reduced N-acetyl aspartate, elevated choline, sometimes elevated lactate
TB granuloma Elevated lipid
HIV Decreased N-acetyl aspartate, increased choline and myo-inositol
Toxoplasmosis Elevated lactate and lipid
Mucormycosis Elevated lactate, decreased N-acetyl aspartate
Bacterial abscess Succinate, acetate, alanine, amino acids, and lactate peaks
Acta Radiol 2007 (8)
Imaging of Intracranial Infections 877
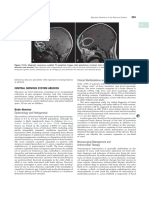
Fig. 1. Bacterial meningitis. A. Pre-contrast CT is unremarkable. B. Post-contrast CT shows meningeal enhancement (black arrows). C.
Axial FLAIR MR imaging with high signal in the subarachnoid space (black arrows). D. Axial post-contrast T1-weighted MR image shows
extensive meningeal enhancement (black arrows).
Viral encephalitis and arachnoid membranes that generally occur in
Encephalitis is distinguished from meningitis based the setting of sinusitis or otitis media (3, 13). In
on the presence of abnormal brain function. Nuchal addition to fever, vomiting, and meningismus,
rigidity is usually absent in encephalitis in contra- patients typically present with focal neurologic
distinction from meningitis. Patients can present signs, including hemiparesis. Since many of the
with focal neurologic deficits and seizures. Viral typical presenting symptoms overlap with those of
encephalitis can be either primary or postinfectious. meningitis, the radiologist must diligently search for
In postinfectious encephalitis, an active virus cannot extra-axial fluid collections, particularly in the
be isolated and is secondary to an immune-mediated setting of paranasal sinus disease.
process. MR is more sensitive than CT for the The pathogenesis includes phlebitic bridging veins
detection of intracranial findings. The predominant (from meningitis), hematogenous spread, and direct
MR imaging characteristic of viral encephalitis is extension of infection from adjacent structures.
parenchymal signal abnormality on T2-weighted Venous thrombosis or brain abscess develops in
imaging. more than 10% of patients. Delays in appropriate
antimicrobial therapy and surgical drainage result in
high mortality rates, as well as serious neurologic
Brain subdural empyema sequelae in those who do survive.
Brain subdural empyemas are infected CSF collec- In the early stages of the disease, small sub-
tions in the potential space between the cranial dura dural empyemas can be very subtle, particularly on
Acta Radiol 2007 (8)
878 B. R. Foerster et al.
non-contrast CT (74). Subdural empyemas do not hematomas (usually high signal on T1WI). A thin
cross the midline, distinguishing them from epidural rim of enhancement may be seen, which is usually
abscesses. Subdural empyemas, like subdural hema- more prominent along the inner table of the skull.
tomas, also tend to have crescent-like configurations Fig. 2 illustrates some of these MRI findings in a
rather than lentiform configurations. patient with a subdural empyema. DWI can be
On CT, subdural empyemas appear as iso- useful in distinguishing between empyemas that are
attenuation to low-attenuation extra-axial collec- bright with low apparent diffusion coefficient
tions compared to brain parenchyma with rim (ADC) values and subdural effusions, which have
enhancement (65, 68). MRI is the study of choice low signal and ADC values similar to CSF (75).
for detection of subdural empyema, as MRI has a
higher sensitivity for detection of small subdural
fluid collections. On MRI, subdural empyemas have Brain epidural abscess
iso-intense signal on T1-weighted imaging (T1WI), Brain epidural abscesses are usually caused by the
likely secondary to increased protein content and contiguous spread of infection from adjacent
high signal on T2WI (33, 39, 47). MRI can also help structures, such as the mastoids or paranasal
to differentiate subdural empyemas from other sinuses, into the epidural space located between
extra-axial fluid collections, such as sterile effusions the dura and the overlying bone. Compared to
(usually low signal on T1WI) and chronic subdural subdural empyema, epidural abscess presents in a
Fig. 2. Subdural empyema. A. Coronal T2-weighted MR image shows hyperintense crescent-shaped subdural collection. B. On axial FLAIR
imaging, the subdural collection has CSF intensity in the anterior portion and iso-intensity in the posterior portion. In addition, high signal is
present in the subarachnoid spaces (black arrowheads). Pre-contrast (C) and post-contrast (D) T1-weighted MR images show peripheral
enhancement (white arrows), as well as meningeal enhancement (black arrowheads).
Acta Radiol 2007 (8)
Imaging of Intracranial Infections 879
more subtle fashion, usually with several days of cerebritis or early capsule formation stage, second-
fever along with mental status changes and neck ary to mass effect. Hematogenous abscesses, which
pain. can be seen in the setting of endocarditis, cardiac
Epidural abscesses can cross the midline, helping shunts, or pulmonary vascular malformations, are
to distinguish them from subdural empyema. In usually multiple, identified at the gray–white junc-
addition, the adjacent brain parenchyma tends to tion, and located in the middle cerebral artery
appear normal, whereas abnormal signal in border- territory.
ing brain tissue can be seen in subdural empyema. In the earlier phases, a non-contrast head CT may
On CT, epidural abscess typically appears as a low- show only low-attenuation abnormalities with mass
attenuation extra-axial mass. On MRI, epidural effect. In later phases, a complete peripheral ring
abscesses have iso-signal on T1WI and high signal may be seen. On contrast CT, uniform ring
on T2WI, with enhancement of the thickened dural enhancement is virtually always present in later
surface (14). Two epidural abscesses with lentiform phases. MRI findings also depend on the stage of
configuration are shown in Fig. 3. the infection. In the early phase, MRI can have low
T1WI signal and high T2WI signal with patchy
Brain abscess enhancement. In later phases, the low T1WI signal
Brain abscesses are a focal, intracerebral infection becomes better demarcated, with high T2WI signal
that begin with a localized region of cerebritis, both in the cavity and surrounding parenchyma.
evolving into a discrete collection of pus surrounded The abscess cavity shows a hyperintense rim on non-
by a well-vascularized capsule. Such infections result contrast T1-weighted images and a hypointense rim
from either hematogenous dissemination or local on T2-weighted images (26). As on CT, MRI
extension from an odontogenic, sinus, or otic usually demonstrates a ring of enhancement sur-
source. The most common organisms involved in rounding the abscess (58). Fig. 4 demonstrates an
brain abscesses include Staphylococcus and abscess centered in the right occipital lobe.
Streptococcus species. Abscesses tend to grow toward the white matter,
Imaging features of a brain abscess depend on the away from the better-vascularized grey matter, with
stage at the time of imaging, as well as the etiology thinning of the medial wall (29). However, the
of infection (10). Brain abscess development can be enhancing-ring sign is nonspecific and must be
divided into four stages: 1) early cerebritis (1 to 4 evaluated in the context of the clinical history.
days); 2) late cerebritis (4 to 10 days); 3) early Thickness, irregularity, and nodularity of the
capsule formation (11 to 14 days); and 4) late enhancing ring are suggestive of tumor (majority
capsule formation (w14 days) (26). The majority of of cases) or, possibly, fungal infection (26). As seen
abscesses demonstrate considerable surrounding in Fig. 5, DWI may show restricted diffusion (bright
edema, which generally presents during the late signal) that helps to differentiate abscesses from
Fig. 3. Epidural abscess. A. Coronal post-contrast T1-weighted MR imaging shows a lentiform, peripherally enhancing, extra-axial fluid
collection adjacent to the inferior right frontal lobe (white arrow). B. Axial post-contrast T1-weighted MR imaging depicts a lentiform,
peripherally enhancing, extra-axial fluid collection adjacent to the left frontal lobe (white arrow). Additional intraparenchymal abscesses are
also shown (black arrows).
Acta Radiol 2007 (8)
880 B. R. Foerster et al.
Fig. 4. Intraparenchymal abscess. A. Axial FLAIR MR imaging shows a high-signal lesion (black arrow) with surrounding edema and mass
effect. Axial pre-contrast (B) and post-contrast (C) T1-weighted MR images display a low-signal intraparenchymal lesion with peripheral
post-contrast enhancement.
necrotic neoplasms, which are not usually restricted showed a significant overlap in ADC values in
(18, 25), although not all abscesses follow this rule. toxoplasmosis and lymphoma (50). The authors
Fungal and tuberculous abscesses may have ele- concluded that, in the majority of patients, ADC
vated diffusivity and low signal on DWI (40). ratios are not definitive in making the distinction
Several studies demonstrate the utility of DWI to between toxoplasmosis and lymphoma. DWI has a
differentiate between necrotic or cystic lesions and high sensitivity to detect early acute ischemic
brain abscesses (18, 25). The latter demonstrates changes in cortical and deep white matter that can
increased signal on the trace images and reduced occur in the setting of infectious vasculitis.
ADC, while necrotic neoplasms demonstrate Intracerebral abscesses are characterized by spe-
decreased signal on the trace image and high ADC cific resonances on MRS that are not detected in
values. Initially, DWI was thought to be helpful in normal or sterile pathologic human tissue. MRS has
differentiation of toxoplasmosis from lymphoma. been shown to be specifically beneficial in differ-
One study proposed an ADC threshold of 0.8, entiating between brain abscesses and other cystic
where ADC ratios less than 0.8 would favor lesions (9), which can be used to expedite imple-
lymphoma over toxoplasmosis; however, the study mentation of the appropriate antimicrobial therapy.
Fig. 5. Intraparenchymal abscess with restricted diffusion. A. Coronal post-contrast T1-weighted MR image shows a peripherally enhancing,
low-signal lesion (black arrow) in the left cerebellum. B. Diffusion-weighted imaging shows the abscess has restricted diffusion with bright
signal (black arrow).
Acta Radiol 2007 (8)
Imaging of Intracranial Infections 881
Metabolic substances, such as succinate (2.4 ppm), encephalitis. Many radiological findings offer sup-
acetate (1.9 ppm), alanine (1.5 ppm), amino acids port for a presumptive diagnosis.
(0.9 ppm), and lactate (1.3 ppm), can all be present CT findings are usually subtle, with lower-
in untreated bacterial abscesses or soon after the attenuation areas in the temporal lobe and insular
initiation of treatment (32). cortex (51). Mass effect on the lateral ventricle can
sometimes be present (63). Petechial hemorrhage is
possible and may be more easily detected on MRI
Specific pathogens than CT. On MRI, there is high signal on T2WI,
with a predilection for the limbic system (temporal
After localizing the infectious process to a specific lobes, cingulate gyri, inferior frontal lobes).
compartment, there may be certain distinguishing Enhancement varies, and mass effect may persist
characteristics that may help suggest a specific (58). Fig. 6 shows the typical asymmetric involve-
pathogen. Of course, lumbar puncture, in most ment of the frontal and temporal lobes. HSV type 2
cases, clinches the diagnosis, but certain infections, infection, more commonly seen in neonates rather
in particular, require specific laboratory testing, for than adults, can demonstrate subtle regions of low
example, the polymerase chain reaction (PCR) test attenuation on CT in various regions of the brain,
for herpes simplex virus. In addition, the immune with subsequent enlargement and meningeal and
status of the patient must be considered, which gyriform enhancement. Thalamic hemorrhage is
affects the differential diagnosis. Table 2 sum- possible, and calcification can be seen several weeks
marizes some of the typical findings for different after disease onset (63).
infections, with a discussion of the clinical and Metabolic alterations have been demonstrated in
imaging features. HSV using MR spectroscopy, and are characterized
by reduced N-acetyl aspartate (NAA), elevated
Herpes simplex virus encephalitis choline compounds (Cho), and, sometimes, eleva-
Herpes simplex virus (HSV) is a common cause of tion of lactate (Lac) with normalization over time.
encephalitis. Both type 1 and type 2 HSV produce These findings correspond to histopathological
encephalitis, with varying epidemiology, depending findings and are thought to reflect neuronal or
primarily on patient age. The virus most often axonal injury (NAA), demyelination (Cho, Lip),
invades the brain after reactivation of latent virus and anaerobic metabolism, or the presence of
that resides in the trigeminal ganglion. Clinical macrophages (Lac) (36, 48, 62). In general, the
features that distinguish HSV disease from other usual microbiologic diagnostic tests offer reasonable
intracranial infections include the findings of red sensitivity and specificity; thus, the routine use of
blood cells in the CSF. The gold standard for MR spectroscopy is limited.
diagnosis is either PCR or viral culture that
demonstrates HSV in the CSF. Treatment with West Nile virus encephalitis
antiviral therapy is generally initiated any time there West Nile virus (WNV) encephalitis is a potentially
is a suggestion of possible viral meningitis and/or fatal viral intracranial infection acquired from
Table 2. Typical imaging findings of specific pathogens
Infection Anatomic predilection CT MRI
HSV Temporal/inferior frontal lobes Subtle low density High T2WI signal, variable enhancement
West Nile virus Parenchyma Negative Restricted diffusion, high T2WI signal
TB Basal cisterns Poor visualization High FLAIR signal, enhancement
Cystercercosis Parenchyma, occasionally ventricles Off-center, spherical Enhancing cysts with variable signal
calcifications characteristics
Coccidioidomycosis Meninges and parenchyma Negative Dilated VR spaces, poorly visualized cisterns
HIV White matter—frontal and parietal Negative High T2WI signal
PML White matter—asymmetric occipital Negative High T2WI signal
and parietal
Toxoplasmosis Basal ganglia, corticomedullary Low- to iso-attenuation High T2WI signal, edema, and ring
junction nodules enhancement
Cryptococcus Subarachnoid spaces infiltrating Normal Dilated VR spaces, non-enhancing cystic
basal ganglia lesions
Aspergillus Basal ganglia and thalami Varying attenuation Low T2WI signal, variable enhancement
Mucormycosis Invades along cavernous sinus Paranasal disease High T2WI signal in frontal/temporal lobes
VR spaces: Virchow-Robin spaces.
Acta Radiol 2007 (8)
882 B. R. Foerster et al.
Fig. 6. Herpes simplex virus encephalitis. A. Axial T2-weighted MR imaging depicts asymmetric increased signal (leftwright) in the
frontotemporal lobes (white arrows). B. Axial post-contrast T1-weighted MR imaging shows low T1 signal in the same regions and meningeal
enhancement (white arrows).
infected mosquitoes. This infection has been the reported in the lobar gray and white matter, as well
source of several epidemics across the United States as the cerebellum, basal ganglia, thalamus, and
during the past several years, beginning in 1999 in brainstem. Isolated restricted diffusion can also be
New York City. The incubation time period of seen (Fig. 7); these patients have a better prognosis
WNV is estimated to range from 3 to 14 days. One than patients with T2WI signal abnormalities.
in 150 people infected with WNV will develop T1WI signal abnormalities and enhancement are
meningoencephalitis, with the immunocompro- rarely present (1, 45).
mised, elderly, and very young at highest risk.
Symptoms include fever, headache, neck stiffness,
mental status changes, muscle weakness, and flaccid Lyme disease
paralysis. Death can occasionally result (7, 42). Lyme disease, or neuroborrelia, is a multisystemic
Imaging findings with WNV infection have disorder caused by the tick-borne spirochete Borrelia
generally been unremarkable. MR imaging findings burgdorferi. The disease is seen worldwide, and is
can be normal. Increased T2WI signal has been common in Europe. The underlying pathogenesis is
Fig. 7. West Nile virus. A. Diffusion-weighted imaging with increased signal (black arrows) in the bilateral thalami in a patient with proven
West Nile virus. B. Corresponding ADC map confirms restricted diffusion in the bilateral thalami (white arrows). (Images courtesy of Nafi
Aygun, MD)
Acta Radiol 2007 (8)
Imaging of Intracranial Infections 883
poorly understood, and different etiologies such as dense exudates, which can subsequently enhance
vasculitis, immune complex mechanisms, and post- with contrast (13). On MRI, the basal cisterns can
viral demyelination have been suggested. About 10 to have high FLAIR signal and meningeal enhance-
15% of patients with Lyme disease develop neurolo- ment secondary to proteinaceous exudate. The
gic complications with cranial nerve palsies and cisterns can enhance, with enhancement extending
peripheral neuropathies being common. over cortical surfaces. Hydrocephalus, either com-
MRI findings may vary from being normal to the municating or obstructive, is a common finding in
presence of extensive superficial and/or deep white tuberculous meningitis. Fig. 8 demonstrates some of
matter lesions that can be more discrete or confluent the CT and MRI findings seen in tuberculous
in appearance. Some lesions may enhance after meningitis. Infarction secondary to panarteritis
contrast administration (2, 59). The lesions are not can also be seen in CNS tuberculosis.
characteristic and cannot be differentiated from Tuberculomas can appear as low- or high-
those seen in acute disseminated encephalomyelitis attenuation nodules on CT (4). On CT, tuberculo-
(ADEM) or multiple sclerosis. Diagnosis is based on mas can also present with a ‘‘target sign:’’ central
clinical findings, CSF laboratory testing, and calcification or a central region of enhancement, as
response to antibiotics. well as a peripheral ring of enhancement (67).
Tuberculomas have a varied clinical course, ranging
Tuberculosis from complete resolution to rupture with menin-
Tuberculosis (TB) remains one of the most goencephalitis. Noncaseating tuberculomas have
common and important infections around the high signal on T2WI, with peripheral nodular
world, with millions infected annually and thou- enhancement. The patient’s immune response then
sands dying directly of complications related to TB. creates a granulomatous reaction, with central
HIV infection and issues of drug resistance add to caseation and a solid center, and eventually,
the importance of TB infection. Central nervous progression to a liquid center (52). Caseating
system (CNS) involvement is a serious manifesta- tuberculomas with a solid center have low to
tion of chronic infection and includes meningitis,
intermediate signal on T1WI and central low signal
intracranial tuberculoma, and spinal tuberculous
on T2WI, with ring-like enhancement (6, 49, 58).
arachnoiditis. The mortality rate of those cases
Caseating granulomas with a liquid center have low
remains high for these complications despite
signal on T1WI and high signal on T2WI, and can
effective treatment. For CNS TB, simple micro-
be indistinguishable from true tuberculous
biology remains the method of choice for diagnosis,
abscesses (seen in immunocompromised patients)
since the areas of greatest prevalence generally lack
or pyogenic abscesses (6). Fig. 9 shows a patient
resources for widespread imaging, especially
MRI. with multiple caseating tuberculomas. MRS typi-
Tubercles (scattered tuberculosis foci) develop in cally shows an elevated lipid peak in tuberculosis
the CNS or adjacent bony structures during the granulomas. This can be helpful in the differential
bacillemia that follows primary TB infection or late diagnosis from neurocysticercosis (27).
reactivation of TB elsewhere in the body. Meningitis
most commonly occurs after primary infection in
infants and young children. Among adults with Cysticercosis
intracranial infection, this develops from chronic Cysticercosis results from infection by Taenia
reactivation, almost always in the setting of some solium, the pork tapeworm. This parasite is
type of immune deficiency (aging, malnutrition, endemic in Mexico, South America, Asia, Africa,
HIV, medications, alcoholism). and Eastern Europe, and is generally acquired by
Tuberculomas are intracranial lesions that ingestion of undercooked pork. The larvae develop
develop from deep-seated tubercles acquired during into the tapeworm in the gastrointestinal tract and
bacillemia. This complication of TB shows a then enter the blood stream to spread to other
variable clinical course ranging from complete regions, including the CNS. Patients are typically
resolution to rupture with associated meningoence- asymptomatic until the larvae die, which incites an
phalitis. Patients typically present with confusion, acute inflammatory reaction. The larvae progress
fevers, headache, lethargy, and meningismus. through different stages, with varying degrees of
Symptoms can progress to stupor, coma, decere- edema and enhancement. While most cases of
brate rigidity, cranial nerve palsy, and stroke. neurocysticercosis are asymptomatic, seizures, other
On CT, the basal and sylvian cisterns can be focal neurologic signs, and increased intracranial
poorly visualized without contrast secondary to pressure can result.
Acta Radiol 2007 (8)
884 B. R. Foerster et al.
Fig. 8. Tuberculosis meningitis. A, B. Non-contrast CT shows dilated lateral ventricles with transependymal migration of CSF. The basilar
cisterns are not seen, and the fourth ventricle is patent (white arrow), indicating communicating hydrocephalus. C. Axial FLAIR MR imaging
shows increased subarachnoid signal (black arrows) seen with proteinaceous material. D. Sagittal post-contrast T1-weighted MR imaging
with enhancement of the basilar meninges (white arrow), as well as a ring-enhancing lesion.
The presence of calcifications, as well as non- Coccidioidomycosis
enhancing cysts and enhancing ring lesions Coccidioidomycosis results from infection by the
(Fig. 10), are typical findings in a patient with dimorphic fungi of the genus Coccidioides (C.
appropriate exposure, and should suggest this immitis and C. posadasii). These are endemic fungi
diagnosis (33). Characteristic calcifications are well present in the southwestern United States as well as
demonstrated on CT, and are slightly off-center and in Central and South America. The spore is inhaled,
spherical in shape. MRI can show multiple cysts, setting up a primary pulmonary infection that can
with changing signal characteristics as the larvae then be hematogenously spread. While the clinical
progress through different stages. In the early manifestations are protean, meningitis is an impor-
vesicular stage, small non-enhancing cysts can be tant complication that must be recognized and
seen, which are iso-intense to CSF on T1WI and treated aggressively. Without appropriate therapy
T2WI, with a mural nodule seen on T1WI. As the with systemic antifungals, the clinical course of
larvae mature, a cyst wall becomes visible with coccidioidal meningitis is fatal.
increasing T1WI signal within the cyst, relative to The initial head CT can be negative. Imaging
CSF. Edema and ring-like enhancement can then be findings include dilated Virchow-Robin spaces,
visualized as the larvae die and incite an inflamma- poor visualization of basal and sylvian cisterns
tory response. Eventually, the cysts decrease in size secondary to dense exudates, and, occasionally,
and become calcified, and are best detected on CT. hydrocephalus (20, 71). As seen in Fig. 11, enhan-
Intraventricular cysticercal cysts occasionally cing nodules that are nonspecific can also be seen.
require surgical intervention if obstructive hydro- Infarction can also be a common finding, which
cephalus occurs (31, 60, 73). may be secondary to direct invasion or vasospasm.
Acta Radiol 2007 (8)
Imaging of Intracranial Infections 885
Fig. 9. Tuberculoma. A. Axial FLAIR MR image shows multiple central low-signal lesions with mild associated edema in the cerebellum. B.
Axial post-contrast T1-weighted MR image shows multiple ring-enhancing lesions with central low T1 signal.
Immunocompromised hosts now rarely seen in areas of the world where patients
have access to highly active antiretroviral therapy
Intracranial infections are important manifestations (HAART). Over time, HIV infection results in
of disease in immunocompromised hosts, especially subacute encephalitis and a syndrome of progressive
in patients with HIV infection and those with dementia, with cognitive, motor, and behavioral
neutropenia related to hematologic malignancies abnormalities. Pathology shows microglial nodules
and/or bone marrow transplantation. Patients with and multinucleated cells in the white matter. CT
solid-organ transplantation are another group where imaging may show brain atrophy, but is typically
serious CNS infection is seen fairly often. Using unremarkable. MRI findings include bilateral
imaging to help narrow the differential diagnosis is patchy and confluent, moderately high T2WI
critical, as treatments differ for different infections. signal changes (Fig. 12) in the white matter,
In addition, therapies for many infections carry predominantly affecting the frontal and parietal
significant toxicity, as well as drug interactions, lobes without contrast enhancement (8, 12, 70, 43).
making prolonged empiric therapy impractical. Proton MR spectroscopy demonstrates metabolic
changes in HIV-infected brains. NAA reduction and
Human immunodeficiency virus (HIV) a low NAA/Cr can be seen in patients with early
Many HIV/AIDS-related intracranial manifesta- disease, even before conventional MRI shows any
tions seen frequently in the pre-retroviral era are changes. Increases in Cho and myo-inositol (MI) are
Fig. 10. Cysticercosis. A. Non-contrast CT images show calcified lesions (white arrows), as well as fluid attenuation lesions, one with a
central calcification (black arrows). B. Contrast-enhanced CT shows no significant enhancement of the fluid attenuation lesions (black
arrows). (Images courtesy of Nafi Aygun, MD)
Acta Radiol 2007 (8)
886 B. R. Foerster et al.
Fig. 11. Coccidioidomycosis. A. Contrast-enhanced CT shows nodular meningeal enhancement in the right suprasellar region and adjacent
to the left sylvian fissure (white arrows) in a patient who had recently traveled to the southwestern United States. B. Coronal post-contrast
T1-weighted MR image shows nodular meningeal enhancement in the region of the left ambient cistern/choroid fissure (white arrow).
seen in virtually all cases of HIV infection in the Imaging findings include high T2WI signal in the
early stages, and a decrease in NAA occurs in HIV white matter that is typically asymmetric and
encephalopathy (37, 38). commonly affects the occipital and parietal lobes,
HIV patients can develop progressive multifocal as depicted in Fig. 13. PML does not exhibit mass
leukoencephalopathy (PML) caused by the JC virus. effect and does not tend to show contrast enhance-
The JC virus is a type of human polyomavirus that ment (12, 23, 28).
primarily infects oligodendrocytes, resulting in a Toxoplasmosis results from Toxoplasma gondii,
demyelinating process. Unlike HIV encephalitis, an intracellular protozoan parasite. Reactivation of
dementia is not the main feature in PML; rather, latent infection is seen in advanced HIV, usually
patients characteristically present with rapidly when CD4 counts fall below 100 cells/ml. Typical
progressive focal neurologic deficits without signs imaging findings include multiple abscess for-
of increased intracranial pressure. Specific deficits mation with a propensity for the basal ganglia,
can include visual field deficits, ataxia, weakness, corticomedullary junction, white matter, and peri-
and hemiparesis, as well as cognitive deficits. ventricular regions. CT can demonstrate areas of
Fig. 12. HIV encephalopathy. A, B. A 46-year-old, HIV-positive patient with clinical signs of dementia. Axial FLAIR MR imaging shows
severe atrophy and diffuse, increased signal abnormalities in the white matter without mass effect.
Acta Radiol 2007 (8)
Imaging of Intracranial Infections 887
low-attenuation or iso-attenuation nodules, both of Cryptococcus neoformans is an endemic fungus
which can show varying degrees of enhancement that results in meningoencephalitis among patients
(24, 44). MRI features include multiple, high T2WI with AIDS. The diagnosis is made by staining the
signal lesions with vasogenic edema and ring or organism from the CSF with India ink, detecting
nodular enhancement (19). Fig. 14 shows some of cryptococcal antigen in the CSF or blood, or
the imaging findings seen in toxoplasmosis. growing the organism in culture. Clinically, the
Clinically, toxoplasmosis and intracranial lym- patients present with elevated intracranial pressure
phoma are often indistinguishable. Newer imaging and often need repeat lumbar puncture in order to
techniques are sometimes useful in differentiating improve clinical symptoms, such as pain and mental
between these processes. High-attenuation masses on status changes. Because the infection results in
non-contrast CT, and periventricular lesions with relatively mild inflammatory changes, many patients
subependymal spread, suggest lymphoma. Thallium- have normal contrast-enhanced CT scan (12). MRI
201 single photon emission computed tomography can also be normal.
(SPECT) has been shown to have increased uptake in Cryptococcomas can present as focal parenchy-
lymphoma, but not in toxoplasmosis; there is, mal masses most commonly located in the basal
however, significant overlap, resulting in false ganglia, thalamus, and midbrain. Leptomeningeal
positives and false negatives (57). Fluoro- nodules, with involvement of the choroid plexus and
deoxyglucose positron emission tomography spinal cord, can also be seen, although less
(18FDG-PET) can also be used to discriminate frequently (64). Some patients demonstrate mixed
between toxoplasmosis and lymphoma, as lym- findings, including dilated Virchow-Robin spaces,
phoma exhibits increased 18FDG uptake (35). Some focal masses, and leptomeningeal nodules (69).
studies suggest that MRS may help differentiate Cryptococcosis can also present with basal ganglia
between toxoplasmosis and lymphoma, whereas lesions (Fig. 15), with the differential diagnosis
other studies have suggested that MRS is less able including toxoplasmosis and lymphoma, which
to discriminate between these two entities (15, 16, 54). more typically show enhancement relative to cryp-
A previous report showed that lymphomas were tococcal infection (28).
characterized by lower NAA/Cr and NAA/Cho
ratios, and by more frequent lipid signals, compared
to toxoplasmosis (55). Other studies have demon- Other immunocompromised hosts
strated the presence of a lactate/lipid peak and the Immunocompromised hosts, including those with
absence of the other metabolites in toxoplasmosis, solid-organ transplants or neutropenia related to
while lymphoma shows increased choline levels hematologic malignancy and/or bone marrow
similar to those present in malignant tumors (15). transplantation, can develop serious fungal
Fig. 13. Progressive multifocal leukoencephalopathy. Axial T2-weighted (A) and FLAIR MR (B) imaging shows asymmetric increased signal
in the bilateral occipital lobes without mass effect. C. Axial post-contrast T1-weighted MR image shows no associated pathologic contrast
enhancement.
Acta Radiol 2007 (8)
888 B. R. Foerster et al.
Fig. 14. Toxoplasmosis. A. Non-contrast CT shows a low-attenuation lesion with a subtle peripheral ring in the left basal ganglia/thalamus
(black arrow). B. Axial FLAIR MR imaging re-demonstrates the lesion (black arrow) with surrounding edema. Axial pre-contrast (C) and
post-contrast (D) T1-weighted MR imaging demonstrates a low-attenuation lesion (black arrow) with peripheral enhancement.
infections of the CNS. Generally, these infections progressing to stupor, coma, decerebrate rigidity,
begin in the respiratory tract or sinuses. Intracranial cranial nerve palsy, and stroke. Aspergillus invades
involvement in this setting usually portends a poor the vasculature walls, which can result in vascular
prognosis. While biopsy, along with culture, is thrombosis, hemorrhage, infarctions, and propaga-
needed to definitively identify most infecting organ- tion of the infection in the infarcted tissue.
isms, imaging modalities offer a means to define the Aspergillus has a predilection for the basal ganglia,
extent of involvement and to track progression. thalami, and corpus callosum (17). On CT, the
Imaging, along with clinical variables, helps suggest abnormalities are usually subtle, with varying
the overall prognosis and is essential for medical densities and minimal mass effect, poor contrast
decision-making. enhancement, and no ring formation (41). On MRI,
The Aspergillus species is an important pathogen the signs are nonspecific and include high-signal
that can produce meningitis and meningoencepha- lesions on T2WI and, at times, on T1WI, with
litis among highly compromised hosts. Fungal variable enhancement (5). However, involvement of
organisms gain entry to the CNS, either via direct the basal ganglia, thalami, corpus callosum, and
extension from the sinuses or, less commonly, other perforator artery territories are suggestive of
hematogenously. Patients may present with confu- aspergillus infection in the immunocompromised
sion, fevers, headache, lethargy, and meningismus, patient. Enhancing soft tissue in the sinuses can
Acta Radiol 2007 (8)
Imaging of Intracranial Infections 889
Fig. 15. Cryptococcus. Axial T2-weighted MR image shows patchy and more focal high T2 signal abnormality present in the bilateral basal
ganglia.
offer further support for the presence of an produces proptosis, chemosis, superior ophthalmic
intracranial aspergillus infection. MRI findings vein thrombosis with extension, and subsequent
from a patient with aspergillosis are shown in thrombosis of the cavernous sinus. The diagnosis of
Fig. 16. mucormycosis must be made clinically. The pro-
gression of infection is often so rapid that imaging
does not offer much beyond demonstrating the
Mucormycosis extent of involvement. Intracranial involvement is
Rhinocerebral mucormycosis is a devastating infec- almost invariably fatal in this infection. CT imaging
tion that results from zygomycetes. Such infection can demonstrate a rim of soft tissue along the walls
carries a very grim prognosis and results invariably of the paranasal sinuses. On MRI, low intensity of
in patients with altered cellular immunity, including the sinuses may be present on T1WI and T2WI.
those with diabetes mellitus and hematologic Intracranially, MRI findings can include high T2WI
malignancies. Mucormycosis spreads from the signal in the basal portions of the frontal and
paranasal sinuses to the skull base or cribiform temporal lobes with mild mass effect; this likely
plate into the orbits, frontal lobes, and basal represents a combination of inflammation and
ganglia. Infection progresses rapidly, spreading infarction secondary to vascular invasion (34, 46,
along vascular structures and often involving the 72). Fig. 17 shows a patient with intracranial
cavernous sinus. Infarction is seen frequently in extension of paranasal sinus mucor.
advanced disease. Spectroscopy has also been studied for the
Clinical symptoms associated with mucormycosis evaluation of mucor. In a previously published case
include facial pain, bloody nasal discharge, chemo- report, proton MRS showed markedly elevated
sis, exophthalmos, and cranial nerve palsy, progres- lactate, depleted NAA, and metabolite resonances
sing rapidly to stroke, encephalitis, and death. attributable to succinate and acetate. The spectro-
Orbital extension from the ethmoid sinuses scopy profile is essentially similar to that of bacterial
Acta Radiol 2007 (8)
890 B. R. Foerster et al.
Fig. 16. Aspergillosis. A. Axial FLAIR MR imaging shows high-signal lesions in the bilateral white matter (black arrows). Axial pre-contrast
(B) and post-contrast (C) T1-weighted MR imaging demonstrates several low-attenuation lesions with peripheral enhancement (black
arrows).
Fig. 17. Mucormycosis. A. Coronal non-contrast sinus CT shows extensive opacification of the right paranasal sinuses, with destruction of
the nasal septum and cribiform plate (white arrows). B. Coronal post-contrast T1-weighted MR image shows enhancing, infiltrating lesion in
the right cavernous sinus (white arrowhead). C. Diffusion-weighted image shows restricted diffusion of the bilateral frontal lobes and right
basal ganglia. D. Magnetic resonance arteriography shows asymmetric decreased caliber of the cavernous portion of the right internal carotid
artery compared to the left internal carotid artery (black arrows). (Images courtesy of Stephen Gebarski, MD)
Acta Radiol 2007 (8)
Imaging of Intracranial Infections 891
abscess, but without the commonly seen resonances 10. Calfee DP, Wispelwey B. Brain abscess. Semin Neurol
of the amino acids valine, leucine, and isoleucine 2000;20:353–60.
11. Castillo M. Magnetic resonance imaging of meningitis
(53). and its complications. Top Magn Reson Imaging 1994;6:
53–8.
12. Castillo M. Brain infections in human immunodeficiency
Conclusion
virus positive patients. Top Magn Reson Imaging
1994;6:3–10.
The radiologist plays a central role in the diagnosis 13. Chang KH, Han MH, Roh JK, Kim IO, Han MC, Choi
and management of patients with intracranial KS et al. Gd-DTPA Enhanced MR imaging in
infections. Different imaging modalities offer dif- intracranial tuberculosis. Neuroradiology 1990;32:
ferent advantages in the diagnostic paradigm. CT is 19–25.
helpful in rapidly excluding a focal mass lesion in 14. Chang KH, Han MH, Roh JK, Kim IO, Han MC,
Kim C. Gd-DTPA-enhanced MR imaging of the brain
the acute setting, prior to lumbar puncture. MRI is in patients with meningitis: comparison with CT. Am J
much more sensitive for defining the extent of Neuroradiol 1990;11:69–76.
infection, and for identifying infection-related com- 15. Chang L, Miller BL, McBride D, Cornford M,
plications, such as infected subdural effusions and Oropilla G, Buchthal S, et al. Brain lesions in patients
venous sinus thrombosis. Many investigators have with AIDS: H-1 MR spectroscopy. Radiology
1995;197:525–31.
demonstrated the value of MR spectroscopy to aid 16. Chinn RJ, Wilkinson ID, Hall-Craggs MA, Paley MN,
in the differentiation of abscesses and neoplasms. A Miller RF, Kendall BE, et al. Toxoplasmosis and
thorough understanding of the imaging patterns primary central nervous system lymphoma in HIV
associated with common intracranial infections infection: diagnosis with MR spectroscopy. Radiology
allows the radiologist to help narrow the differential 1995;197:649–54.
17. DeLone DR, Goldstein RA, Petermann G, Salamat MS,
diagnosis and facilitate timely implementation of Miles JM, Knechtle SJ, et al. Disseminated aspergillosis
appropriate therapies. involving the brain: distribution and imaging character-
istics. Am J Neuroradiol 1999;20:1597–604.
18. Desprechins B, Stadnik T, Koerts G, Shabana W,
References Breucq C, Osteaux M. Use of diffusion-eeighted MR
imaging in differential diagnosis between intrace-
1. Agid R, Ducreux D, Halliday WC, Kucharczyk W, rebral necrotic tumors and cerebral abscesses. Am J
terBrugge KG, Mikulis DJ. MR Diffusion-weighted Neuroradiol 1999;20:1252–7.
imaging in a case of West Nile virus encephalitis. 19. Dina TS. Primary central nervous system lymphoma
Neurology 2003;61:1821–3. versus toxoplasmosis in AIDS. Radiology 1991;179:823–8.
2. Agosta F, Rocca MA, Benedetti B, Capra R, Cordioli C, 20. Erly WK, Bellon RJ, Seeger JF, Carmody RF. MR
Filippi M. MR imaging assessment of brain and cervical imaging of acute coccidioidal meningitis. Am J
cord damage in patients with neuroborreliosis. Am J Neuroradiol 1999;20:509–14.
Neuroradiol 2006;27:892–4. 21. Falcone S, Post MJ. Encephalitis, cerebritis, and brain
3. Anslow P. Cranial bacterial infection. Eur Radiol abscess: pathophysiology and imaging findings.
2004;14:E145–54. Neuroimaging Clin N Am 2000;10:333–53.
4. Artopoulos J, Chalemis Z, Christopoulos S, Manios S, 22. Fukui M, Williams RL, Mudigonda S. CT and MR
Kelekis L. Sequential computed tomography in tuber- imaging features of pyogenic ventriculitis. Am J
culous meningitis in infants and children. Comput Neuroradiol 2001;22:1510–6.
Radiol 1984;8:271–7. 23. Garrels K, Kucharczyk W, Wortzman G, Shandling M.
5. Ashdown BC, Tien RD, Felsberg GJ. Aspergillus of the Progressive multifocal leukoencephalopathy: clinical and
brain and the paranasal sinuses in immunocompromised MR response to treatment. Am J Neuroradiol
patients: CT and MR imaging findings. Am J 1996;17:597–600.
Roentgenol 1994;162:155–9. 24. Gaston A, Gheradi R, N’Guyen JP, Perroud AM,
6. Bernaerts A, Vanjonacker FM, Parizel PM, Van Wechsler J, Wallman J, et al. Cerebral toxoplasmosis in
Goethem JWM, van Altena R, Laridon A, et al. acquired immunodeficiency syndrome. Neuroradiol
Tuberculosis of the central nervous system: overview of 1985;27:83–6.
neuroradiological findings. Eur Radiol 2003;13:1876–90. 25. Guzman R, Barth A, Lovblad K, El-Koussy M, Weis J,
7. Brilla R Block M, Geremia. , Wichter M. Clinical and Schroth G, et al. Use of diffusion-weighted magnetic
neuroradiologic features of 39 consecutive cases of West resonance imaging in differentiating purulent brain
Nile virus meningoencephalitis. J Neurol Sci 2004;220: processes from cystic brain tumors. J Neurosurg
37–40. 2002;97:1101–7.
8. Broderick DF, Wippold FJ, Clifford DB, Kido D, 26. Haimes AB, Zimmerman RD, Morgello S,
Wilson BS. White matter lesions and cerebral atrophy on Weingarten K, Becker RD, Jennis R, et al. MR imaging
MR images in patients with and without AIDS dementia of brain abscesses. Am J Neuroradiol 1989;10:279–
complex. Am J Roentgenol 1993;161:177–81. 91.
9. Burtscher IM, Holtas S. In vivo 1H-MR spectroscopy in 27. Jayasundar R, Singh VP, Raghunathan P, Jain K, A,
untreated and treated brain abscesses. Am J Neuroradiol Banerji AK. Inflammatory granulomas: evaluation with
1999;20:1049–53. proton MRS. NMR Biomed 1999;12:139–44.
Acta Radiol 2007 (8)
892 B. R. Foerster et al.
28. Jensen MC, Brant-Zawadzki M. MR imaging of the 47. Ramsey DW, Mohammad A, Cherryman GR.
brain in patients with AIDS: value of routine use of IV Diffusion-weighted imaging of cerebral abscess and
gadopentetate dimeglumine. Am J Roentgenol subdural empyema. Am J Neuroradiol 2000;21:1172.
1993;160:153–7. 48. Sämann PG, Schlegel J, Müller G, Prantl F,
29. Karampekios S, Hesselink J. Cerebral infections. Eur Emminger C, Auer DP. Serial proton MR spectroscopy
Radiol 2005;15:485–93. and diffusion imaging findings in HIV-related herpes
30. Kastrup O, Wanke I, Maschke M. Neuroimaging of simplex encephalitis. Am J Neuroradiol 2003;24:2015–
infections. NeuroRx 2005;2:324–32. 9.
31. Kramer LD, Locke GE, Byrd SE, Daryabagi J. Cerebral 49. Schoeman J, Hewlett R, Donald P. MR of childhood
cystercercosis: documentation of natural history with tuberculosis meningitis. Neuroradiol 1988;30:473–7.
CT. Radiology 1989;171:459–62. 50. Schroeder PC, Post MJD, Oschatz E, Stadler A, Bruce-
32. Lai PH, Ho JT, Chen WL, Hsu SS, Wang JS, Pan HB, Gregorios J, Thurnher MM. Analysis of the utility of
et al. Brain abscess and necrotic brain tumor: discrimi- diffusion-weighted MRI and apparent diffusion coeffi-
nation with proton MR spectroscopy and diffusion- cient values in distinguishing central nervous system
weight imaging. Am J Neuroradiol 2002;23:1369–77. toxoplasmosis from lymphoma. Neuroradiology
33. Latchaw RE, Hirsch WL, Yock DH. Imaging of 2006;48:715–20.
intracranial infection. Neurosurg Clin N Am 51. Schroth G, Kretzschmar K, Gawehn J, Voight K.
1992;3:303–22. Advantage of magnetic resonance imaging in the
34. McLean FM, Ginsberg LE, Stanton CA. Perineural diagnosis of cerebral infections. Neuroradiology
spread of rhinocerebral mucormycosis. Am J 1987;29:120–6.
Neuroradiol 1996;17:114–6. 52. Shah GV. Central nervous system tuberculosis imaging
35. Menendez JA, Lilien DL, Nanda A, Polin RS. Use of manifestations. Neurosurg Clin N Am 2000;10:355–74.
fluorodeoxyglucose-positron emission tomography for 53. Siegal JA, Cacayorinb ED, Sami-Nassef A, Rizk D,
the differentiation of cerebral lesions in patients with Galambos C, Levy B, et al. Cerebral mucormycosis:
acquired immune deficiency syndrome. Neurosurg proton MR spectroscopy and MR imaging. Magn
Focus 2000;8:1–4. Reson Imaging 2000;18:915–20.
36. Menon DK, Sargentoni J, Peden CJ, Bell JD, Cox IJ, 54. Simone IL, Federico F, Trojano M, Tortorella C,
Coutts GA, et al. Proton MR spectroscopy in herpes Liguori M, Giannini P, et al. High resolution proton
simplex encephalitis: assessment of neuronal loss. J MR spectroscopy of cerebrospinal fluid in MS patients.
Comput Assist Tomogr 1990;14:449–52. Comparison with biochemical changes in demyelinating
37. Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, plaques. J Neurol Sci 1996;144:182–90.
Weiner MW, Fein G. Elevated subcortical choline 55. Simone IL, Federico F, Tortorella C, Anddreula C-F,
metabolites in cognitively and clinically asymptomatic Zimatore G-B, Giannini P, et al. Localised 1H-MR
HIV patients. Neurology 1999;52:995–1003. spectroscopy for metabolic characterization of diffuse
38. Möller HE, Vermathen P, Lentschig MG, Schuierer G, and focal brain lesions in patients infected with HIV. J
Schwarz S, Wiedermann D, et al. Metabolic character- Neurol Neurosurg Psychiatry 1998;64:516–23.
ization of AIDS-dementia complex by spectroscopic 56. Singer MB, Atlas SW, Drayer BP. Subarachnoid space
imaging. J Magn Reson Imaging 1999;9:10–8. disease: diagnosis with fluid-attenuated inversion recov-
39. Moseley IF, Kendall BE. Radiology of intracranial ery MR Imaging and comparison with gadolinium-
empyemas, with special reference to computed tomo- enhanced spin-echo MR imaging-blinded reader study.
graphy. Neuroradiol 1984;26:333–45. Neuroradiology 1998;208:417–22.
40. Mueller-Mang C, Castillo M, Mang TG, Cartes- 57. Skiest DJ, Erdman W, Chang WE, Oz OK, Ware A,
Zumelzu F, Weber M, Thurnher MM. Fungal versus Fleckenstein J. SPECT Thallium-201 combined with
bacterial brain abscesses: is diffusion-weighted MR toxoplasma serology for the presumptive diagnosis of
imaging a useful tool in the differential diagnosis? focal central nervous system mass lesions in patients with
Neuroradiology on-line. 2007, 27 June. AIDS. J Infect 2000;40:274–81.
41. Nadkarni T, Goel A. Aspergilloma of the brain: an 58. Smith RR. Neuroradiology of intracranial infection.
overview. J Postgrad Med 2005;51:37–41. Pediatr Neurosurg 1992;18:92–104.
42. Nash D, Mostasharai F, Fine A, Miller J, O’Leary D, 59. Steinbach JP, Melms A, Skalej M, Dichgans J. Delayed
Murray K, et al. The outbreak of West Nile virus resolution of white matter changes following therapy of
infection in the New York City area in 1999. N Engl J B burgdorferi encephalitis. Neurology 2005;64:758–9.
Med 2001;344:1807–14. 60. Suh DC, Chang KH, Han MH, Lee SR. Han MC, Kim
43. Olsen WL, Longo FM, Mills CM, Norman D. White CW. Unusual MR manifestations of neurocysticercosis.
matter disease in AIDS: findings at MR imaging. Neuroradiology 1989;31:396–402.
Radiology 1988;169:445–8. 61. Sze G. Disease of the intracranial meninges: MR
44. Ostrow TD, Hudgins PA. Magnetic resonance imaging imaging features. Am J Roentgenol 1993;170:727–33.
of intracranial fungal infections. Top Magn Reson 62. Takanashi J, Sugita K, Ishii M, Aoyagi M, Niimi H.
Imaging 1994;6:22–31. Longitudinal MR imaging and proton MR spectroscopy
45. Petropoulou KA, Gordon SM, Prayson RA, in herpes simplex encephalitis. J Neurol Sci 1997;149:
Ruggierri PM. West Nile virus meningoencephalitis: 99–102.
MR imaging findings. Am J Neuroradiol 2005;26: 63. Tien RD, Feisberg. , Osumi AK. Herpesvirus infections
1986–95. of the CNS: MR findings. Am J Roentgenol
46. Press GA, Weindling SM, Hesselink JR, Ochi JW, 1993;161:167–76.
Harris JP. Rhinocerebral mucormycosis: MR manifesta- 64. Tien RD, Chu PK, Hesselink JR, Duberg A, Wiley C.
tions. J Comput Assist Tomogr 1988;12:744–9. Intracranial cryptococcosis in immunocompromised
Acta Radiol 2007 (8)
Imaging of Intracranial Infections 893
patients: CT and MR findings in 29 cases. Am J 70. Whiteman ML, Post MJ, Berger JR, Tate LG, Bell MD,
Neuroradiol 1991;12:283–9. Limonte LP. Progressive multifocal leukoencephalo-
65. Tsai Y, Chang W, Shen C, Lin Y, Lu C, Liliang P, et al. pathy in 47 HIV-seropositive patients: neuroimaging
Intracranial suppuration: a clinical comparison of with clinical and pathologic correlation. Radiology
subdural empyemas and epidural abscesses. Surg 1993;187:233–40.
Neurol 2003;59:191–6. 71. Wrobel CJ, Meyer S, Johnson RH, Hesselink JR. MR
66. Tsuchiya K, Inaoka S, Mizutani Y, Hachiya J. Fast Findings in acute and chronic coccidioidomycosis
fluid-attenuated inversion-recovery MR of intracranial meningitis. Am J Neuroradiol 1992;13:1241–5.
infections. Am J Neuroradiol 1997;18:909–13. 72. Yousem DM, Galetta SL, Gusnard DA, Goldberg HI.
67. van Dyk A. CT of intracranial tuberculomas with MR findings in rhinocerebral mucormycosis. J Comput
specific reference to the ‘‘target sign’’. Neuroradiol Assist Tomogr 1989;13:878–82.
1988;30:329–36. 73. Zee C, Segall HD, Boswell W, Admadi J, Nelson M,
68. Weingarten K, Zimmerman RD, Becker RD, Heier LA, Colletti P. MR imaging of neurocysticercosis. J Comput
Haimes AB, Deck MD. Subdural and epidural empye- Assist Tomogr 1988;12:927–34.
mas: MR imaging. Am J Roentgenol 1989;152:615–21. 74. Zimmerman RD, Leeds NE, Danziger A. Subdural
69. When SM, Heinz ER, Burger PC, Boyko OB. Dilated empyema: CT findings. Radiology 1984;150:417–22.
Virchow-Robin spaces in cryptococcal meningitis asso- 75. Zimmerman RD, Weingarten K. Neuroimaging of
ciated with AIDS: CT and MR findings. J Comput cerebral abscesses. Neuroimaging Clin N Am 1991;1:
Assist Tomogr 1989;13:756–62. 1–16.
Acta Radiol 2007 (8)
You might also like
- War Surgery - Field ManualDocument880 pagesWar Surgery - Field ManualTromsø Mine Victim Resource Centre100% (20)
- 【Zybio】IFU-Disposable Virus Sampling Tube 20200915Document2 pages【Zybio】IFU-Disposable Virus Sampling Tube 20200915Daniel Huachani Coripuna100% (1)
- Local Literature of Sleep DeprivationDocument3 pagesLocal Literature of Sleep DeprivationEunsang Lee82% (11)
- Diabetic Foot Ulcer - Yandriyane Stephanie Robiady - 131621190504Document28 pagesDiabetic Foot Ulcer - Yandriyane Stephanie Robiady - 131621190504Yohelio Priawan SibuNo ratings yet
- The Challenge of Managing Tendinopathy in Competing AthletesDocument7 pagesThe Challenge of Managing Tendinopathy in Competing AthletesMichele MarengoNo ratings yet
- Imaging of Intracranial InfectionsDocument12 pagesImaging of Intracranial Infectionsrafael rocha novaesNo ratings yet
- Imaging in EncephalitisDocument10 pagesImaging in Encephalitisrafael rocha novaesNo ratings yet
- Cerebral Abscesses Imaging A Practical ApproachDocument14 pagesCerebral Abscesses Imaging A Practical Approachhasbi.alginaaNo ratings yet
- Mold Infections of The Central Nervous SystemDocument15 pagesMold Infections of The Central Nervous SystemJohn TusselNo ratings yet
- Neuroimaging in The Brain in HIV-1-Infected PatientsDocument25 pagesNeuroimaging in The Brain in HIV-1-Infected PatientsYanina Pérez de VillarrealNo ratings yet
- Management of Abcess CerebriDocument6 pagesManagement of Abcess Cerebricamelia musaadNo ratings yet
- Magnetic Resonance Imaging - 2010 - Kumar - Eccentric Target Sign in Cerebral Toxoplasmosis Neuropathological Correlate ToDocument4 pagesMagnetic Resonance Imaging - 2010 - Kumar - Eccentric Target Sign in Cerebral Toxoplasmosis Neuropathological Correlate ToMartha OktaviaNo ratings yet
- AbcesoDocument2 pagesAbcesoRocio LedesmaNo ratings yet
- Nej MR A 1216008Document11 pagesNej MR A 1216008guillosarahNo ratings yet
- Chapter 2Document11 pagesChapter 2fiskiyeyikimkirdi06No ratings yet
- (10920684 - Neurosurgical Focus) Neurogenic Thoracic Outlet Syndrome - Current Diagnostic Criteria and Advances in MRI DiagnosticsDocument5 pages(10920684 - Neurosurgical Focus) Neurogenic Thoracic Outlet Syndrome - Current Diagnostic Criteria and Advances in MRI DiagnosticsGeorgios StathisNo ratings yet
- Neuroimaging Patterns of Intracranial Infections Meningitis, Cerebritis, and Their ComplicationsDocument31 pagesNeuroimaging Patterns of Intracranial Infections Meningitis, Cerebritis, and Their ComplicationsMauro Oscar Soares De Souza LimaNo ratings yet
- Infogrfico5 ArtigoderefernciaDocument21 pagesInfogrfico5 Artigoderefernciagiovannacollins404No ratings yet
- Rar Vol11 Nro3Document21 pagesRar Vol11 Nro3Valentine WijayaNo ratings yet
- MeningoencephalitisDocument8 pagesMeningoencephalitisjohza al-thafifNo ratings yet
- Tuberculomas of The Brain With and Without Associated Meningitis: A Cohort of 28 Cases Treated With Anti-Tuberculosis Drugs at A Tertiary Care CentreDocument4 pagesTuberculomas of The Brain With and Without Associated Meningitis: A Cohort of 28 Cases Treated With Anti-Tuberculosis Drugs at A Tertiary Care CentreDestyNo ratings yet
- WJR 6 716Document11 pagesWJR 6 716laraviNo ratings yet
- Imaging Findings of Intraventricular and Ependymal LesionsDocument16 pagesImaging Findings of Intraventricular and Ependymal Lesionsanggi abNo ratings yet
- Neuroimaging Patterns of Intracranial InfectionsDocument31 pagesNeuroimaging Patterns of Intracranial InfectionsPaola LunaNo ratings yet
- GM 04015Document6 pagesGM 04015Ikhsan FebriansyahNo ratings yet
- Lee 2007Document6 pagesLee 2007Luis PerezNo ratings yet
- Brain AbcesDocument6 pagesBrain AbcesGintar Isnu WardoyoNo ratings yet
- Intracranial CystsDocument9 pagesIntracranial CystsjosephNo ratings yet
- Infections Diseases of The Central Nervous System: Chapter VI - J. RuscalledaDocument11 pagesInfections Diseases of The Central Nervous System: Chapter VI - J. RuscalledaArif BudimanNo ratings yet
- Magnetic Resonance Imaging of Meningiomas: A Pictorial ReviewDocument10 pagesMagnetic Resonance Imaging of Meningiomas: A Pictorial ReviewKevin EdroNo ratings yet
- Clinicalsigns, Imagingfeatures, Neuropathology, Andoutcome Incatsanddogswithcentralnervoussystemcryptococcosis FromcaliforniaDocument12 pagesClinicalsigns, Imagingfeatures, Neuropathology, Andoutcome Incatsanddogswithcentralnervoussystemcryptococcosis FromcaliforniaMicheleBerselliNo ratings yet
- Cerebral Leukimia 1Document9 pagesCerebral Leukimia 1Cika HanandiyaNo ratings yet
- Tuberculous Meningitis: Basal Cistern Enhancement Pattern On CT ImagingDocument9 pagesTuberculous Meningitis: Basal Cistern Enhancement Pattern On CT ImagingDeei RizalNo ratings yet
- Coles TBIDocument12 pagesColes TBIhalamadrid77No ratings yet
- Current Epidemiological Trends of Brain Abscess: A Clinicopathological StudyDocument9 pagesCurrent Epidemiological Trends of Brain Abscess: A Clinicopathological StudyNur Fadhilah KusnadiNo ratings yet
- Bodil Sen 2018Document15 pagesBodil Sen 2018Sannita Mayusda BadiriNo ratings yet
- CNS InfectionDocument17 pagesCNS InfectionEdward PuteraNo ratings yet
- Necrosis and Brain Invasion Predict Radio-Resistance and Tumor Recurrence in Atypical Meningioma - A Retrospective Cohort StudyDocument7 pagesNecrosis and Brain Invasion Predict Radio-Resistance and Tumor Recurrence in Atypical Meningioma - A Retrospective Cohort StudyHastrina MailaniNo ratings yet
- Diagnosis Dan Pengobatan MeningiomaDocument7 pagesDiagnosis Dan Pengobatan Meningiomasilvia erfanNo ratings yet
- Chronic MeningitisDocument7 pagesChronic Meningitisneurojuancarfab100% (1)
- Infeccion BacterianaDocument10 pagesInfeccion BacterianaMonica Aleja FernándezNo ratings yet
- Cranial CT in Acquired Immunodeficiency Syndrome:: Spectrum of Diseases and Optimal Contrast Enhancement TechniqueDocument12 pagesCranial CT in Acquired Immunodeficiency Syndrome:: Spectrum of Diseases and Optimal Contrast Enhancement Techniqueudayana kramasanjayaNo ratings yet
- Prepared and Presented by Marc Imhotep Cray, M.DDocument30 pagesPrepared and Presented by Marc Imhotep Cray, M.DMarc Imhotep Cray, M.D.100% (1)
- Article ReviewDocument3 pagesArticle ReviewBrigetNo ratings yet
- Bacterial Brain Abscess: An Outline For Diagnosis and ManagementDocument10 pagesBacterial Brain Abscess: An Outline For Diagnosis and ManagementWIWI HRNo ratings yet
- SpondilodiscytisDocument16 pagesSpondilodiscytischristinawiyaniputriNo ratings yet
- Review Article: Central Nervous System Tuberculosis: An Imaging-Focused Review of A Reemerging DiseaseDocument9 pagesReview Article: Central Nervous System Tuberculosis: An Imaging-Focused Review of A Reemerging DiseaseJhoselinOrbegosoNo ratings yet
- Carpio 2018Document14 pagesCarpio 2018María Fernanda Calvo VázquezNo ratings yet
- Sherly 2Document41 pagesSherly 2asa mutiaNo ratings yet
- Original Research Paper General MedicineDocument10 pagesOriginal Research Paper General MedicineKriti KumariNo ratings yet
- Wjarr 2024 0786Document4 pagesWjarr 2024 0786mcvallespinNo ratings yet
- Magnetic Resonance Imaging in Differentatial Diagnosis of Pyogenic Spondylodiscitis and Tuberculous SpondylodiscitisDocument17 pagesMagnetic Resonance Imaging in Differentatial Diagnosis of Pyogenic Spondylodiscitis and Tuberculous SpondylodiscitisHaniNo ratings yet
- KLP 10 - Jurnal - MeningiomaDocument11 pagesKLP 10 - Jurnal - Meningiomawahy udiNo ratings yet
- Intracranial Tuberculoma 5Document1 pageIntracranial Tuberculoma 5Arief RahmatullahNo ratings yet
- Cerebral Toxoplasmosis: ClinicalpictureDocument2 pagesCerebral Toxoplasmosis: ClinicalpictureRahul RaiNo ratings yet
- Contents NCLDocument3 pagesContents NCLdewi najiraNo ratings yet
- Spec MRI Distinguish Acue TM of NMOSD N Infarction. Kister Et Al 2015Document21 pagesSpec MRI Distinguish Acue TM of NMOSD N Infarction. Kister Et Al 2015Ido BramantyaNo ratings yet
- AbscessDocument12 pagesAbscesslittlecandiesNo ratings yet
- Inflammatory DisordersDocument4 pagesInflammatory DisordersArouetNo ratings yet
- (10920684 - Neurosurgical Focus) Pyogenic Brain AbscessDocument10 pages(10920684 - Neurosurgical Focus) Pyogenic Brain AbscesschiquitaputriNo ratings yet
- Cerebral Toxoplasmosis - 1Document2 pagesCerebral Toxoplasmosis - 1hasbi.alginaaNo ratings yet
- Brain Abscess: Current Management: Go ToDocument17 pagesBrain Abscess: Current Management: Go ToretnoNo ratings yet
- (10920684 - Neurosurgical Focus) Intracranial Infections - Lessons Learned From 52 Surgically Treated CasesDocument8 pages(10920684 - Neurosurgical Focus) Intracranial Infections - Lessons Learned From 52 Surgically Treated CasesIsmail MuhammadNo ratings yet
- Echography and Doppler of the BrainFrom EverandEchography and Doppler of the BrainChiara RobbaNo ratings yet
- RD TIPS - 10 Choose Ans From A ListDocument2 pagesRD TIPS - 10 Choose Ans From A ListJess NguyenNo ratings yet
- Caffeine and The Kidney EvidenceDocument10 pagesCaffeine and The Kidney EvidencePaloma GBNo ratings yet
- Assessment of Immune FunctionDocument3 pagesAssessment of Immune Functionhalloween candyNo ratings yet
- Empa-Kidney 1Document5 pagesEmpa-Kidney 1api-660408385No ratings yet
- 10 Symptoms of PneumoniaDocument3 pages10 Symptoms of PneumoniaYidnekachew Girma AssefaNo ratings yet
- Laboratory Tests of Renal FunctionDocument5 pagesLaboratory Tests of Renal Functiongiselle155204No ratings yet
- Lesson Plan On: Growth and Development of ChildrenDocument43 pagesLesson Plan On: Growth and Development of ChildrenPinkymekala HasanparthyNo ratings yet
- Yerba BuenaDocument2 pagesYerba BuenaLloydNo ratings yet
- BingeeatingworkbookDocument153 pagesBingeeatingworkbookAngel MarieNo ratings yet
- Report On Drug AddictionDocument30 pagesReport On Drug AddictionChirag GoyalNo ratings yet
- NCM 108 - Ethical PrinciplesDocument17 pagesNCM 108 - Ethical PrinciplesRica ParcasioNo ratings yet
- CASEDocument9 pagesCASELeah SorianoNo ratings yet
- Soft Tissue Tumors of The Neck: David E. Webb, DDS, Brent B. Ward, DDS, MDDocument15 pagesSoft Tissue Tumors of The Neck: David E. Webb, DDS, Brent B. Ward, DDS, MDCynthia BarrientosNo ratings yet
- NonMalignant Leukocyte DisordersDocument43 pagesNonMalignant Leukocyte DisordersKei Ef SiNo ratings yet
- Delphi Consensus Anti TNF in Crohns DiseaseDocument13 pagesDelphi Consensus Anti TNF in Crohns DiseaseAliabdulghaniNo ratings yet
- Machine Coolant Health and SafetyDocument6 pagesMachine Coolant Health and SafetyhemakumarsNo ratings yet
- Netter's Internal Medicine 2nd Ed 13Document9 pagesNetter's Internal Medicine 2nd Ed 13Panagiotis SouldatosNo ratings yet
- Brainstorming-Emerald SeasonDocument11 pagesBrainstorming-Emerald SeasonHaydi Pineda MedinaNo ratings yet
- Hyundai Sierra 5en1 ManualDocument60 pagesHyundai Sierra 5en1 ManualITZEL GUADALUPE DELGADO DELFINNo ratings yet
- Third Year Class Bone-Loss-PatternsDocument49 pagesThird Year Class Bone-Loss-PatternsDrAjey BhatNo ratings yet
- Nadiparikshan 1Document42 pagesNadiparikshan 1AmitNo ratings yet
- NEJMNotable Articlesof 2021Document53 pagesNEJMNotable Articlesof 2021Federico Ariel RodriguezNo ratings yet
- 600-Article Text-2449-1-10-20210630Document13 pages600-Article Text-2449-1-10-20210630Amlia -No ratings yet
- Kymriah Car T Cell Therapy FaqDocument4 pagesKymriah Car T Cell Therapy FaqVahid MansouriNo ratings yet
- Science p.5 Kolfram Bot Term One Examinations 2023Document6 pagesScience p.5 Kolfram Bot Term One Examinations 2023lazorus LEVITICUSNo ratings yet