Professional Documents
Culture Documents
Hemocue WBC Diff: Operating Manual
Hemocue WBC Diff: Operating Manual
Uploaded by
LowayOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hemocue WBC Diff: Operating Manual
Hemocue WBC Diff: Operating Manual
Uploaded by
LowayCopyright:
Available Formats
HemoCue® WBC DIFF
Operating Manual
Downloaded from www.Manualslib.com manuals search engine
Table of Content
HemoCue WBC DIFF system.................................................... 3
Components................................................................................. 4
Start-Up....................................................................................... 6
Data Entry & Navigation.......................................................... 10
Buttons................................................................................................. 10
Keyboard............................................................................................. 10
Barcode Reader................................................................................... 10
Symbols..................................................................................... 12
Navigation Symbols............................................................................12
Procedure Symbols.............................................................................. 14
Display Symbols.................................................................................. 15
Set-Up........................................................................................ 18
Settings................................................................................................ 18
Printer..................................................................................................26
Keyboard.............................................................................................28
Barcode Reader...................................................................................30
Connectivity........................................................................................ 32
Blood Collection........................................................................ 34
Capillary Sample.................................................................................34
Control Material and Venous Sample.................................................40
Routine Use............................................................................... 42
Patient Test...........................................................................................42
QC Test................................................................................................48
Review/Delete Results.............................................................. 54
Maintenance.............................................................................. 58
Daily Maintenance..............................................................................58
Optical Parts........................................................................................60
Troubleshooting Guide.............................................................. 62
Specifications............................................................................ 68
Downloaded from www.Manualslib.com manuals search engine
HemoCue® WBC DIFF system
GB Thank you for choosing the HemoCue® WBC DIFF system.
The HemoCue WBC DIFF system is an In-Vitro diagnostic system designed for quantitative
determination of white blood cells (WBC) in capillary or venous whole blood. The system
provides values for a total white blood cell count and a differential white blood cell count in-
cluding neutrophil count, lymphocyte count, monocyte count, eosinophil count and basophil
count.
The HemoCue WBC DIFF system is indicated for use in clinical laboratories and for point of
care testing in professional health care settings on pediatric (≥ 3 months) and adult patients.
The HemoCue WBC DIFF Analyzer is only to be used with HemoCue WBC DIFF Microcu-
vettes for measurement of a total white blood cell count and a differential white blood cell
count or with HemoCue WBC Microcuvettes for measurement of a total white blood cell
count only.
All system components are designed and manufactured to provide maximum safety.
Any other use of the system than indicated may impair the safety.
Downloaded from www.Manualslib.com manuals search engine
Components
1 2 3 4
5a 5b 6
Downloaded from www.Manualslib.com manuals search engine
1. HemoCue WBC DIFF Analyzer
2. AC adapter (country specific)
3. Six type C (LR14/HR14) batteries*
4. HemoCue WBC DIFF Quick Reference Guide
5. a. HemoCue WBC DIFF Microcuvettes*
b. HemoCue WBC Microcuvettes*
6. HemoCue WBC DIFF Operating Manual
7. HemoCue® Cleaner WBC
Place analyzer and accessories on a rigid surface (non-vibrating).
NOTE: Do not open the cover of the analyzer. The warranty is void if the cover of the
analyzer is opened.
Only WBC DIFF cuvette holder (marked WBC DIFF) is to be used with the
WBC DIFF analyzer.
*Not included.
For infomation about HemoCue WBC DIFF Microcuvettes and HemoCue WBC
Microcuvettes, please contact your HemoCue distributor.
Downloaded from www.Manualslib.com manuals search engine
Start-Up
1 2
Downloaded from www.Manualslib.com manuals search engine
Only use AC adapters listed under Specifications in the AC Adapters section.
The analyzer can be powered by either AC adapter or batteries.
Connect the AC Adapter
1. Plug the supplied AC adapter into the power inlet at the back of the analyzer.
Insert Batteries
2. Press the ribbed marked button on the back of the analyzer to open the battery compart-
ment on the left side.
3. Gently take out the battery unit. Insert six type C (LR14/HR14) batteries, 1.5 V.
Observe the indication of polarity. Put the battery unit back in the analyzer.
Consult local environmental authorities for proper disposal of batteries.
Downloaded from www.Manualslib.com manuals search engine
4 5,8 6
Downloaded from www.Manualslib.com manuals search engine
Start the Analyzer
4. Pull the cuvette moving arm out to loading position.
5. Press and hold the left button until the display is activated. A start-up window shows
analyzer software version for approximately 15 seconds.
6. The analyzer performs a self test and the hourglass is shown for approximately
30 seconds.
7. The analyzer is ready for use when the main menu is shown.
Turn off the Analyzer
8. Press and hold the left button until the display goes blank.
The analyzer will automatically turn off when not in use.
• after 5 min with battery supply
• after 8 hours with AC adapter
Downloaded from www.Manualslib.com manuals search engine
Data Entry & Navigation
1 2 3
10
Downloaded from www.Manualslib.com manuals search engine
Analyzer buttons
1. The three buttons below the display are for navigating and confirming the choices/sym-
bols displayed above each button. The left button is also for turning on/off.
External keyboard
2. Use the keyboard for data input e.g. Patient ID, Operator ID, Lab ID, Control ID and
Site ID. It can also navigate by using F1 for left, F2 for up/down and F3 for right.
For installation of external keyboard see Set-Up/Keyboard section.
External barcode reader
3. Use the barcode reader for data input e.g. Patient ID, Operator ID, Lab ID, Control ID
and Site ID.
For installation of the external barcode reader see Set-Up/Barcode Reader section.
11
Downloaded from www.Manualslib.com manuals search engine
GB
Symbols
Navigation Symbols Designation Function
Home Go to main menu.
Right Continue to next number/window.
Left Return to previous number/window.
Up Scroll up.
Down Scroll down.
12
Downloaded from www.Manualslib.com manuals search engine
Navigation Symbols Designation Function
Accept Accept input.
Reject Reject input.
Switch Jump between result and data.
13
Downloaded from www.Manualslib.com manuals search engine
Procedure Symbols Designation Function
Patient Test Go to Patient Test procedure.
Menu Go to “Menu” window for Review/Delete/Settings/QC test.
Review/Delete Go to “Review/Delete menu” window.
Review Go to “Review” window.
Delete Go to “Delete Results” window.
Settings Go to settings menu.
14
Downloaded from www.Manualslib.com manuals search engine
Display Symbols Designation Function
Connected Analyzer connected to a PC application.
Unsent Results Unsent results stored in the analyzer.
Accepted Accepted result.
Rejected Rejected result.
Auto Accepted Auto accepted result.
Battery Level Critical battery level.
15
Downloaded from www.Manualslib.com manuals search engine
Display Symbols Designation Function
Battery Level Battery level 30%. Battery level indicates 10-100%.
AC Adapter Analyzer connected with AC adapter.
Insert Cuvette Analyzer ready for measurement. Insert a microcuvette to start measuring.
Remove Cuvette Pull the cuvette moving arm to laoding position and remove the cuvettte.
Measuring Measuring.
Hourglass The analyzer performs a selftest.
16
Downloaded from www.Manualslib.com manuals search engine
Display Symbols Designation Function
Page Number Current page number/total amount of pages.
Caution The results for the WBC differential count are uncertain. The sample may contain
pathological, abnormal or unidentified cells. For more information see the Trouble-
shooting Guide section. The print-out will show an asterisk (*) instead of the display
symbol
Non-critical Error An error has occurred. For more information see Troubleshooting Guide section.
Critical Error A critical error has occurred. For more information see Troubleshooting Guide sec-
tion.
17
Downloaded from www.Manualslib.com manuals search engine
Set-Up Settings
1 2 3
4 5a 5b
18
Downloaded from www.Manualslib.com manuals search engine
Settings are made in the Set-Up/Settings menu. Use the left button to confirm/accept input
and move backwards. Use the right button to confirm/accept input and move forward. The
center button scrolls up/downwards and ticks boxes.
1. Turn on the analyzer as described in the Start-Up section.
2. Press the right button.
3. Press right button to chose menu and move to next window.
4. Enter PIN Code window is shown. The Pin Code is used to prevent unauthorized users
from entering/changing input in the settings menu. As default, the PIN Code is set to
0000. For change of PIN Code see step 19, Settings section.
Enter the PIN code by pressing the center button to change digit. Press right button to
confirm the input and move to the next. After entering the four digit PIN Code press
right button to accept and move to next window.
5. If the analyzer is configured for USB - window 5a is shown. If the analyzer is config-
ured for ethernet - window 5b is shown. The display shows: Home, Review/Delete and
Settings. Press the right button to enter Settings.
19
Downloaded from www.Manualslib.com manuals search engine
6 7 8
9 10 11
20
Downloaded from www.Manualslib.com manuals search engine
6. Language set up window is shown. Select language by pressing the center button. Press
right button to confirm and move forward.
7. Time Zone window is shown. Select Time Zone input by pressing the button below the
up arrow. Press the right button to confirm and move forward.
8. Date Format window is shown. Select Date format by pressing the button below the
down arrow. Press right button to confirm and move forward.
9. Date input window is shown. Select Date input by pressing the button below the up ar-
row to change numbers. Press right button to confirm and move to next number. Press
right button to confirm and move forward.
10. Time Format window is shown. Select Time format by pressing the button below the
down arrow. Press right button to confirm and move forward.
11. Time input window is shown. Set hours by pressing the button below the up arrow.
Press right button to confirm the input and move to minutes. Press right button to con-
firm and move forward.
21
Downloaded from www.Manualslib.com manuals search engine
12 13 14
15 16
22
Downloaded from www.Manualslib.com manuals search engine
12. Patient ID window is shown. Select Patient ID on/off by pressing the button below the
up arrow. Press right button to confirm and move forward.
13. Operator ID window is shown. Select Operator ID on/off by pressing the button below
the up arrow. Press right button to confirm and move forward.
14. Lab ID window is shown. Select Lab ID on/off by pressing the button below the up ar-
row. Press right button to confirm and move forward.
15. Site ID window is shown. Select Site ID on/off by pressing the button below the up ar-
row. Press right button to confirm and move forward.
16. Control ID window is shown. Select Control ID on/off by pressing the button below the
up arrow. Press right button to confirm and move forward.
23
Downloaded from www.Manualslib.com manuals search engine
17 18 19
20 21
24
Downloaded from www.Manualslib.com manuals search engine
17. Unit window is shown. The results can be presented in
• absolute numbers together with percentage; 109/L, %
• absolute numbers only; 109/L
• percentage only; %
Select how to present the result by pressing the button below the down arrow. Press
right button to confirm and move forward.
18. Audio Signal window is shown. A soft signal is heard when buttons are pressed, one
short signal is heard when a test is ready and a distinct signal is heard when an error has
occured. Select Audio Signal on/off by pressing the button below the up arrow. Press
right button to confirm and move forward.
19. PIN Code Setting window is shown. Change digits by pressing the button below the up
arrow. Press right button to confirm and move to next digit. Press right button to accept
the four digit PIN Code.
20. If the PIN Code is changed the Verify Change of PIN Code is shown. Press right button
to accept the change. Press left button to reject the change.
21. Main menu is shown.
25
Downloaded from www.Manualslib.com manuals search engine
Set-Up Printer
1 2a 2b
26
Downloaded from www.Manualslib.com manuals search engine
1. Connect the cable* to the analyzer and ASCII printer* before performing the analysis.
Perform the analysis as described in the Routine Use Patient Test or Routine Use QC
Test sections.
2. The result is shown on the display (2a) and will be printed automatically (2b).
Only current result can be transferred directly to the printer. Stored results can not be
printed.
*Must be purchased separately.
Read and follow the printer instructions for use to adjust the following COM-port settings.
• Baud rate 9600
• Databits 8
• Parity None
• Stopbits 1
• Flow control None
27
Downloaded from www.Manualslib.com manuals search engine
Set-Up Keyboard
28
Downloaded from www.Manualslib.com manuals search engine
The keyboard can be used for data entry of Patient ID, Operator ID, Lab ID, Control ID and
Site ID. To navigate use F1 for left, F2 for up/down and F3 for right.
1. Connect cable from the keyboard* to the USB port connection on the analyzer before
performing analysis.
*Must be purchased separately.
29
Downloaded from www.Manualslib.com manuals search engine
Set-Up Barcode Reader
30
Downloaded from www.Manualslib.com manuals search engine
The barcode reader can be used for data entry of Patient ID, Operator ID, Lab ID, Control
ID and Site ID
1. Connect cable from the barcode reader* to the USB port connection on the analyzer
before performing analysis.
For barcode reader settings refer to barcode reader operating manual.
*Must be purchased separately.
31
Downloaded from www.Manualslib.com manuals search engine
Set-Up Connectivity
1 2
32
Downloaded from www.Manualslib.com manuals search engine
The analyzer can be connected to a PC/Server for transfer of data via USB cable* or LAN
cable*.
1. Connection via USB: Connect cable* from the PC* to the USB B connection on the ana-
lyzer.
2. Connection via LAN: Connect cable* from the LAN port to the LAN connection on the
analyzer.
The data is sent according to the POCT1-A standard. For more information about the con-
nectivity and how to configure the IP address settings when connecting via the LAN port,
please contact HemoCue AB.
*Must be purchased separately.
33
Downloaded from www.Manualslib.com manuals search engine
Blood Collection Capillary Sample
1 2 3
34
Downloaded from www.Manualslib.com manuals search engine
Important! Always handle blood specimens with care, they might be infectious.
Consult local environmental authorities for proper disposal. Always wear protective
gloves when handling blood specimens. The microcuvette is for single use only.
For measuring procedure see Routine Use/Patient Test section.
1. Make sure the patient’s hand is warm and relaxed. Use only the middle or ring finger
for sampling. Avoid fingers with rings on.
2. Clean fingertip with disinfectant and allow to dry completely or wipe off with a dry,
lint-free wipe.
3. Using your thumb, lightly press the finger from the top of the knuckle towards the tip.
35
Downloaded from www.Manualslib.com manuals search engine
4 5 6
36
Downloaded from www.Manualslib.com manuals search engine
4. Sample at the side of the fingertip.
5. While applying light pressure towards the fingertip, puncture the finger using a lancet*.
6. Wipe away the first two or three drops of blood.
7. Re-apply light pressure towards the fingertip until another drop of blood appears.
*In order to obtain a sufficient blood flow and representative sample, high flow lancet with
blade is preferred.
37
Downloaded from www.Manualslib.com manuals search engine
8 9 10
38
Downloaded from www.Manualslib.com manuals search engine
8. When the blood drop is large enough, fill the microcuvette in one continuous process.
Do NOT refill!
NOTE: Make sure that the microcuvette is filled from the tip, forming an angle (about
45 °) with the blood drop.
9. Wipe off excess blood from the outside of the microcuvette with a clean, lint-free wipe.
Do not touch the open end of the microcuvette.
10. Look for air bubbles in the filled microcuvette. If present, discard the microcuvette and
fill a new microcuvette from a new drop of blood.
NOTE: Make sure that the microcuvette is filled correctly as an improper filling angle might
cause air bubbles.
NOTE: If a second sample is to be taken, it is important that this is done after the measure-
ment of the first sample is completed. Wipe away the remains of the drop of blood and fill
the second microcuvette from a new drop of blood as per steps 8–10 above.
NOTE: HemoCue WBC DIFF is not validated for heel stick.
39
Downloaded from www.Manualslib.com manuals search engine
Blood Collection Venous Sample and Control Material
1 2a 2b
3 4 5
40
Downloaded from www.Manualslib.com manuals search engine
Important! Always handle blood specimens with care, they might be infectious.
Consult local environmental authorities for proper disposal. Always wear protective
gloves when handling blood specimens. The microcuvette is for single use only.
For the measuring procedure see Routine Use/QC Test or Routine Use/Patient Test.
Control material shall always be measured using QC test procedure.
1. Venous blood samples shall be stored at room temperature (18–30 °C, 64–86°F) and
analyzed within eight hours after sample collection. Mix the venous sample tubes
thoroughly using a roller mixer for 1-2 minutes or invert the tube 10–20 times by hand.
Samples may not be diluted. For control material always follow instructions for use
provided by the manufacturer.
2. Place a drop of blood or control material onto a hydrophobic surface using a pipette (2a)
or other suitable transfer device (2b).
3. Fill the microcuvette in one continuous process. Do NOT refill!
NOTE: Make sure that the microcuvette is filled from the tip, forming an angle (about
45 °) with the blood drop.
4. Wipe off excess blood from the outside of the microcuvette with a clean, lint-free wipe.
Do not touch the open end of the microcuvette.
5. Look for air bubbles in the filled microcuvette. If present, discard the microcuvette and
fill a new microcuvette from a new drop of blood.
41
Downloaded from www.Manualslib.com manuals search engine
Routine Use Patient Test
1 2 3
4 5
42
Downloaded from www.Manualslib.com manuals search engine
Patient Test stands for performing tests on samples from patients.
1. Start the analyzer as described in Start-Up section. Place the cuvette moving arm in
loading position.
2. The display will show the main menu. Take a microcuvette from the package
(HemoCue WBC DIFF Microcuvette or HemoCue WBC Microcuvette). For single
packaged microcuvettes; open the inner wrapping and take out the microcuvette.
3. Press the button below Patient Test symbol.
4. Enter required data (selected in settings) e.g. Operator ID, Patient ID, Lab ID, and Site
ID. To enter data see Data Entry section.
5. If data has been entered the data confirmation window is shown. Press right button to
confirm. To change information, press left button until the desired window is shown.
43
Downloaded from www.Manualslib.com manuals search engine
6 7a 7b
44
Downloaded from www.Manualslib.com manuals search engine
6. Insert cuvette symbol is shown.
7. Take a sample according to Blood Collection Capillary Sample or Blood Collection
Venous Sample and Control Material sections. Place the microcuvette into the cuvette
holder (7a) and start measurement as soon as possible but no later than 1 minute after
filling the microcuvette by gently pushing the cuvette holder to its measuring position
(7b).
8. During measurement the measuring window is shown.
45
Downloaded from www.Manualslib.com manuals search engine
9a 9b 9c
10
46
Downloaded from www.Manualslib.com manuals search engine
9. The results are displayed within < 5 minutes,
9a. WBC DIFF result window
9b. WBC result window
9c. Entered data
The result windows may differ due to data entered and choice of microcuvette. To jump
between the result and data entered windows press button below the Switch symbol.
Confirm result by pressing button below the Accept symbol. Reject result by pressing
button below the Reject symbol. A rejected result will be saved but marked with a flag
as rejected. The display shows the main menu.
NOTE: Do not remeasure the filled microcuvette.
10. Always handle blood specimens with care, they might be infectious. Consult local envi-
ronmental authorities for proper disposal. Always wear protective gloves when handling
blood specimens. The microcuvette is for single use only.
47
Downloaded from www.Manualslib.com manuals search engine
Routine Use QC Test
1 2 3
4 5 6
48
Downloaded from www.Manualslib.com manuals search engine
QC Test means performing tests on control material.
NOTE: Do not analyse patient samples in QC Test mode.
1. Start the analyzer as described in the Start-Up section. Place the cuvette moving arm in
loading position.
2. The display will show the main menu. Take a microcuvette from the package
(HemoCue WBC DIFF Microcuvette or HemoCue WBC Microcuvette). For single
packaged microcuvettes; open the wrapping and take out the microcuvette.
3. Press right button for Menu/QC Test.
4. Press center button to scroll down and choose QC Test. Press right button to accept QC
Test and move to the next window.
5. Press right button to confirm QC test.
6. Enter required data (selected in settings) e.g. Operator ID, Control ID.
49
Downloaded from www.Manualslib.com manuals search engine
7 8 9a
9b 10
50
Downloaded from www.Manualslib.com manuals search engine
7. If data has been entered the data confirmation window is shown. Press right button to
confirm. To change information, press left button until the desired window is displayed.
8. Insert cuvette symbol is shown.
9. Take a sample according to Blood Collection Venous Sample and Control Material sec-
tions. Place the microcuvette into the cuvette holder (9a) and start measurement as soon
as possible but no later than 1 minute after filling the microcuvette by gently pushing
the cuvette holder to its measuring position (9b). It will automatically slide into the cor-
rect position and the measurement starts.
10. During measurement the measuring window is displayed.
51
Downloaded from www.Manualslib.com manuals search engine
11a 11b 11c
12
52
Downloaded from www.Manualslib.com manuals search engine
11. The results are displayed within < 5 minutes,
11a. WBC DIFF result window
11b. WBC result window
11c. Entered data
The result windows may differ due to data entered and choice of microcuvette. To jump
between result and data windows; press the center button.
Confirm result by pressing button below the Accept symbol. Reject result by pressing
button below the Reject symbol. A rejected result will be saved but marked with a flag
as rejected. After rejecting the result main menu is shown.
NOTE: Do not remeasure the filled microcuvette.
12. Always handle blood specimens with care, they might be infectious. Consult local envi-
ronmental authorities for proper disposal. Always wear protective gloves when handling
blood specimens. The microcuvette is for single use only.
53
Downloaded from www.Manualslib.com manuals search engine
Review/Delete Results
1 2 3
4 5 6
54
Downloaded from www.Manualslib.com manuals search engine
Use Set-Up/Settings menu to review/delete results. The analyzer can store up to 600 results.
1. Start the analyzer as described in the Start-Up section.
2. Press the button below the Menu/QC Test symbol.
3. Press right button to accept menu and to move to next window.
4. Enter PIN Code window is shown. The Pin Code is used to prevent unauthorized users
from entering/changing input in the settings menu. As default, the PIN Code is set to
0000. For changing the PIN Code see step 19 in the Settings section.
Enter PIN code by pressing the button below up arrow to change digit. Press right
button to confirm and move to next digit. After entering the four digit PIN Code press
right button to accept and move to next window.
5. The display shows: Home, Review/Delete and Settings. Press center button to enter
Review/Delete.
6. The display shows Review/Delete window. Select review by pressing the button below
Review symbol and move to number 7. Select Delete by pressing the button below
Delete symbol and move to number 9.
55
Downloaded from www.Manualslib.com manuals search engine
7 8 9
56
Downloaded from www.Manualslib.com manuals search engine
Review Results
7. The latest results are displayed. To jump between result and data windows; press center
button. The result windows may differ due to data entered and type of microcuvette.
Return to main menu by pressing center button and then left button.
8. To jump between result and data windows; press center button.
Press left button to return to main menu.
Delete Results
9. Delete window is shown. Press right button to delete all results stored in the analyzer.
To cancel press left button. The main menu is shown.
NOTE: Single results can not be deleted.
57
Downloaded from www.Manualslib.com manuals search engine
Maintenance Daily Maintenance
1 2 3
4 5
58
Downloaded from www.Manualslib.com manuals search engine
Clean the cuvette holder after each day of use.
1. Turn off the analyzer. Place the cuvette moving arm in loading position.
2. Remove cuvette holder by lifting it straight up.
3. Clean the cuvette holder with alcohol (20-70%) or mild detergent.
NOTE: Do not autoclave.
If the optical parts are stained an error code is displayed. Clean according to
Maintenance/Optical Parts section.
4. Wait 15 minutes before replacing the cuvette holder.
5. Place the cuvette moving arm in loading position before replacing the cuvette holder.
Clean the cover with alcohol (20-70%) or a mild detergent.
Only the WBC DIFF cuvette holder is to be used with the WBC DIFF analyzer.
The WBC DIFF cuvette holder is marked WBC DIFF.
59
Downloaded from www.Manualslib.com manuals search engine
Maintenance Optical Parts
1 2a 2b
3 4
60
Downloaded from www.Manualslib.com manuals search engine
If the optical parts of the WBC DIFF Analyzer are dirty an error code is displayed. Use the
HemoCue Cleaner WBC for cleaning optical parts.
1. Turn off the analyzer. Place the cuvette moving arm in loading position, leave cuvette
holder in place.
2. Hold HemoCue Cleaner WBC* with the HemoCue logotype facing upwards. Push the
cleaner as far as possible into the opening of the optic unit. Move back and forth along
the cuvette holder 5-10 times (2a). The optical parts are placed on the left in the open-
ing. Move the cleaner alongside of the cuvetteholder to get the right angle for cleaning
the optical parts (2b).
3. Move from side to side 5-10 times.
If the cleaner is stained, repeat using a new cleaner.
4. Wait 15 minutes before performing a new measurement.
*One HemoCue Cleaner WBC is included with the analyzer. Contact your local distributor
to order additional cleaners.
NOTE: Make sure the cuvette moving arm is in loading position with cuvette holder in
place.
NOTE: Dispose of the cleaner as potentially infectious waste. Do not reuse.
Only the WBC DIFF cuvette holder is to be used with the WBC DIFF analyzer.
The WBC DIFF cuvette holder is marked WBC DIFF.
61
Downloaded from www.Manualslib.com manuals search engine
GB
Troubleshooting Guide
The HemoCue WBC DIFF system will display a flag or an Error Code if a problem is detected within the system or the sample. In the
trouble shooting guide are explanations and recommended actions for each flag or Error Code described.
If the Troubleshooting Guide is unable to resolve the problem, please contact your local HemoCue distributor or HemoCue AB. The ana-
lyzer should be cleaned as described in the Maintenance section prior to service or disposal. The analyzer has no serviceable parts.
NOTE: Do not open the cover of the analyzer. The warranty is void if the cover of the analyzer is opened.
62
Downloaded from www.Manualslib.com manuals search engine
Flag Explanation
The results in the differential count are uncertain. The sample
may contain pathological, abnormal or unidentified cells.
The sample should be verified with a suitable laboratory method
and be questioned as to the pathological condition of the patient.
The printout will show an asterisk (*) instead of the display
symbol
The differential count is not presented when the total WBC is
below 1 x 109/L.
If a differential count is required the sample should be verified
with a suitable laboratory method.
63
Downloaded from www.Manualslib.com manuals search engine
Flag Explanation
The measured total white blood cell count is below 0.3 x 109/L.
The sample should be verified with a suitable laboratory method.
The measured white blood cell count is above 30.0 x 109/L.
The sample should be verified with a suitable laboratory method
64
Downloaded from www.Manualslib.com manuals search engine
The HemoCue WBC DIFF system will display an Error Code if a problem occurs impacting the performance of an analysis. Each error
code is numbered and related to a specific cause. If an error code appears, accept the error code and continue according to the action
described for the specific error code in the trouble shooting guide.
Error code Explanation Action
Non-critical error (Err01, Err02, Err04, Err05, Confirm error code. Continue according to action described for
Err31, Err33) error code shown.
Critical error (Err03, Err30, Err34, Err35, Err60, Confirm error code. The analyzer will automatically turn off.
Err69, Err90) Restart the analyzer and continue according to action described
for error code shown.
Err01 Part of the image area can not be analyzed. 1. Take a new microcuvette, repeat the measurement as descri-
Potential causes may be: bed in Routine Use Patient Test or QC Test sections.
• Airbubbles in the sample. 2. If the problem persists, the sample should be verified with a
• Incorrect handling of the sample suitable laboratory method.
• Abnormalities in sample.
Err02 Uneven spatial distribution of detected cells. Take a new microcuvette, repeat the measurement as described
in Routine Use Patient Test or QC Test sections.
Err03 Image, or part of the image area is out-of-focus. 1. Take a new microcuvette, repeat the measurement as de-
scribed in Routine Use Patient Test or QC Test sections.
2. If the problem persists, the analyzer needs service. Contact
your local distributor.
Err04 Acceptable light level cannot be achieved. 1. Take a new microcuvette, repeat the measurement as de-
scribed in Routine Use Patient Test or QC Test sections.
2. If the problem continues, the analyzer needs service. Contact
your local distributor.
Err05 Cuvette holder is inserted before Patient Test is Remove the microcuvette. Take a new microcuvette, repeat the
selected. measurement as described in Routine Use Patient Test or QC
Test sections.
NOTE: Make sure the “insert cuvette” symbol is displayed
before inserting a new microcuvette.
65
Downloaded from www.Manualslib.com manuals search engine
Error code Explanation Action
Err30 The optical parts are dirty or wet after cleaning. 1. Clean the optical parts as described in the Maintenance sec-
tion. Wait 15 minutes before starting the analyzer after cleaning
to make sure that the optical parts are dry.
2. If the problem persists, the analyzer needs service. Contact
your local distributor.
Err31 Memory error. 1. Restart the analyzer as described in the Start-Up section.
2. If the problem persists, choose one of the alternatives:
· Remove all measurements as decribed in Review/Delete Re-
sults section. NOTE: All stored data will be deleted.
· The analyzer needs service. Contact your local distributor.
Err33 Empty microcuvette, not filled with sample. Take a new microcuvette, repeat the measurement as described
in Routine Use Patient Test or QC Test sections. Make sure that
the microcuvette is filled with sample.
Err34 Stray light detected. 1. Make sure that the analyzer is not exposed to any bright light
sources.
2. If the problem continues, the analyzer needs service. Contact
your local distributor.
Err35 The battery power is too low. Replace the batteries, six type C (LR14/HR14) batteries, 1.5 V
or use the AC adapter. All described in the Start-Up section.
Err60 General hardware error. Try one or more of the following:
1a) Clean the optical parts as described in Maintenance section.
1b) If an error code appears when connecting a USB device,
remove the device and start the analyzer as described in Start-
Up section.
1c) Wait 30 seconds and start the analyzer.
2 If the problem persists, the analyzer needs service. Contact
your local distributor.
Err69 Configuration error. The analyzer needs service. Contact your local distributor.
66
Downloaded from www.Manualslib.com manuals search engine
Error code Explanation Action
Err90 Internal error. 1. Wait 30 seconds, start the analyzer as decribed in Start-Up
section.
Take a new microcuvette, repeat the measurement as described
in Routine Use Patient Test or QC Test sections
2. If the problem persists, the analyzer needs service. Contact
your local distributor.
Other Errors
No characters on the display 1. No power. 1a. Check that the AC adapter is properly connected to the
2. If on battery power, the batteries need to be analyzer.
replaced. 1b. Check the cable for damages.
3. The display is not working. 2. Replace the batteries, six type C (LR14/HR14) batteries, 1.5
V as described in Start-Up section.
3. The analyzer needs service. Contact your local distributor.
The display shows 1. The display is out of order. The analyzer needs service. Contact your local distributor.
erroneous characters 2. The microprocessor is out of order.
The cuvette holder is not mov- The magnet in the cuvette holder is missing. The analyzer needs service. Contact your local distributor.
ing into correct position.
Measurement on patient 1. Improper sampling technique. 1. Take a new microcuvette, repeat the measurement, as de-
samples are higher or lower 2. The microcuvettes are damaged, not properly scribed in Routine Use Patient Test section.
than anticipated. stored or have passed the expiry date. 2. Check expiry date and storage conditions of the microcvettes.
3. The sample has not been properly stored. 3. Check storage conditions of the sample.
67
Downloaded from www.Manualslib.com manuals search engine
GB
Specifications
Intended Purpose/Intended Use Warning and Precaution
The HemoCue WBC DIFF system is an In-Vitro diagnostic system The microcuvettes are for In Vitro Diagnostic use only. Always
designed for quantitative determination of white blood cells handle blood specimens with care, they may be infectious. Consult
(WBC) in capillary or venous whole blood. The system provides local environment authorities for proper disposal. Always wear
values for a total white blood cell count and a differential count protective gloves when handling blood specimens. The micro-
including neutrophil count, lymphocyte count, monocyte count, cuvettes are for single-use only. Do not analyse patient samples in
eosinophil count and basophil count. QC test mode.
The HemoCue WBC DIFF system is indicated for use in clinical Storage and Handling
laboratories and for point of care testing in professional health Microcuvettes
care settings on pediatric (≥ 3 months) and adult patients. The microcuvettes are to be stored at 15–35 °C (59–95 °F), <90%
The HemoCue WBC DIFF Analyzer is only to be used with non-condensing humidity. An unopened vial/capsule of micro-
HemoCue WBC DIFF Microcuvettes for measurement of a total cuvettes can be stored for a shorter period of time (4 weeks)
white blood cell count and a differential white blood cell count or outside the specified storage conditions, down to 4 °C (39 °F) and
with HemoCue WBC Microcuvettes for measurement of a total up to 50 °C (122 °F). Allow the microcuvettes to reach 18-30° C
white blood cell count only. (64-86 °F) before use. For individually packaged microcuvettes:
once the wrapping of a single packaged microcuvette is opened it
IVD Medical Device Directive shall be used within 10 minutes. For microcuvettes packaged in
The HemoCue WBC DIFF system complies with the IVD Medical vial: once the seal of the vial is broken, the microcuvettes shall be
Device Directive 98/79/EC and carries the CE mark. used within 3 months. Always keep the vial properly closed.
Use the microcuvettes prior to the expiry date which is printed on
Principles of the Method/Procedure the package. The microcuvettes should be stored in the original
Principle of the method package.
A hemolyzing agent lyses the red cells in the microcuvette and a Analyzer
staining agent colours the white cells. Several images are taken of The analyzer can be stored at 4–50 °C (39–122 °F), <90% non-
the stained white cells and cells are classified. The number of cells condensing humidity for four weeks.
are counted by image analysis in the analyzer. Allow the analyzer to reach ambient temperature before use.
Principle of the procedure Operating temperature
The microcuvette is for single-use only and serves as a sample For optimal performance of the HemoCue WBC DIFF system, the
container and reaction chamber. A blood sample of approximately operating temperature should be:
10 µL is drawn into the cavity by capillary action. The micro Venous/capillary sample in EDTA tube: 18 - 30° C (64-86 °F),
cuvette is placed in the analyzer and within <5 minutes the result <90% non-condensing humidity
is obtained. The system is designed to establish agreement with: Capillary sample from fingerstick: 18 - 25° C (64-77 °F), <90%
• Manual method for the differential white blood cell count. non-condensing humidity.
• Manual method for total white blood cell count.
No calibration is needed as the system is factory calibrated
68
Downloaded from www.Manualslib.com manuals search engine
Specimen Collection and Preparation
Capillary or venous whole blood may be used. EDTA anti-coagu-
lant should be used. Do not dilute sample.
Venous blood samples and capillary blood samples in EDTA
tubes shall be stored at room temperature 18–30°C, (64–86°F).
Stability: venous samples 8h, capillary samples 4h.
Materials Required
• HemoCue WBC DIFF Analyzer
• HemoCue WBC DIFF Microcuvettes and/or HemoCue WBC
Microcuvettes
• Lancet (capillary samples)
• Pipette or other transfer device (venous samples)
• Lint-free tissue (non-fraying)
Quality Control
When the HemoCue WBC DIFF Analyzer is started, a quality
control (self-test) is automatically performed to verify perfor-
mance. If the test fails, an error code will be displayed.
For each measurement, quality controls are made on:
• The HemoCue WBC DIFF Analyzer.
• The HemoCue WBC DIFF Microcuvettes or WBC Microcu-
vettes.
• The sample.
• The handling of microcuvettes and sample.
No additional quality controls performed by the operator are
required for functionality verification.
69
Downloaded from www.Manualslib.com manuals search engine
Expected Values (Dacie and Lewis Practical Haematology)
White Blood Cell values for normal children expressed as a mean ±2SD (95% Range)
3-6 Months 1 Year 2-6 Years 6-12 Years
White Blood Cell Count x109/L 12±6 11±5 10±5 9±4
9
Neutrophils x10 /L 1-6 1-7 1.5-8 2-8
Lymphocytes x109/L 4-12 3.5-11 6-9 1-5
9
Monocytes x10 /L 0.2-1.2 0.2-1.0 0.2-1.0 0.2-1.0
Eosinophils x109/L 0.1-1.0 0.1-1.0 0.1-1.0 0.1-1.0
70
Downloaded from www.Manualslib.com manuals search engine
Expected Values (Dacie and Lewis Practical Haematology)
White Blood Cell values for normal adults expressed as a mean ±2SD (95% Range)
Adults (x109/L) Adults (%)
White Blood Cell Count 4.0-10.0 NA
Neutrophils 2.0-7.0 40-80
Lymphocytes 1.0-3.0 20-40
Monocytes 0.2-1.0 2-10
Eosinophils 0.02-0.5 1-6
Basophils 0.02-0.1 <1-2
The values above may vary due to a wide range of factors, such as sex, diurnal variations, exercise, physical stress or trauma, pregnancy,
indigestion of food, and cigarette smoking.
71
Downloaded from www.Manualslib.com manuals search engine
Measuring Range Capillary Sampling
Displayed range for total WBC count: 0.3–30.0 x109/L (300– When a capillary skin puncture is performed, several
30000/mm 3, 300–30000/µL) Results above the measuring range defense systems in the body are activated very quickly.
will be displayed as HHH. Results below measuring range will be These defense systems cause an increase in the number
displayed as LLL. of WBCs in the blood closest to the wound, leading to
greater differences in results from several samples taken
The differential cell count will be presented when the total white from the same finger stick.
blood cell count is between 1.0–30.0 x 109/L.
Limit of Detection
Limit of Detection is defined as the lowest amount of analyte that
can be detected with probability.
Limit of Detection has been determined to be 0.3 x 109/L. (CLSI
Document EP17-A) for WBC count.
Limitations of the Method/Procedure
a) The measurement needs to be started no later than 1 minute
after filling the microcuvette.
b) Do not remeasure a filled microcuvette.
c) Mixing samples for an extended period may affect the result.
d) Results above the measuring range will be displayed as
HHH. Results below the measuring range will be displayed
as LLL.
e) Discard sample if clots.
f) For interference studies see chapter Known Interferences.
Specific Performance Characteristics
Linearity
The white blood cell count for the WBC DIFF system has accord-
ing to the FDA Guidance Premarket Notification for Automated
Differential Cell Counters for Immature or Abnormal Blood
Cells, been demonstrated to be linear between 0.3 – 30.0 x 109/L
with a correlation coefficient (r) to 0.999.
72
Downloaded from www.Manualslib.com manuals search engine
Repeatability
A study has been performed according to CLSI Document H26-A2 using fresh blood samples to determine repeatability at low, normal
and high concentrations. The study was performed on venous whole blood samples tested with 31 replicates per sample using 1 analyzer
and 1 batch of cuvettes.
WBC Total WBC Neutrophils Lymfocytes Monocytes Eosinophils
N Level
Mean SD CV Mean SD CV Mean SD CV Mean SD Mean SD
(x109/L) (x109/L) (%) (x109/L) (x109/L) (%) (x109/L) (x109/L) (%) (x109/L) (x109/L) (x109/L) (x109/L)
31 Low 2.8 0.14 5.0 1.5 0.11 7.3 1.0 0.08 7.8 0.2 0.03 0.1 0.02
31 Normal 6.1 0.17 2.8 3.4 0.13 3.7 2.2 0.10 4.6 0.3 0.07 0.1 0.03
31 High 18.1 0.57 3.1 14.4 0.61 4.3 2.8 0.26 9.5 0.8 0.14 0.1 0.05
73
Downloaded from www.Manualslib.com manuals search engine
Method Comparison Study - Venous Samples
Results from a method comparison study performed according to CLSI Document EP 9-A2 on the HemoCue WBC DIFF system and the
Beckman Coulter LH750 are shown for total WBC count in Figure 1, for neutrophil count in Figure 2 and for lymphocytes in Figure 3.
30 30 30
Coefficients: Coefficients: Coefficients:
y = -0.24 + 1.01x y = 0.16 + 0.96x y = 0.20 + 0.90x
25 n = 762 25 n = 596 25
n = 596
r2 = 0.994 r2 = 0.968
HemoCue WBC DIFF, Lymphocytes (x109/L)
r2 = 0.930
HemoCue WBC DIFF, Neutrophils (x109/L)
r = 0.997 r = 0.984
HemoCue WBC DIFF total WBC (x109/L)
r = 0.964
20 y=x 20 y=x 20
y=x
15 15 15
10 10 10
5 5 5
0 0 0
0 5 10 15 20 25 30 0 5 10 15 20 25 30 0 5 10 15 20 25 30
Beckman Coulter LH750, total WBC (x109/L) Beckman Coulter LH750, Neutrophils (x109/L) Beckman Coulter LH750, Lymphocytes (x109/L)
Figure 1 Total WBC Figure 2 Neutrophils Figure 3 Lymphocytes
74
Downloaded from www.Manualslib.com manuals search engine
Method Comparison Study - Capillary Samples Direct from Finger
Results from a method comparison study performed on the HemoCue WBC DIFF system and the Sysmex XS-1000i are shown for total
WBC count in Figure 1, for neutrophil count in Figure 2 and for lymphocytes in Figure 3. Capillary sample for Sysmex was taken in a
microtube.
Coefficients: Coefficients: Coefficients:
y = 0.47 + 0.94x y = 0.33 + 0.88x y = 0.27 + 1.10x
n = 103 n = 96 n = 96
r2 = 0.859 r2 = 0.867
HemoCue WBC DIFF, Lymphocytes (x109/L)
r2 = 0.807
HemoCue WBC DIFF, Neutrophils (x109/L)
r = 0.927 r = 0.931
HemoCue WBC DIFF total WBC (x109/L)
r = 0.898
y=x y=x y=x
Sysmex XS-1000i, total WBC (x109/L) Sysmex XS-1000i, Neutrophils (x109/L) Sysmex XS-1000i, Lymphocytes (x109/L)
Figure 1 Total WBC Figure 2 Neutrophils Figure 3 Lymphocytes
75
Downloaded from www.Manualslib.com manuals search engine
Known Interference Essential Performance
Nucleated red blood cells (NRBC) may be counted as white The HemoCue WBC DIFF system is an In-Vitro diagnostic system
blood cells and give falsely elevated total leukocyte count. This designed for quantitative determination of white blood cells
will mainly be reflected in the differential count as an elevated (WBC) in capillary or venous whole blood. The system provides
lymphocyte count. values for a total white blood cell count and a differential white
Cold agglutination or cryoglobulin may interfere. blood cell count including neutrophil count, lymphocyte count,
Low Hb (<80 g/L) may cause an increased level of flags. monocyte count, eosinophil count and basophil count.
NOTE: Discard sample if clots.
Technical Specifications
Dimensions: 188x157x155 mm (7.40 x 6.18 x 6.10 inches)
Weight: 1300 g (2.87 pounds) (with 6 C (LR14/HR14) batteries
installed)
AC adapter: CE marked
Only use AC adapters listed under AC Adapters.
Pollution degree: 2
Overvoltage category: II
Atmospheric pressure: 700 hPa to 1060 hPa.
Equipment not suitable for use in the presence of flammable
mixtures.
The HemoCue WBC DIFF system has been tested for electrical
safety and EMC according to the following standards:
• IEC 61010-1 Safety requirements for electrical equipment for
measurement, control, and laboratory use - Part 1: General
requirements
• IEC 61010-2-101 Safety requirements for electrical equipment
for measurement, control and laboratory use – Part 2-101:
Particular requirements for in vitro diagnostic (IVD) medical
equipment.
• UL 61010/CSA-C22.2 No. 61010-1 Safety requirements for
electrical equipment for measurement, control and laboratory
use – Part 1: General requirements.
• IEC/EN 60601-1-2 Ed 3.0 Medical electrical equipment-Part 1:
General requirements for Safety – Part 1-2: Collateral Standard:
Electromagnetic compability – requirements and tests.
76
Downloaded from www.Manualslib.com manuals search engine
Recommended separation distance between Portable and NOTE 1 At 80 MHz and 800 MHz, the separation distance for
mobile RF communications equipment and HemoCue WBC the higher frequencyrange applies.
DIFF Analyzer
The HemoCue systems are intended for use in an electromagnetic NOTE 2 These guidelines may not apply in all situations. Elec-
environment in which radiated RF disturbances are controlled. tromagnetic propagation is affected by absorption and reflection
The customer or the user of HemoCue systems can help prevent from structures, objects and people.
electromagnetic interference by maintaining a minimum distance
between portable and RF communications equipment (transmit-
ters) and HemoCue systems as recommended below, according to
the maximum output power of the communications equipment.
Rated maxi- Separation distance according to fre-
mum output quency of transmitter (m)
power of
150 kHz 80 MHz to 800 MHz to
transmitter
to 80 MHz 800 MHz 2.5 GHz
(W)
d=1.2√P d=1.2√P d=2.3√P
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at maximum output power not listed above,
the recommended separation distances (d) in meters (m) can be
estimated using the equation applicable to the frequency of the
transmitter, where (P) is the maximum output power rating of the
transmitter in watts (W) according to transmitter manufacturer.
77
Downloaded from www.Manualslib.com manuals search engine
Guidance and manufacturer’s declaration – Electromagnetic immunity
The HemoCue systems are intended for use in the electromagnetic environment specified below. The customer or user of the
HemoCue systems should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment – guidance
Electrostatic discharge ±6 kV contact ±6 kV contact Floors should be wood, concrete or ceramic tile.
(ESD) If floors are covered with synthetic material, the
IEC 61000-4-2 ±8 kV air ±8 kV air relative humidity should be at least 30 %.
Electrical fast transient/ ±2 kV for power supply 2 kV for power Mains power quality should be that of a typical
burst lines supply lines commercial or hospital environment.
IEC 61000-4-4 ±1 kV for input/output lines ±1 kV for input/
output lines
Surge ±1 kV differential mode ±1 kV differential Mains power quality should be that of a typical
mode commercial or hospital environment.
IEC 61000-4-5
Voltage dips, short interrup- <5%U (>95% dip in U) for <5%U (>95% dip Mains power quality should be that of a typical
tions and a 0.5 cycle in U) for a 0.5 cycle commercial or hospital environment.
voltage variations on power
supply lines 40%U (60% dip in U) for 40%U (60% dip in If the user of the HemoCue systems image
5 cycles U) for 5 cycles intensifier requires continued operation during
IEC 61000-4-11 power mains interruptions, it is recommended
70%U (30% dip in U) for 70%U (30% dip in that the HemoCue systems image intensifier be
25 cycles U) for 25 cycles powered from an uninterruptible power supply
or a battery.
<5%U (>95% dip in U) for <5%U (>95% dip
5 seconds in U) for 5 seconds
For explanation of U see
NOTE 1
78
Downloaded from www.Manualslib.com manuals search engine
Immunity test IEC 60601 test level Compliance level Electromagnetic environment – guidance
Conducted RF 3 Vrms 3 Vrms Portable and mobile RF communications equip-
IEC 61000-4-6 150 kHz to 80 MHz ment should be used no closer to any part of the
HemoCue systems, including cables, than the
Radiated RF 3V/m 3 V/m recommended separation distance calculated
IEC 61000-4-3 80 MHz to 2.5 GHz from the equation applicable to the frequency of
the transmitter
See NOTE 2 and NOTE 3 Recommended separation distance
d=1.2√P
d=1.2√P 80 MHz to 800 MHz
d=2.3√P 800 MHz to 2.5 GHz
Where (P) is the maximum output power rating
of the transmitter in watts (W) according to the
transmitter manufacturer and (d) is the recom-
mended separation distance in meters (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey (a),
should be less than the compliance level in each
frequency range (b).
Interference may occur in the vicinity of equip-
ment marked with the following symbol.
79
Downloaded from www.Manualslib.com manuals search engine
NOTE 1 U is the a.c. mains voltage prior to application of the test level.
NOTE 2 At 80MHz and 800MHz, the separation distance for the higher frequency range applies.
NOTE 3 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects and people.
a) Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios,
amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the elec-
tromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field
strength in the location in which the HemoCue systems are used exceeds the applicable RF compliance level above, the HemoCue
systems should be observed to verify normal operation. If abnormal performance is observed, additional measures may be neces-
sary, such as re-orienting or relocating the systems.
b) Over the frequency range 150 KHz to 80 Mhz, field strength should be less than 3 V/m.
80
Downloaded from www.Manualslib.com manuals search engine
Technical specifications (EMC-RF)
Use only cables with the following specification:
USB shielded maximum 2 m
Serial shielded maximun 1.5 m
Guidance and manufacturer´s declaration – electromagnetic emissions
The HemoCue systems are intended for use in the electromagnetic environment specified below. The customer or the user of the
HemoCue systems should assure that it is used in such an environment.
Emission test Compliance Electromagnetic environment – guidance
RF emissions Group 1 The HemoCue systems uses RF energy only for its
internal function. Therefore, its RF emissions are
very low and not likely to cause any interference in
nearby electronic equipment
RF emissions Class B The HemoCue systems are suitable for use in all es-
tablishments, including domestic establishments and
those directly connected to the public low-voltage
Harmonic emissions Class A
power supply network that supplies buildings used
IEC 61000-3-2
for domestic purposes.
Voltage fluctuations/flicker emissions Complies
IEC 61000-3-3
81
Downloaded from www.Manualslib.com manuals search engine
AC Adapters
Country: EU/US/GB Spare Parts and Accessories
Type: HCA01 The following accessories and spare parts are available:
Input: 100V~ - 240V~/50-60 Hz/< 500mA • AC adapter
• Cuvette holder
Warning • HemoCue Cleaner WBC
The device is tested according to standard IEC 61010-2-101 and is • Printer
found to comply with the standard.
Despite this compliance it is impossible to predict any possible Patents
effects by other nearby standing instruments, (stationary, portable The product is protected by the following patents (or patents
or mobile units) or the possible impact of electromagnetic radi- pending):
ance. This is the reason we need to inform users of this analyzer SE 0601575-4, SE 0700958-2, US 11/822,159, EP 07808762.4,
that disturbance from other equipment might affect the perfor- SE 0601576-2, US 7,633,615, EP 07768988.3, SE 0800117-4,
mance of the analyzer. Should you note that this is the fact, please US 8,009,894, EP 09702056.4, SE 0500549-1, US 7,521,243,
contact your local HemoCue distributor. US 8,092,758
The HemoCue WBC DIFF system is intended for use in the elec-
tromagnetic environment specified in Technical specifications.
The customer or user of the HemoCue WBC DIFF system should
assure that it is used in such an environment. The HemoCue
WBC DIFF Analyzer uses RF energy only for its internal
function. Therefore, its RF emissions are very low and not likely
to cause any interference in nearby electronic equipment.
The HemoCue WBC DIFF system is suitable for use in all estab-
lishments, including domestic establishments and those directly
connected to the public low-voltage power supply network that
supplies buildings used for domestic purposes.
Warranty
The analyzer carries a 24-month warranty from the day of receipt.
After the warranty period service/repair is carried out at fixed
prices. Any other use of the system than recommended by the
manufacturer will void the warranty.
Service and Disposal
The analyzer should be cleaned as recommended under Mainte-
nance prior to service or disposal. Consult local environmental
authorities for proper disposal.
82
Downloaded from www.Manualslib.com manuals search engine
Symbols Used
To maintain safety use only AC adapter marked
Caution HCA01.
CE mark
References
Class II equipment • HemoCue WBC DIFF Microcuvettes package insert
• HemoCue WBC Microcuvettes package insert
• Dacie and Lewis, Practical Haematology Tenth edition.
Only valid within the European Community. Indi- • Class II Special Controls Guidance Document: Premarket
cates seperate collection for waste of electrical and Notifications for Automated Differential Cell Counters for
electronic equipment. I mmature or Abnormal Blood Cells; Final Guidance for Indus
try and FDA, Document issued at December 4, 2001
• CSLI Document H26-A2, Vol. 30, No. 14, Validation, Veri-
Serial port fication, and Quality Assurance of Automated Hematology
Analyzers
USB • CLSI Document EP09-A2-IR, Vol. 30, No. 17, Method Com-
parison and Bias Estimation Using Patient Samples
90 • CLSI Document EP17-A, Vol. 24, No. 34, Protocols for Deter-
5 Relative humidity, non-condensing mination of Limits of Detection and Limits of Quantitation
DC inlet
Manufacturer
HemoCue AB
Box 1204
Temperature SE-262 23 Ängelholm
Sweden
Phone: +46 77 570 02 10
Efficiency Level Fax: +46 77 570 02 12
E-mail: info@HemoCue.se
www.hemocue.com
Consult instructions for use.
83
Downloaded from www.Manualslib.com manuals search engine
HemoCue AB | Box 1204 | SE-262 23 Ängelholm | Sweden | Phone +46 77 570 02 10 | Fax +46 77 570 02 12 BERGSTENS, HBG
903501 140726 GB
info@hemocue.se | hemocue.com
Downloaded from www.Manualslib.com manuals search engine
You might also like
- 3BUA000146-600 A en System 800xa Batch Management 6.0 ConfigurationDocument564 pages3BUA000146-600 A en System 800xa Batch Management 6.0 ConfigurationVidmantasČergelisNo ratings yet
- Freud Bose CorrespondenceDocument17 pagesFreud Bose CorrespondenceSiddhi JangidNo ratings yet
- BioConsole 560 Operator and Reference ManualDocument150 pagesBioConsole 560 Operator and Reference Manualagnithium71% (7)
- BD FACSVia Instructions For UseDocument162 pagesBD FACSVia Instructions For UseGi MarieNo ratings yet
- CNC Machining Handbook: Building, Programming, and ImplementationFrom EverandCNC Machining Handbook: Building, Programming, and ImplementationNo ratings yet
- Swelab Alfa Plus User Manual - V11, 2015-05-20 - EN PDFDocument98 pagesSwelab Alfa Plus User Manual - V11, 2015-05-20 - EN PDFAlfonso Peñaranda100% (1)
- BH-70P Service ManualDocument48 pagesBH-70P Service ManualFreddy YandúnNo ratings yet
- XN-L x30 BasicOperation 2004 enDocument262 pagesXN-L x30 BasicOperation 2004 enmaha muhammedNo ratings yet
- Biobase Elisa Microplate Reader BK-EL10C User Manual 202103Document48 pagesBiobase Elisa Microplate Reader BK-EL10C User Manual 202103Victor BlancoNo ratings yet
- Decomat 4656 UserDocument16 pagesDecomat 4656 UserLowayNo ratings yet
- Electrical Properties of Normal and Scarred Skin: Y. T. Zhang ofDocument4 pagesElectrical Properties of Normal and Scarred Skin: Y. T. Zhang ofLowayNo ratings yet
- Allelic Discrimination Getting Started Guide: Applied Biosystems 7900HT Fast Real-Time PCR SystemDocument118 pagesAllelic Discrimination Getting Started Guide: Applied Biosystems 7900HT Fast Real-Time PCR SystemCristina CapitaoNo ratings yet
- Operator Manual Coaguchek XS En.144f0c61Document232 pagesOperator Manual Coaguchek XS En.144f0c61Kmo mastnNo ratings yet
- Neumodx™ Laboratory Developed Test (LDT) Supplement: Implementation of Ldts On The Neumodx™ 96 and 288 Molecular SystemsDocument114 pagesNeumodx™ Laboratory Developed Test (LDT) Supplement: Implementation of Ldts On The Neumodx™ 96 and 288 Molecular SystemsClaudia NeacșuNo ratings yet
- UM-263639-001 - B 2102-2104 PH - Cond - AnalyzerDocument101 pagesUM-263639-001 - B 2102-2104 PH - Cond - AnalyzerUlises TovarNo ratings yet
- BD FACSCelesta™ Flow Cytometer User's GuideDocument152 pagesBD FACSCelesta™ Flow Cytometer User's Guideservice iyadMedicalNo ratings yet
- DF745SGMax v5.9.1 09-19-2014Document90 pagesDF745SGMax v5.9.1 09-19-2014Batik DávidNo ratings yet
- Downloaded From Manuals Search EngineDocument41 pagesDownloaded From Manuals Search EngineJajang OonNo ratings yet
- Man 065361 AS AP Autosampler Man065361 ENDocument276 pagesMan 065361 AS AP Autosampler Man065361 EN84nguyenlehuyNo ratings yet
- DTR 8510Document64 pagesDTR 8510Wilkinson PereiraNo ratings yet
- Dionex ASE 350 Accelerated Solvent Extractor Operator's ManualDocument268 pagesDionex ASE 350 Accelerated Solvent Extractor Operator's ManualAhmad HamdounNo ratings yet
- Manual-55 009Document93 pagesManual-55 009zakariaNo ratings yet
- BD Facspresto™ Near-Patient Cd4 Counter: 651000 Instructions For UseDocument96 pagesBD Facspresto™ Near-Patient Cd4 Counter: 651000 Instructions For UseOSCAR RUHWEZANo ratings yet
- BD FACS Canto II Users GuideDocument250 pagesBD FACS Canto II Users GuideAlexandre ChavesNo ratings yet
- H-1000-5057 UCC Programmers ManualDocument92 pagesH-1000-5057 UCC Programmers ManualYobany MatosNo ratings yet
- 300 SeriesDocument86 pages300 SeriesDumisani Tawanda DubeNo ratings yet
- MedonicM32 User Manual V4 2015-04-01Document97 pagesMedonicM32 User Manual V4 2015-04-01Stefan JovanovicNo ratings yet
- Gas Monitor BeaconDocument53 pagesGas Monitor Beaconenriquepm06iNo ratings yet
- UserManual B600Document319 pagesUserManual B600manhdtvt.mtaNo ratings yet
- BDVDocument84 pagesBDVMuhammad NurhudaNo ratings yet
- E4401 90473 PDFDocument100 pagesE4401 90473 PDFDenny PerezNo ratings yet
- Mai DLM 77000-08 ENDocument420 pagesMai DLM 77000-08 ENSalvador Valero BermejoNo ratings yet
- Axiom Microbiome UGDocument94 pagesAxiom Microbiome UGTrần Thị Hồng ThịnhNo ratings yet
- CMV Quant PCRDocument82 pagesCMV Quant PCRyousrazeidan1979No ratings yet
- UC-3500 BO 1909 enDocument80 pagesUC-3500 BO 1909 enОлександрNo ratings yet
- Operator Manual: Nanotrace Moisture AnalyzerDocument112 pagesOperator Manual: Nanotrace Moisture AnalyzerDolfin ChefNo ratings yet
- 3BSE035981-600 A en System 800xa Control 6.0 AC 800M Binary and Analog Handling PDFDocument670 pages3BSE035981-600 A en System 800xa Control 6.0 AC 800M Binary and Analog Handling PDFFelix Eduardo Ossorio GarciaNo ratings yet
- Cobas c111Document38 pagesCobas c111Kiwon ShimNo ratings yet
- Instruction Manual HAAKE Viscotester 550Document90 pagesInstruction Manual HAAKE Viscotester 550Trung Bui100% (1)
- Register Assistant User Manual Release v5.1 © 2010-2018 Mentor Graphics CorporationDocument274 pagesRegister Assistant User Manual Release v5.1 © 2010-2018 Mentor Graphics Corporationdupipi100% (1)
- OLS 85 - UserMan - 2019 12 11 enDocument74 pagesOLS 85 - UserMan - 2019 12 11 enRenis RevajNo ratings yet
- Catalog Roche 2012Document180 pagesCatalog Roche 2012MrmohanakNo ratings yet
- Standard Operating Procedure For Manual Dispensing ToolsDocument90 pagesStandard Operating Procedure For Manual Dispensing ToolsPaaahMullerNo ratings yet
- HPV Qual PCRDocument53 pagesHPV Qual PCRyousrazeidan1979No ratings yet
- Beacon 200Document53 pagesBeacon 200fullmarineNo ratings yet
- Autotrol Logix 764 Owners ManualDocument66 pagesAutotrol Logix 764 Owners Manualitstayyaba477No ratings yet
- Drive Server - Bus Server S7 Getting Started - v1-0 - ENDocument63 pagesDrive Server - Bus Server S7 Getting Started - v1-0 - ENmih abdouNo ratings yet
- Synergy HTX Operators Manual - 1341000 Rev ADocument184 pagesSynergy HTX Operators Manual - 1341000 Rev Ataniamariaguzman_353No ratings yet
- Technical Manual XeniaDocument414 pagesTechnical Manual XeniaSIELAB C.A.No ratings yet
- Olp-87 Manuel Utilisateur Anglais 0Document128 pagesOlp-87 Manuel Utilisateur Anglais 0Didier HOUNDETONNo ratings yet
- Drichem 4000Document121 pagesDrichem 4000Irvin VillaltaNo ratings yet
- CLINITEK Status Plus Operators Manual Rev C-2011-12 Eng-US 1800000005980302Document170 pagesCLINITEK Status Plus Operators Manual Rev C-2011-12 Eng-US 1800000005980302Malcolm LeeNo ratings yet
- User Guide FlowscannerDocument137 pagesUser Guide FlowscannerJDavid NavaNo ratings yet
- Direct Probe User GuideDocument80 pagesDirect Probe User Guidepae242729No ratings yet
- Operator Manual: Nanotrace Moisture AnalyzerDocument98 pagesOperator Manual: Nanotrace Moisture AnalyzerDolfin ChefNo ratings yet
- Unicel DxI Operator's GuideDocument436 pagesUnicel DxI Operator's Guidesantosh100% (1)
- DPA-4.1 e 20071016 v4Document97 pagesDPA-4.1 e 20071016 v4Williams Medina100% (1)
- DOC00301 Verix EVo Volume I Operating System Programmers Manual PDFDocument900 pagesDOC00301 Verix EVo Volume I Operating System Programmers Manual PDFh432fh9832hf98hdsjfhNo ratings yet
- XN-550/XN-450 /XN-350: Basic OperationDocument266 pagesXN-550/XN-450 /XN-350: Basic Operationtartaglia2002No ratings yet
- Cell-Dyn 1600 System: Operator'S ManualDocument189 pagesCell-Dyn 1600 System: Operator'S ManualNguyễnVănLượngNo ratings yet
- Vanquish Charged Aerosol Detectors (VH-D20, VF-D20) - Operating ManualDocument182 pagesVanquish Charged Aerosol Detectors (VH-D20, VF-D20) - Operating ManualNguyễnHoàngDanhNo ratings yet
- In Ven Sys License Manager GuideDocument80 pagesIn Ven Sys License Manager GuideantonioherediaNo ratings yet
- A255 User Guide For C500CDocument80 pagesA255 User Guide For C500CKen DizzeruNo ratings yet
- instrukcija СО 7Document23 pagesinstrukcija СО 7LowayNo ratings yet
- Heat Sealer Repair and Maintenance Kit IFU 2019-02-06Document27 pagesHeat Sealer Repair and Maintenance Kit IFU 2019-02-06LowayNo ratings yet
- The Validation of The Sterinis Machine: at Adelaide Meath Hospital, Tallaght, DublinDocument21 pagesThe Validation of The Sterinis Machine: at Adelaide Meath Hospital, Tallaght, DublinLowayNo ratings yet
- Hiperfet Power Mosfets: Ixfh14N80 Ixfh15N80Document6 pagesHiperfet Power Mosfets: Ixfh14N80 Ixfh15N80LowayNo ratings yet
- Online Neurowerk Product-Brochure-Eegemg 2018-02 enDocument10 pagesOnline Neurowerk Product-Brochure-Eegemg 2018-02 enLowayNo ratings yet
- Polaris 600Document96 pagesPolaris 600LowayNo ratings yet
- Bioimpedance Measurement System For Smart ClothingDocument10 pagesBioimpedance Measurement System For Smart ClothingLowayNo ratings yet
- FSN Monitor - FS-P2601D-User-GuideDocument48 pagesFSN Monitor - FS-P2601D-User-GuideLowayNo ratings yet
- PDF KBU6A-KBU6M FairchildDocument3 pagesPDF KBU6A-KBU6M FairchildLowayNo ratings yet
- Redacted For PrivacyDocument38 pagesRedacted For PrivacyLowayNo ratings yet
- Acculan 3ti Ga677Document146 pagesAcculan 3ti Ga677LowayNo ratings yet
- Nellcor N395 User ManualDocument110 pagesNellcor N395 User ManualLowayNo ratings yet
- Piezoelectric Properties Dry Human SkinDocument7 pagesPiezoelectric Properties Dry Human SkinLowayNo ratings yet
- Heart Rate Detection From Plantar Bioimpedance Measurements: R. González Landaeta, O. Casas, R. Pallàs-ArenyDocument4 pagesHeart Rate Detection From Plantar Bioimpedance Measurements: R. González Landaeta, O. Casas, R. Pallàs-ArenyLowayNo ratings yet
- Sharp WQ 268eDocument22 pagesSharp WQ 268eLowayNo ratings yet
- Sharp GF 777Document34 pagesSharp GF 777Loway100% (1)
- Sharp GF 700h SCHDocument4 pagesSharp GF 700h SCHLowayNo ratings yet
- GF 6363Document1 pageGF 6363LowayNo ratings yet
- The Employees' Compensation Act, 1923Document23 pagesThe Employees' Compensation Act, 1923Charan Kamal SinghNo ratings yet
- Chapter 21 Electric Charge - Gui SVDocument5 pagesChapter 21 Electric Charge - Gui SVHậu Vũ100% (1)
- Creep and Shrinkage in ConcreteDocument18 pagesCreep and Shrinkage in Concretesarthakpuri21No ratings yet
- Weathering and Organic Processes Form Soil.: What Makes Soils Different?Document8 pagesWeathering and Organic Processes Form Soil.: What Makes Soils Different?Precious BalgunaNo ratings yet
- MIL-I-17563C - Impregnation StdsDocument18 pagesMIL-I-17563C - Impregnation StdsMohanrajMJNo ratings yet
- Caring For Your African American/Biracial Child's HairDocument32 pagesCaring For Your African American/Biracial Child's Hair4kidslikemeNo ratings yet
- Beer & Wine Osd PresDocument18 pagesBeer & Wine Osd PresAnand100% (1)
- Nameplate PDFDocument8 pagesNameplate PDFobida adailehNo ratings yet
- Datasheet DD2603Document3 pagesDatasheet DD2603RONALD TERRAZASNo ratings yet
- Definition of Terms Mine SurveyingDocument2 pagesDefinition of Terms Mine Surveyingaquariuspj25100% (3)
- Purkey Suggestions of Self ConceptDocument3 pagesPurkey Suggestions of Self ConceptLorene bbyNo ratings yet
- Pre-Test in Science 6: M.S. Garcia Elementary SchoolDocument3 pagesPre-Test in Science 6: M.S. Garcia Elementary SchoolAnepsu HohoNo ratings yet
- Prolapse Lumbar DiscDocument40 pagesProlapse Lumbar DiscAlfred BantigueNo ratings yet
- Roach PreviewDocument14 pagesRoach PreviewDomênico GayNo ratings yet
- Evolution of Life On Earth: General Biology 2Document10 pagesEvolution of Life On Earth: General Biology 2Geox NikeeNo ratings yet
- 2-Subject Verb Agreement CWDocument2 pages2-Subject Verb Agreement CWAmir NagyNo ratings yet
- AIA Future Builder FAQsDocument3 pagesAIA Future Builder FAQsjulianraphaelrazonNo ratings yet
- Nutrition Factor in Women in Backward AreasDocument7 pagesNutrition Factor in Women in Backward AreaspranjalNo ratings yet
- Exercise Induced Neuroplasticity - Rogge 2018Document38 pagesExercise Induced Neuroplasticity - Rogge 2018Juani Cantellanos0% (1)
- Review of WAG Field ExperienceDocument10 pagesReview of WAG Field ExperienceJavier E. Guerrero ArrietaNo ratings yet
- Singapore Math Worksheets Grade 3 MeasurementDocument8 pagesSingapore Math Worksheets Grade 3 MeasurementKungfu Math100% (1)
- Beyond The Five Stages of Grief - Class 6 DocumentDocument2 pagesBeyond The Five Stages of Grief - Class 6 DocumentoksanaNo ratings yet
- Monograph COBIOGUM I Rev00Document1 pageMonograph COBIOGUM I Rev00DIANELANo ratings yet
- Wastewater Characteristics: Table 7.1.5 Table 7.1.6Document4 pagesWastewater Characteristics: Table 7.1.5 Table 7.1.6Amin EnviroNo ratings yet
- Past Life Regression Therapist Training WorkshopDocument3 pagesPast Life Regression Therapist Training WorkshopDrManjiree Gokhale50% (2)
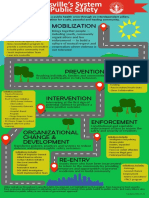
- Reimagining Public SafetyDocument1 pageReimagining Public SafetyWAVE 3 NewsNo ratings yet
- Build 443Document5 pagesBuild 443mh_tadayonNo ratings yet
- The Best Things To Do in Binghamton NYDocument4 pagesThe Best Things To Do in Binghamton NYShashank SangwanNo ratings yet
- ERBEJETDocument8 pagesERBEJETHossain TanjilaaNo ratings yet