Professional Documents
Culture Documents
Experiment On Vitamins - CONGSON
Experiment On Vitamins - CONGSON
Uploaded by
Shayne Angelique CongsonOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Experiment On Vitamins - CONGSON
Experiment On Vitamins - CONGSON
Uploaded by
Shayne Angelique CongsonCopyright:
Available Formats
Shayne Angelique B.
Congson
BSMLS-2B
Experiment on Vitamins
Laboratory Report in Biochemistry
Group 1 & 2 - Solubility of Vitamins
Vitamins are organic substances found in trace amounts in natural foods that are
required for optimal cell function, growth, and development. They are necessary
nutrients that allow our bodies to operate correctly and reduce the chance of acquiring
certain health problems. Vitamins are classified into two types: water-soluble vitamins
and fat-soluble vitamins. The majority of vitamins are obtained through diet. The primary
distinction between water-soluble vitamins and fat-soluble vitamins is how they are
absorbed and used by our bodies. Water-soluble vitamins are those that dissolve in
water and are absorbed into the body's tissues. Vitamins C and B are included (B1, B2,
B6, B3, pantothenic acid, folic acid, biotin, lipoic acid). Fat-soluble vitamins, on the other
hand, are absorbed with lipids and stored in the body. These are vitamins A, D, E, and
K.
The solubility of vitamins is determined by the solvent in which they are
dissolved. Some vitamins are claimed to be water soluble, whereas others are fat
soluble. As we all know, the notion of solubility centers around two key statements:
organic solutes dissolve in organic solvents, inorganic solutes dissolve in inorganic
solvents, non-polar solutes dissolve in non-polar solvents, and polar solutes dissolve in
polar solvents. These assertions are critical for gaining a better knowledge of vitamin
solubility. To put it simply, it dissolves the complementary substance. Excess water-
soluble vitamins are excreted in the urine, but excess fat-soluble vitamins are stored in
body fat and can be toxic in large quantities. Any vitamin shortage causes certain
symptoms. We utilize water and dichloromethane (methylene chloride) as a reagent in
the experiment to test if the vitamins are water-soluble or fat-soluble. Vitamins B3, C,
and Folic acid dissolved entirely in water with no residue left in the sample, indicating
that the vitamins are water soluble. Since of the residue and layers in the sample,
Vitamin D3 and Vitamin E are entirely insoluble in water because they were completely
dissolved in an organic solvent dichloromethane, indicating that Vitamin D3 and Vitamin
E are fat-soluble vitamins.
Group 3 & 4 – Standardization of Vitamins
Vitamin C (ascorbic acid) is a water-soluble vitamin. It has a somewhat acidic
flavor and appears as a white or slightly yellow crystal or powder. It is, in fact, an
antiscorbutic supplement. It gradually darkens when it is exposed to light. When dry, it is
somewhat stable in air, but it quickly oxidizes in solution. Ascorbic acid dissolves water,
alcohol, chloroform, ether, and benzene, but not chloroform, ether, or benzene. The
reduction-oxidation or redox process between iodine and ascorbic acid is crucial in
determining the Vitamin C content in fruit juices. Iodine is reduced or acquires electrons
through this chemical reaction. Ascorbic acid, on the other hand, loses electrons or is
oxidized. This leads in the creation of dehydroascorbic acid, hydrogen ions, and iodide
ions. Iodine is also regarded as an oxidizing agent, whereas ascorbic acid is regarded
as a reducing agent. The major rationale for include starch in the reaction mixture is that
it acts as a signal of the titration process's endpoint. When all of the ascorbic acid
molecules have been oxidized, the extra iodine tends to react with the starch molecules.
This, in turn, results in the formation of a bluish-black starch-iodine complex, signaling
the completion of the process. Vitamin C is a necessary nutrient that humans must
receive from food or supplements in order to satisfy our needs. According to the
National Institutes of Health, adults should consume 75 milligrams (mg) of vitamin C per
day for women and 90 mg for men; smokers should consume an additional 35 mg per
day, and pregnant and nursing women should consume 85 and 120 mg, respectively.
Supplementing with vitamin C may also help with the healing of more common
problems. Vitamin C is advantageous to the heart in a variety of ways.
Group 5 – Analysis in Fruits
Vitamins are chemical substances that are needed for a variety of biological
processes. In general, vitamins are not created in the human body, but their absence or
deficiency may result in certain disorders. The relative amount of ascorbic acid
contained in fruit juices (apple, orange, cranberry, and grape) was measured in this
observational research utilizing a redox titration analysis employing an iodometric
approach. Titrations were performed three times for each therapy, and the identification
of the titration endpoints were found using starch as a visual signal. According to our
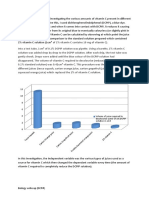
findings, apple juice (4.8 mg / 10 mL) provided the most vitamin C, followed by orange
juice (4.0 mg / 10 mL), grape juice (2.2 mg / 10 mL), and cranberry juice (2.0 21mg / 10
mL). Many fruits and commercial drinks contain ascorbic acid. The quantity of ascorbic
acid in various fruit juices was determined using the iodometric titration technique.
Determining the precise quantity of ascorbic acid contained in fruit juices necessitates
the use of adequate scientific equipment while controlling for physical conditions such
as temperature and light.
Determining the precise quantity of ascorbic acid contained in fruit juices
necessitates the use of adequate scientific equipment while controlling for physical
conditions such as temperature and light. Other aspects, such as human and
environmental influences, must also be considered. Using iodometric titration, it was
discovered that a higher concentration of ascorbic acid necessitates a larger
concentration of iodine in order to detect the precise quantity of Vitamin C in a given
sample. Furthermore, in terms of applications, determining the quantity of ascorbic acid
in fruit juices has been critical in the fields of biochemistry and the food business.
Group 6 – Heat Destruction
The redox titration technique was then used to determine vitamin C levels. The
sample solution for titration was made by combining 25 mL of vegetable extract with 5
mL of starch solution (indicator) and potassium iodide (KI). After then, the solution was
titrated with 0.009M potassium iodate (titrant) until a blue-black color shift was seen.
The vitamin C was oxidized when the iodine was added, and the excess iodine
interacted with the indicator to produce a blue-black hue in the liquid. The shift in color
from blue to black in the sample denotes our end point, and the titre quantity (in mL) is
subsequently recorded at this point. The results revealed that all the vegetables contain
vitamin C, however, the pepper sample gave the highest value of 61.56mg/100ml
implying it is a good source of vitamin C while the carrot sample gave the least vitamin
C amount of 6.43mg/100ml. The vitamin C content of the raw vegetables is generally
high when compared with those of the heated. Pepper had the greatest amount of
vitamin C (61.56mg/100ml) and the highest percentage loss of vitamin C in its sample of
all the veggies (64.17 percent loss of vitamin C in 30 mins heating). This implies that
denaturation of vitamin C due to heating is dependent on its availability in the vegetable,
with a larger percentage loss when denatured by heating for a vegetable with a higher
vitamin C content available. The purpose of this experiment was to see how heating
affected the vitamin C content of the selected veggies. Temperature variations had a
substantial influence on the vitamin C concentrations of the various vegetable samples.
It has been found that heating time has a substantial influence on the vitamin C content
of all vegetables; as heating time increases, so does the percentage loss of vitamin C.
The longer the samples (pepper, green peas, spinach, pumpkin, and carrot) were
cooked at high temperatures, the less vitamin C was detected.
You might also like
- Vitamin C Core Practical Write Up PDFDocument2 pagesVitamin C Core Practical Write Up PDFMichael Collin0% (1)
- Vitamin C Determination by IodineDocument4 pagesVitamin C Determination by IodineGrace Emphasis100% (3)
- RESULT & Discussion Exp 6 AnalyDocument3 pagesRESULT & Discussion Exp 6 AnalyAlimah AzeliNo ratings yet
- Laboratory Experiment No. 8 Advanced Acid-BaseDocument4 pagesLaboratory Experiment No. 8 Advanced Acid-BaseShayne Angelique CongsonNo ratings yet
- Chem Project PresentationDocument23 pagesChem Project PresentationSarah LeeNo ratings yet
- Analysis Vitamin C Fruit JuicesDocument9 pagesAnalysis Vitamin C Fruit Juiceskhalifa1122100% (1)
- Lab 4 LipidDocument8 pagesLab 4 Lipidapi-384770852No ratings yet
- Iodometric Analysis For Vitamin C Lab ReportDocument9 pagesIodometric Analysis For Vitamin C Lab ReportAlleia Mae Urbano Mazo80% (10)
- Biology November ReportDocument3 pagesBiology November ReportIndrani GoswamiNo ratings yet
- How Does Exercise Affect Heart Rate Alvin PDFDocument13 pagesHow Does Exercise Affect Heart Rate Alvin PDFapi-134712575No ratings yet
- Litreature Review On Vitamin CDocument14 pagesLitreature Review On Vitamin CPredeswaran Ponniah100% (2)
- How To Make TE BufferDocument2 pagesHow To Make TE BufferJoanne HodgsonNo ratings yet
- (Welding) MIG-MAG Welding Guide - Lincoln Electric (Ebook, 48 Pages)Document48 pages(Welding) MIG-MAG Welding Guide - Lincoln Electric (Ebook, 48 Pages)Ahmad Arafa100% (10)
- Vitmin C ReportDocument14 pagesVitmin C ReportOdongo TonnyNo ratings yet
- Chemistry Project 3.0Document15 pagesChemistry Project 3.0Lubna Khalid67% (3)
- Quantitative Analysis of Vitamin C Contained in FoodsDocument8 pagesQuantitative Analysis of Vitamin C Contained in FoodsCleve Hines100% (1)
- TitrationlabreportDocument7 pagesTitrationlabreportapi-272775120No ratings yet
- Foods That Contain Vitamin CDocument11 pagesFoods That Contain Vitamin CLam YanWen0% (1)
- Chem Project NewDocument17 pagesChem Project NewSrishti KumariNo ratings yet
- Vitamin C Write-UpDocument5 pagesVitamin C Write-UpannafiiNo ratings yet
- Determination of Vitamin C Concentration by TitrationDocument5 pagesDetermination of Vitamin C Concentration by TitrationMaryam JabiyevaNo ratings yet
- Chem Project - Class 12Document12 pagesChem Project - Class 12M AdithyaNo ratings yet
- Ascorbic Acid PDFDocument18 pagesAscorbic Acid PDFHarshNo ratings yet
- Vitaminc IodineDocument3 pagesVitaminc IodineMuh. Ma'arifNo ratings yet
- Vit C Experiment Write UpDocument9 pagesVit C Experiment Write UpSanngeeta100% (2)
- Vitamin C Lab PDFDocument7 pagesVitamin C Lab PDFJohn Baptist John Bosco100% (1)
- DCPIP Write UpDocument2 pagesDCPIP Write UpHashim Chishty0% (1)
- Determination of Vitamin CDocument7 pagesDetermination of Vitamin Capi-487208181No ratings yet
- Bio ReportDocument8 pagesBio ReportTharshini_Indr_6713No ratings yet
- Exp. 8 (Iodimetric Analysis For Vitamin C)Document4 pagesExp. 8 (Iodimetric Analysis For Vitamin C)Nikko Gabriel AquinoNo ratings yet
- EXP 5 Determination For Ascorbic Acid AnalysisDocument2 pagesEXP 5 Determination For Ascorbic Acid AnalysisthirafauziNo ratings yet
- Tesis vITAMIN C CONTENT IN FRUITSDocument24 pagesTesis vITAMIN C CONTENT IN FRUITSHema JothyNo ratings yet
- Vitamin C in Fruit JuicesDocument5 pagesVitamin C in Fruit JuicesANMOL journeyNo ratings yet
- Practical Vitiman CDocument14 pagesPractical Vitiman CLee da DonNo ratings yet
- The Extraction of Benzoic AcidDocument7 pagesThe Extraction of Benzoic AcidChenling NiNo ratings yet
- Amount of Vitamin C in Different Flavours of Marigold Yogurt DrinkDocument13 pagesAmount of Vitamin C in Different Flavours of Marigold Yogurt DrinkHema Jothy0% (1)
- Investigating Vitamin C Concentration in Homemade and Store Bought SmoothiesDocument7 pagesInvestigating Vitamin C Concentration in Homemade and Store Bought SmoothiesisabelleNo ratings yet
- Oxidation Reactions of AlcoholsDocument1 pageOxidation Reactions of AlcoholsxantogenatNo ratings yet
- Measuring The Vitamin C Content of Foods and Fruit JuicesDocument3 pagesMeasuring The Vitamin C Content of Foods and Fruit JuicesJohnNo ratings yet
- Iodometric Titration of Vitamin C PDFDocument6 pagesIodometric Titration of Vitamin C PDFDr. Rajni GargNo ratings yet
- Estimation of Vitamin C in Fruit and Vegetable JuiceDocument9 pagesEstimation of Vitamin C in Fruit and Vegetable JuiceAvnish BhasinNo ratings yet
- Analysis of Concentration of Vitamin C IDocument21 pagesAnalysis of Concentration of Vitamin C IMahamud Hasan Prince100% (2)
- CHEM 22161 Lab 1 :synthesis of Medicinal Agent: Synthesis of AspirinDocument8 pagesCHEM 22161 Lab 1 :synthesis of Medicinal Agent: Synthesis of AspirinKasun WekasingheNo ratings yet
- Determination of Vitamin Content in Sample Tablets by TitrationDocument2 pagesDetermination of Vitamin Content in Sample Tablets by TitrationmagicianchemistNo ratings yet
- Determination of The Concentration of Vitamin C by Using The DCPIP TestDocument2 pagesDetermination of The Concentration of Vitamin C by Using The DCPIP TestBlaireNo ratings yet
- Analysis of Vitamin C in Fruit JuiceDocument17 pagesAnalysis of Vitamin C in Fruit JuiceManav RajeshNo ratings yet
- Vitamin CDocument15 pagesVitamin Czaiy67% (3)
- Lab Report 4 sbl1023Document7 pagesLab Report 4 sbl1023api-3850387010% (1)
- Which Type of Fruit Juice Provides The Most Vitamin C?Document5 pagesWhich Type of Fruit Juice Provides The Most Vitamin C?Aswathy BijuNo ratings yet
- Folic AcidDocument4 pagesFolic AcidBal Ri MekoleuNo ratings yet
- Measuring The Vitamin C Content of Foods and Fruit Juices Ss 36Document1 pageMeasuring The Vitamin C Content of Foods and Fruit Juices Ss 36Aji CuteMadNo ratings yet
- The Determination of Ascorbic AcidDocument5 pagesThe Determination of Ascorbic AcidCarina JLNo ratings yet
- Enzymes Formal ReportDocument4 pagesEnzymes Formal ReportJenelle Jane Quilaneta75% (4)
- Determination of Vitamin C (Ascorbic Acid)Document8 pagesDetermination of Vitamin C (Ascorbic Acid)Syahirrashahar100% (1)
- CHEMISTRY ProjectDocument17 pagesCHEMISTRY ProjectMerin MariamNo ratings yet
- Lab Report 5: Lipid Analysis Sbl102 Group A Technique in Biology and Biochemistry Laboratory SEM 2 2022/2023 Lecture'S Name: DR Remmy Keong Bun PohDocument5 pagesLab Report 5: Lipid Analysis Sbl102 Group A Technique in Biology and Biochemistry Laboratory SEM 2 2022/2023 Lecture'S Name: DR Remmy Keong Bun Pohmuhammad hafizuddinNo ratings yet
- EXPERIMENT FIX KomangDocument27 pagesEXPERIMENT FIX KomangKomang AriyaniNo ratings yet
- Experiment # 7 "Investigating Vitamin C"Document2 pagesExperiment # 7 "Investigating Vitamin C"Denisse Angelie CastroNo ratings yet
- Vitamins and HormonesDocument10 pagesVitamins and HormonesNooraNo ratings yet
- Biochemistry Practical ReportDocument10 pagesBiochemistry Practical ReportChan HuiqiNo ratings yet
- Vitamin CDocument18 pagesVitamin CrashmiNo ratings yet
- Purpcomm IntroDocument4 pagesPurpcomm IntroMegan AmethystNo ratings yet
- Infographic in PrinDocument1 pageInfographic in PrinShayne Angelique CongsonNo ratings yet
- Laboratory Experiment #5 - Chemical EquilibriumDocument3 pagesLaboratory Experiment #5 - Chemical EquilibriumShayne Angelique CongsonNo ratings yet
- Laboratory Experiment No.9 - Neutralization TitrationDocument4 pagesLaboratory Experiment No.9 - Neutralization TitrationShayne Angelique CongsonNo ratings yet
- Laboratory Experiment No.10 - Unknown Compound Using Gravimetric AnalysisDocument2 pagesLaboratory Experiment No.10 - Unknown Compound Using Gravimetric AnalysisShayne Angelique CongsonNo ratings yet
- Kartilya NG Katipunan: Kartilya Francis Magalonan Song "Man From Manila"Document2 pagesKartilya NG Katipunan: Kartilya Francis Magalonan Song "Man From Manila"Shayne Angelique Congson100% (1)
- Laboratory Experiment #6A - Acids and BasesDocument2 pagesLaboratory Experiment #6A - Acids and BasesShayne Angelique CongsonNo ratings yet
- Final Activity 2 - Writing An Application LetterDocument1 pageFinal Activity 2 - Writing An Application LetterShayne Angelique CongsonNo ratings yet
- cc2861 PDFDocument6 pagescc2861 PDFArturo Eduardo Huarcaya OntiverosNo ratings yet
- Ionic Bonding Lab ActivityDocument3 pagesIonic Bonding Lab ActivityMBOTAKE LawsonNo ratings yet
- The Stability of Cyclodextrin Complexes in Solution: Kenneth A. ConnorsDocument34 pagesThe Stability of Cyclodextrin Complexes in Solution: Kenneth A. Connorsnganngao2407No ratings yet
- Week 1 - Topic 7 - 1-7 Process Clean UtilitiesDocument10 pagesWeek 1 - Topic 7 - 1-7 Process Clean UtilitiesCelineNo ratings yet
- Well Stimulation, Acidizing - Peyman DaneshfarDocument87 pagesWell Stimulation, Acidizing - Peyman Daneshfarعلی محمودیNo ratings yet
- Edelweiss PRO 2020 en PDFDocument44 pagesEdelweiss PRO 2020 en PDFGuillermo BascurNo ratings yet
- ASTM C185-20, IDT Standard Test Method For Air Content of Hydraulic Cement Mortar.Document2 pagesASTM C185-20, IDT Standard Test Method For Air Content of Hydraulic Cement Mortar.Salman MansurNo ratings yet
- Stone: A Versatile Building MaterialDocument15 pagesStone: A Versatile Building MaterialSUSHAMYNo ratings yet
- Bendezu & Fontboté PDFDocument12 pagesBendezu & Fontboté PDFJesusNo ratings yet
- CN102807192A - Potassium Perchlorate Production Technology With Zero Wastewater Discharge and Products Thereof - Google PatentsDocument4 pagesCN102807192A - Potassium Perchlorate Production Technology With Zero Wastewater Discharge and Products Thereof - Google PatentsThirupathi ThippaniNo ratings yet
- Washer-Extractor: Pocket HardmountDocument62 pagesWasher-Extractor: Pocket HardmountCarlos TrybiecNo ratings yet
- Chandeep Singh XI-A PhysicsDocument16 pagesChandeep Singh XI-A Physicschandeep singhNo ratings yet
- Company Profil - PT Multan Kusuma SaktiDocument32 pagesCompany Profil - PT Multan Kusuma SaktiMariana LopNo ratings yet
- UraniumDocument19 pagesUraniumEdgar Apaza HuallpaNo ratings yet
- MLL242 Lab ManualDocument26 pagesMLL242 Lab Manualyadavtstsy07No ratings yet
- Udvashito Mukh SSC22Document13 pagesUdvashito Mukh SSC22Showmajit SahaNo ratings yet
- The Kinetics of The Adsorption Process of CR (VI) in Aqueous Solution Using Neem Seed Husk (Azadirachta Indica) Activated CarbonDocument13 pagesThe Kinetics of The Adsorption Process of CR (VI) in Aqueous Solution Using Neem Seed Husk (Azadirachta Indica) Activated CarbonNórida Pájaro GómezNo ratings yet
- Performance Qualification of A Vial WasherDocument26 pagesPerformance Qualification of A Vial WasherprakashNo ratings yet
- Review of Date FruitsDocument11 pagesReview of Date FruitsAiysah ArisNo ratings yet
- MSDS RD705Document5 pagesMSDS RD705Empy SumardiNo ratings yet
- New Era University: Pharmacology For MidwiferyDocument11 pagesNew Era University: Pharmacology For MidwiferyHarriegail O. LontocNo ratings yet
- Helios 480 EC MSDSDocument8 pagesHelios 480 EC MSDSasmaamNo ratings yet
- GIL ReportDocument133 pagesGIL ReportHarsh PatelNo ratings yet
- Dr. Chan Kook Weng Dr. Chan Kook Weng Hj. Wahid Omar Hj. Wahid OmarDocument23 pagesDr. Chan Kook Weng Dr. Chan Kook Weng Hj. Wahid Omar Hj. Wahid OmarkoperasiNo ratings yet
- Madani Sirait FormatingDocument8 pagesMadani Sirait FormatingAdnan ArsvnNo ratings yet
- For Teachers' Use Only: with 弋 mark eπ0Document22 pagesFor Teachers' Use Only: with 弋 mark eπ0Copper ShortswordNo ratings yet
- Demonstration of A Voltaic CellDocument5 pagesDemonstration of A Voltaic CellAgung Aulia RahmanNo ratings yet
- MPN Untuk AlgaDocument8 pagesMPN Untuk AlgaIyak LectureNo ratings yet
- Super Five Backpack Catalogue 20210120Document20 pagesSuper Five Backpack Catalogue 20210120Mulya LaiNo ratings yet