Professional Documents
Culture Documents
Organic Chemistry Poster
Organic Chemistry Poster
Uploaded by
텅텅Copyright:
Available Formats
You might also like
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDocument2 pagesOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- Experiment 4Document7 pagesExperiment 4Pratik PatelNo ratings yet
- Empirical Formula Problems and KeyDocument4 pagesEmpirical Formula Problems and KeyJime Ryle Althea GazzinganNo ratings yet
- Organic Chemistry Synthesis IedxcelDocument10 pagesOrganic Chemistry Synthesis IedxcelAliya Rahman100% (2)
- Synthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsDocument6 pagesSynthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsJunior GonzalesNo ratings yet
- Bab 02 - Families of Carbon CompoundsDocument79 pagesBab 02 - Families of Carbon Compoundscindy wiriaatmadjaNo ratings yet
- Organic Chemistry Chart PDFDocument3 pagesOrganic Chemistry Chart PDFJayjayjay 5100% (2)
- Algebra Translating Algebraic Phrases 002Document2 pagesAlgebra Translating Algebraic Phrases 002ronil_sukhuNo ratings yet
- 12 Chemistry Notes Ch11 Alcohols Phenols and EthersDocument10 pages12 Chemistry Notes Ch11 Alcohols Phenols and Etherskamla kamlaNo ratings yet
- AQA GCSE Chemistry AnswersDocument14 pagesAQA GCSE Chemistry AnswersErvin MetushiNo ratings yet
- Alcohols, Phenols, EthersDocument9 pagesAlcohols, Phenols, Ethersjane kangNo ratings yet
- CHE-501Carboranes and Boranes Metal ClusterDocument40 pagesCHE-501Carboranes and Boranes Metal Clusteranuthakur100% (1)
- AQA AS Level Chemistry Data SheetDocument4 pagesAQA AS Level Chemistry Data SheetA100% (1)
- ProteinDocument16 pagesProteinRosnadia RosliNo ratings yet
- Organic ChemistryDocument236 pagesOrganic ChemistryAbdallahNo ratings yet
- First LE Exam Reviewer Answer KeyDocument2 pagesFirst LE Exam Reviewer Answer KeyLeah Ann Mari BongcoNo ratings yet
- Compounds Containing Nitrogen: Questions & AnswersDocument9 pagesCompounds Containing Nitrogen: Questions & AnswersAakashNo ratings yet
- Organic Chemistry Lab Caffeine ExtractionDocument8 pagesOrganic Chemistry Lab Caffeine Extractionrubu azuNo ratings yet
- ADVANCED CHEMISTRY Q3 Module Jan 2021 PDFDocument48 pagesADVANCED CHEMISTRY Q3 Module Jan 2021 PDFLouis C. GutierrezNo ratings yet
- Chem TB PDFDocument173 pagesChem TB PDFPrudence SitholeNo ratings yet
- 12 Organic SynthesisDocument8 pages12 Organic SynthesisDanyal AhmadNo ratings yet
- 6 14 Organic SynthesisDocument8 pages6 14 Organic SynthesisPedro Moreno de SouzaNo ratings yet
- 20 Organic Chemistry Synthesis Iedxcel PDFDocument10 pages20 Organic Chemistry Synthesis Iedxcel PDFMohammedNo ratings yet
- Summary of Organic ReactionsDocument6 pagesSummary of Organic ReactionsAbudi Alsagoff100% (6)
- MBD Toolkit ADocument1 pageMBD Toolkit AOCRChemistrySaltersNo ratings yet
- 6carboxylic AcidsDocument1 page6carboxylic AcidssharmimiameerasanadyNo ratings yet
- 6 2 5 Revision Guides Organic SynthesisDocument5 pages6 2 5 Revision Guides Organic SynthesisAddan AddanNo ratings yet
- Carboxylic & DerivtDocument7 pagesCarboxylic & DerivtNanda NaimahNo ratings yet
- 2018 l3 Organic Reaction Scheme Version 2Document1 page2018 l3 Organic Reaction Scheme Version 2NUR SYAFIQAH BINTI MD REJABNo ratings yet
- Alkane and Alkyl Halides PP5Document9 pagesAlkane and Alkyl Halides PP5odubade opeyemiNo ratings yet
- Reactions of Alkene: CH CH Markovnikov AdditionDocument8 pagesReactions of Alkene: CH CH Markovnikov AdditionRaye VolvoNo ratings yet
- 2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Document17 pages2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Anarkin FitriNo ratings yet
- Chemistry Handbook ExportDocument17 pagesChemistry Handbook Exportgauranggunjkar2006No ratings yet
- Organic Chemistry ReactionDocument3 pagesOrganic Chemistry ReactionGAMEPORIUMNo ratings yet
- Organic Chemistry Reaction Summary SheetDocument30 pagesOrganic Chemistry Reaction Summary SheetKylo RenNo ratings yet
- Memory Map AromaticsDocument1 pageMemory Map AromaticsOCRChemistrySaltersNo ratings yet
- 3.14 Revision Guide Organic Synthesis AqaDocument7 pages3.14 Revision Guide Organic Synthesis AqaRutba SafdarNo ratings yet
- Reaction of Ketone CompleteDocument1 pageReaction of Ketone CompleteJoko SusiloNo ratings yet
- Reaksi EliminasiDocument23 pagesReaksi EliminasiAde FadilahNo ratings yet
- 202003291608409347arun Sethi SteroidDocument7 pages202003291608409347arun Sethi SteroidVishva AegonNo ratings yet
- Reaction of Alcohols CompleteDocument1 pageReaction of Alcohols CompleteJoko SusiloNo ratings yet
- Reaction SchemeDocument1 pageReaction SchemesakoakimNo ratings yet
- Organic ConversionDocument9 pagesOrganic ConversionAnonymous lmpvRsaz90% (1)
- Reaction of AldehydesDocument1 pageReaction of AldehydesJoko SusiloNo ratings yet
- Sr. No. Reaction Reagent Condition Mechanism Example NoteDocument3 pagesSr. No. Reaction Reagent Condition Mechanism Example NoteAbbas HaiderNo ratings yet
- Organic RevisionDocument4 pagesOrganic RevisionalicejessicapreesNo ratings yet
- Carboxylic Acids and Its Derivatives PDFDocument58 pagesCarboxylic Acids and Its Derivatives PDFAniruddha Kawade100% (2)
- Alkanes Alkenes AlkynesDocument2 pagesAlkanes Alkenes AlkynesGAMEPORIUMNo ratings yet
- Chemistry - Overview of Aliphatic Organic ChemistryDocument1 pageChemistry - Overview of Aliphatic Organic Chemistryhelixate100% (5)
- X Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLDocument1 pageX Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLEmily McCullochNo ratings yet
- Organic Chemistry I Problem Set AlcoholsDocument2 pagesOrganic Chemistry I Problem Set AlcoholssaddamixoNo ratings yet
- X UV Light or Heat: Reactions in Topic XIDocument3 pagesX UV Light or Heat: Reactions in Topic XImichelsonyip100% (1)
- 12 Chemistry Notes ch11 Alcohols Phenols and EthersDocument8 pages12 Chemistry Notes ch11 Alcohols Phenols and Ethersmv7602456No ratings yet
- Reaction of Aldehyde CompleteDocument1 pageReaction of Aldehyde CompleteJoko SusiloNo ratings yet
- SynrxnsDocument48 pagesSynrxnsRonak MantriNo ratings yet
- AminesDocument24 pagesAminesRajdeep Singh RahiNo ratings yet
- JC1 Chemistry Organic Reagent Practice - HWDocument14 pagesJC1 Chemistry Organic Reagent Practice - HWTesar DzikrullohNo ratings yet
- Organic Mindmap (Condensed) 2021 AnswersDocument2 pagesOrganic Mindmap (Condensed) 2021 AnswersbeverlyyyNo ratings yet
- Chemical Reactions of Alkanes: Mechanism Reaction Reagent Condition Catalysts Product(s)Document9 pagesChemical Reactions of Alkanes: Mechanism Reaction Reagent Condition Catalysts Product(s)Sam LeeNo ratings yet
- Weapon 4Document1 pageWeapon 4md.muhibmusabbir1101No ratings yet
- 11 Alcohols Phenols and EthersDocument2 pages11 Alcohols Phenols and EthersVarun Sankpal100% (1)
- Product ListDocument3 pagesProduct ListjagrutiNo ratings yet
- Solutions To HW #7Document2 pagesSolutions To HW #7Try “Play videos” MusicNo ratings yet
- 13 - BhattiAcademy - Com - Chemistry - 5. Scholar Series (Obj)Document11 pages13 - BhattiAcademy - Com - Chemistry - 5. Scholar Series (Obj)Amir FarooqNo ratings yet
- Carboxylic Acids and Their DerivativesDocument22 pagesCarboxylic Acids and Their DerivativesEugene OkpanteyNo ratings yet
- Ujian Bertulis TJC401 Mac-Jul 2018Document5 pagesUjian Bertulis TJC401 Mac-Jul 2018Siti Maryam UmairahNo ratings yet
- Great Orthogonality TheoremDocument4 pagesGreat Orthogonality TheoremSagar RawalNo ratings yet
- SettingsproviderDocument22 pagesSettingsproviderJose maria MartinezNo ratings yet
- The Elements Science Crossword Puzzle Worksheet in Colorful Lined Style - 20240322 - 174014 - 0000Document3 pagesThe Elements Science Crossword Puzzle Worksheet in Colorful Lined Style - 20240322 - 174014 - 0000Jessica García LópezNo ratings yet
- Project On: Action On A Set: Submission Date 01/10/2019 Sub. Instructor To . (M.SC)Document11 pagesProject On: Action On A Set: Submission Date 01/10/2019 Sub. Instructor To . (M.SC)shambelNo ratings yet
- Alkohol, Eter, Aldehid, KetonDocument21 pagesAlkohol, Eter, Aldehid, KetonFaesal AmrullahNo ratings yet
- IUPAC Short Notes Nitesh DevnaniDocument4 pagesIUPAC Short Notes Nitesh Devnaniunderprocess786No ratings yet
- Chapter 1 - Aldehydes KetonesDocument51 pagesChapter 1 - Aldehydes KetonesSarathy Hari KumarNo ratings yet
- Aldehyde Ketone PPT 4Document9 pagesAldehyde Ketone PPT 4muskan dahiyaNo ratings yet
- VisakhapatnamDocument18 pagesVisakhapatnamStepinconsultancy NamchettyNo ratings yet
- HaloalkaneDocument20 pagesHaloalkaneHediarta Widiana PutraNo ratings yet
- Lectures On Lie Groups, Second EditionDocument161 pagesLectures On Lie Groups, Second Editionfernandega100% (3)
- 12.8 Aldehydes and Ketones Solution - PremiumDocument24 pages12.8 Aldehydes and Ketones Solution - PremiumJonathan ParkerNo ratings yet
- The Geometry of Some Special Arithmetic QuotientsDocument347 pagesThe Geometry of Some Special Arithmetic Quotientslandau1994100% (3)
- Aldehyde Ketone PPT 2Document21 pagesAldehyde Ketone PPT 2muskan dahiyaNo ratings yet
- MATH 3E03 Lagrange's Theorem Questions: SolutionDocument2 pagesMATH 3E03 Lagrange's Theorem Questions: SolutionArtianaNo ratings yet
- Das & Okubo-Lie Groups and Lie Algebras For Physicists PDFDocument358 pagesDas & Okubo-Lie Groups and Lie Algebras For Physicists PDFAlfredo Echegollen Guzmán100% (5)
- Toribio Medina 1930 Bibliografia Quechua AymaraDocument124 pagesToribio Medina 1930 Bibliografia Quechua AymaraOlivia DaltreyNo ratings yet
- Subgroups: Sergei Silvestrov Spring Term 2011, Lecture 9Document12 pagesSubgroups: Sergei Silvestrov Spring Term 2011, Lecture 9prathimaNo ratings yet
- PK# RT Hit Compound Name Match R.Match CAS LibraryDocument11 pagesPK# RT Hit Compound Name Match R.Match CAS LibraryGerson JoelNo ratings yet
- Geometric Group Theory NotesDocument58 pagesGeometric Group Theory NotesAllenNo ratings yet
- Group Theory Calculations Involving Linear MoleculesDocument12 pagesGroup Theory Calculations Involving Linear MoleculesJonathanNo ratings yet
- Presentation For Subgroups PDFDocument32 pagesPresentation For Subgroups PDFMary John Pinuela100% (1)
- CCN MHT CET Synopsis PDFDocument7 pagesCCN MHT CET Synopsis PDFAbhishek Mandlik100% (1)
- Soluble GroupsDocument2 pagesSoluble GroupsJaime GutiérrezNo ratings yet
Organic Chemistry Poster
Organic Chemistry Poster
Uploaded by
텅텅Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organic Chemistry Poster
Organic Chemistry Poster
Uploaded by
텅텅Copyright:
Available Formats
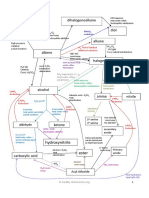
Organic Chemistry
Dibromoalkane
Cracking
5 Thermal: 500°C /20atm Oil
Alkene Cat: Zeolite/200°C Alkane Fractional distillation
350°C
1 H H
C C
Alkyl 10 2 C C
hydrogensulfate H2, Ni
180°C
H H
300°C
70atm 4 180°C Free radical
3 6
Cat:H3PO4 c. H2SO4 9 substitution
180°C Halogen X2
NaBr(s) Amide
K2Cr2O7 11 c. H2SO4
U.V. light O
d. H2SO4 Reflux
Ketone Heat and distil Alcohol 7 Halogenoalkane C
Oxidation
O -X R NH2
-OH 8
C KOH(aq)
NaBH4 or Primary Reflux NaCN (alc)
Acylchloride or
Nucleophilic Reflux
LiAlH4 Acidanhydride
substitution 16 Nucleophilic
reduction 180°C 18
K2Cr2O7 substitution
NaBH4 or
Note: 3° alcohol do not d. H2SO4
LiAlH4
undergo oxidation : the C Heat and distil (reduction)
19
holding the –OH does Oxidation
Nitrile Amine
not have H attach to it Aldehyde 17
H
O R C N R C
H2, Ni cat,200°C
Aldehyde/ketone
I C H Or LiAlH4 H
Brady's reagent 2,4- In ether + hydrolysis
Alcohol
DNPH Reduction
NaBH4 or K2Cr2O7 c.H2SO4
I c.H2SO4 Reflux
Solid derivitive
LiAalH4
Reflux Ester
(reduction) 13
14 O
Carboxylic
R C O R'
15
O acid Reflux, 19
HCl (aq) or NaOH
C OH then HCl(aq)
Mechanisms
1 6 10 13
11
14
15
2
7
16
17
5 9
12
18
Halogenoalkane
With hydroxide ions
If solvent is water:
nucleophilic substitution
Alcohol: elimination
You might also like
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDocument2 pagesOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- Experiment 4Document7 pagesExperiment 4Pratik PatelNo ratings yet
- Empirical Formula Problems and KeyDocument4 pagesEmpirical Formula Problems and KeyJime Ryle Althea GazzinganNo ratings yet
- Organic Chemistry Synthesis IedxcelDocument10 pagesOrganic Chemistry Synthesis IedxcelAliya Rahman100% (2)
- Synthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsDocument6 pagesSynthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsJunior GonzalesNo ratings yet
- Bab 02 - Families of Carbon CompoundsDocument79 pagesBab 02 - Families of Carbon Compoundscindy wiriaatmadjaNo ratings yet
- Organic Chemistry Chart PDFDocument3 pagesOrganic Chemistry Chart PDFJayjayjay 5100% (2)
- Algebra Translating Algebraic Phrases 002Document2 pagesAlgebra Translating Algebraic Phrases 002ronil_sukhuNo ratings yet
- 12 Chemistry Notes Ch11 Alcohols Phenols and EthersDocument10 pages12 Chemistry Notes Ch11 Alcohols Phenols and Etherskamla kamlaNo ratings yet
- AQA GCSE Chemistry AnswersDocument14 pagesAQA GCSE Chemistry AnswersErvin MetushiNo ratings yet
- Alcohols, Phenols, EthersDocument9 pagesAlcohols, Phenols, Ethersjane kangNo ratings yet
- CHE-501Carboranes and Boranes Metal ClusterDocument40 pagesCHE-501Carboranes and Boranes Metal Clusteranuthakur100% (1)
- AQA AS Level Chemistry Data SheetDocument4 pagesAQA AS Level Chemistry Data SheetA100% (1)
- ProteinDocument16 pagesProteinRosnadia RosliNo ratings yet
- Organic ChemistryDocument236 pagesOrganic ChemistryAbdallahNo ratings yet
- First LE Exam Reviewer Answer KeyDocument2 pagesFirst LE Exam Reviewer Answer KeyLeah Ann Mari BongcoNo ratings yet
- Compounds Containing Nitrogen: Questions & AnswersDocument9 pagesCompounds Containing Nitrogen: Questions & AnswersAakashNo ratings yet
- Organic Chemistry Lab Caffeine ExtractionDocument8 pagesOrganic Chemistry Lab Caffeine Extractionrubu azuNo ratings yet
- ADVANCED CHEMISTRY Q3 Module Jan 2021 PDFDocument48 pagesADVANCED CHEMISTRY Q3 Module Jan 2021 PDFLouis C. GutierrezNo ratings yet
- Chem TB PDFDocument173 pagesChem TB PDFPrudence SitholeNo ratings yet
- 12 Organic SynthesisDocument8 pages12 Organic SynthesisDanyal AhmadNo ratings yet
- 6 14 Organic SynthesisDocument8 pages6 14 Organic SynthesisPedro Moreno de SouzaNo ratings yet
- 20 Organic Chemistry Synthesis Iedxcel PDFDocument10 pages20 Organic Chemistry Synthesis Iedxcel PDFMohammedNo ratings yet
- Summary of Organic ReactionsDocument6 pagesSummary of Organic ReactionsAbudi Alsagoff100% (6)
- MBD Toolkit ADocument1 pageMBD Toolkit AOCRChemistrySaltersNo ratings yet
- 6carboxylic AcidsDocument1 page6carboxylic AcidssharmimiameerasanadyNo ratings yet
- 6 2 5 Revision Guides Organic SynthesisDocument5 pages6 2 5 Revision Guides Organic SynthesisAddan AddanNo ratings yet
- Carboxylic & DerivtDocument7 pagesCarboxylic & DerivtNanda NaimahNo ratings yet
- 2018 l3 Organic Reaction Scheme Version 2Document1 page2018 l3 Organic Reaction Scheme Version 2NUR SYAFIQAH BINTI MD REJABNo ratings yet
- Alkane and Alkyl Halides PP5Document9 pagesAlkane and Alkyl Halides PP5odubade opeyemiNo ratings yet
- Reactions of Alkene: CH CH Markovnikov AdditionDocument8 pagesReactions of Alkene: CH CH Markovnikov AdditionRaye VolvoNo ratings yet
- 2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Document17 pages2024 Carboxylic Acid and Derivatives Tutorial (Teacher)Anarkin FitriNo ratings yet
- Chemistry Handbook ExportDocument17 pagesChemistry Handbook Exportgauranggunjkar2006No ratings yet
- Organic Chemistry ReactionDocument3 pagesOrganic Chemistry ReactionGAMEPORIUMNo ratings yet
- Organic Chemistry Reaction Summary SheetDocument30 pagesOrganic Chemistry Reaction Summary SheetKylo RenNo ratings yet
- Memory Map AromaticsDocument1 pageMemory Map AromaticsOCRChemistrySaltersNo ratings yet
- 3.14 Revision Guide Organic Synthesis AqaDocument7 pages3.14 Revision Guide Organic Synthesis AqaRutba SafdarNo ratings yet
- Reaction of Ketone CompleteDocument1 pageReaction of Ketone CompleteJoko SusiloNo ratings yet
- Reaksi EliminasiDocument23 pagesReaksi EliminasiAde FadilahNo ratings yet
- 202003291608409347arun Sethi SteroidDocument7 pages202003291608409347arun Sethi SteroidVishva AegonNo ratings yet
- Reaction of Alcohols CompleteDocument1 pageReaction of Alcohols CompleteJoko SusiloNo ratings yet
- Reaction SchemeDocument1 pageReaction SchemesakoakimNo ratings yet
- Organic ConversionDocument9 pagesOrganic ConversionAnonymous lmpvRsaz90% (1)
- Reaction of AldehydesDocument1 pageReaction of AldehydesJoko SusiloNo ratings yet
- Sr. No. Reaction Reagent Condition Mechanism Example NoteDocument3 pagesSr. No. Reaction Reagent Condition Mechanism Example NoteAbbas HaiderNo ratings yet
- Organic RevisionDocument4 pagesOrganic RevisionalicejessicapreesNo ratings yet
- Carboxylic Acids and Its Derivatives PDFDocument58 pagesCarboxylic Acids and Its Derivatives PDFAniruddha Kawade100% (2)
- Alkanes Alkenes AlkynesDocument2 pagesAlkanes Alkenes AlkynesGAMEPORIUMNo ratings yet
- Chemistry - Overview of Aliphatic Organic ChemistryDocument1 pageChemistry - Overview of Aliphatic Organic Chemistryhelixate100% (5)
- X Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLDocument1 pageX Uv H /PT HX Mno4 H or Oh NH (Alc) Heat NH (Alc) : PCL PCL Socl ZN/HCLEmily McCullochNo ratings yet
- Organic Chemistry I Problem Set AlcoholsDocument2 pagesOrganic Chemistry I Problem Set AlcoholssaddamixoNo ratings yet
- X UV Light or Heat: Reactions in Topic XIDocument3 pagesX UV Light or Heat: Reactions in Topic XImichelsonyip100% (1)
- 12 Chemistry Notes ch11 Alcohols Phenols and EthersDocument8 pages12 Chemistry Notes ch11 Alcohols Phenols and Ethersmv7602456No ratings yet
- Reaction of Aldehyde CompleteDocument1 pageReaction of Aldehyde CompleteJoko SusiloNo ratings yet
- SynrxnsDocument48 pagesSynrxnsRonak MantriNo ratings yet
- AminesDocument24 pagesAminesRajdeep Singh RahiNo ratings yet
- JC1 Chemistry Organic Reagent Practice - HWDocument14 pagesJC1 Chemistry Organic Reagent Practice - HWTesar DzikrullohNo ratings yet
- Organic Mindmap (Condensed) 2021 AnswersDocument2 pagesOrganic Mindmap (Condensed) 2021 AnswersbeverlyyyNo ratings yet
- Chemical Reactions of Alkanes: Mechanism Reaction Reagent Condition Catalysts Product(s)Document9 pagesChemical Reactions of Alkanes: Mechanism Reaction Reagent Condition Catalysts Product(s)Sam LeeNo ratings yet
- Weapon 4Document1 pageWeapon 4md.muhibmusabbir1101No ratings yet
- 11 Alcohols Phenols and EthersDocument2 pages11 Alcohols Phenols and EthersVarun Sankpal100% (1)
- Product ListDocument3 pagesProduct ListjagrutiNo ratings yet
- Solutions To HW #7Document2 pagesSolutions To HW #7Try “Play videos” MusicNo ratings yet
- 13 - BhattiAcademy - Com - Chemistry - 5. Scholar Series (Obj)Document11 pages13 - BhattiAcademy - Com - Chemistry - 5. Scholar Series (Obj)Amir FarooqNo ratings yet
- Carboxylic Acids and Their DerivativesDocument22 pagesCarboxylic Acids and Their DerivativesEugene OkpanteyNo ratings yet
- Ujian Bertulis TJC401 Mac-Jul 2018Document5 pagesUjian Bertulis TJC401 Mac-Jul 2018Siti Maryam UmairahNo ratings yet
- Great Orthogonality TheoremDocument4 pagesGreat Orthogonality TheoremSagar RawalNo ratings yet
- SettingsproviderDocument22 pagesSettingsproviderJose maria MartinezNo ratings yet
- The Elements Science Crossword Puzzle Worksheet in Colorful Lined Style - 20240322 - 174014 - 0000Document3 pagesThe Elements Science Crossword Puzzle Worksheet in Colorful Lined Style - 20240322 - 174014 - 0000Jessica García LópezNo ratings yet
- Project On: Action On A Set: Submission Date 01/10/2019 Sub. Instructor To . (M.SC)Document11 pagesProject On: Action On A Set: Submission Date 01/10/2019 Sub. Instructor To . (M.SC)shambelNo ratings yet
- Alkohol, Eter, Aldehid, KetonDocument21 pagesAlkohol, Eter, Aldehid, KetonFaesal AmrullahNo ratings yet
- IUPAC Short Notes Nitesh DevnaniDocument4 pagesIUPAC Short Notes Nitesh Devnaniunderprocess786No ratings yet
- Chapter 1 - Aldehydes KetonesDocument51 pagesChapter 1 - Aldehydes KetonesSarathy Hari KumarNo ratings yet
- Aldehyde Ketone PPT 4Document9 pagesAldehyde Ketone PPT 4muskan dahiyaNo ratings yet
- VisakhapatnamDocument18 pagesVisakhapatnamStepinconsultancy NamchettyNo ratings yet
- HaloalkaneDocument20 pagesHaloalkaneHediarta Widiana PutraNo ratings yet
- Lectures On Lie Groups, Second EditionDocument161 pagesLectures On Lie Groups, Second Editionfernandega100% (3)
- 12.8 Aldehydes and Ketones Solution - PremiumDocument24 pages12.8 Aldehydes and Ketones Solution - PremiumJonathan ParkerNo ratings yet
- The Geometry of Some Special Arithmetic QuotientsDocument347 pagesThe Geometry of Some Special Arithmetic Quotientslandau1994100% (3)
- Aldehyde Ketone PPT 2Document21 pagesAldehyde Ketone PPT 2muskan dahiyaNo ratings yet
- MATH 3E03 Lagrange's Theorem Questions: SolutionDocument2 pagesMATH 3E03 Lagrange's Theorem Questions: SolutionArtianaNo ratings yet
- Das & Okubo-Lie Groups and Lie Algebras For Physicists PDFDocument358 pagesDas & Okubo-Lie Groups and Lie Algebras For Physicists PDFAlfredo Echegollen Guzmán100% (5)
- Toribio Medina 1930 Bibliografia Quechua AymaraDocument124 pagesToribio Medina 1930 Bibliografia Quechua AymaraOlivia DaltreyNo ratings yet
- Subgroups: Sergei Silvestrov Spring Term 2011, Lecture 9Document12 pagesSubgroups: Sergei Silvestrov Spring Term 2011, Lecture 9prathimaNo ratings yet
- PK# RT Hit Compound Name Match R.Match CAS LibraryDocument11 pagesPK# RT Hit Compound Name Match R.Match CAS LibraryGerson JoelNo ratings yet
- Geometric Group Theory NotesDocument58 pagesGeometric Group Theory NotesAllenNo ratings yet
- Group Theory Calculations Involving Linear MoleculesDocument12 pagesGroup Theory Calculations Involving Linear MoleculesJonathanNo ratings yet
- Presentation For Subgroups PDFDocument32 pagesPresentation For Subgroups PDFMary John Pinuela100% (1)
- CCN MHT CET Synopsis PDFDocument7 pagesCCN MHT CET Synopsis PDFAbhishek Mandlik100% (1)
- Soluble GroupsDocument2 pagesSoluble GroupsJaime GutiérrezNo ratings yet