Professional Documents
Culture Documents
Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not Detected
Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not Detected
Uploaded by
Irvin SamaniegoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not Detected
Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not Detected
Uploaded by
Irvin SamaniegoCopyright:
Available Formats
Covid-19 Report
Safeguard DNA Diagnostics Inc
#3 Iñigo St., Barrio Obrero, Davao City, Philippines
(+63) 968 854 2621
Medical Director: John Patrick C. Padilla, MD, FPSP
#0089855
Patient Information Specimen Information Facility Information
Name: IRVIN BALTAZAR SAMANIEGO Accession Number: COV−40977 Facility Name: Nephrology Center of
Buhangin Dialysis, Inc.
DOB: 12/04/1991 Date Collected: 09/24/2021 09:00
Provider Name: John Patrick C. Padilla,
Gender: M Date Received: 09/24/2021 15:44 M.D., F.P.S.P
Nationality: FILIPINO Report Date: 09/25/2021 04:27 Address: Lot 6 Blk 14, Km. 6, C.P. Garcia
Ave., Buhangin, Davao, Davao del Sur, 8000
Clinical Notes from Ordering Physician: Sample Type: Nasopharyngeal Dry Swab
Covid-19 Test Result Summary
SARS-COV-2 VIRAL RNA - NOT DETECTED
Results Comment: Negative for SARS-CoV-2
Processing and Detection Methodology:
Test Performed:
SARS-CoV-2 (causative agent of COVID-19) virus detection by Real-Time Polymerase Chain Reaction.
Test Description:
The test includes Ribonucleic Acid (RNA) extraction with RT-PCR amplification and detection of SARS-Cov-2 virus gene, with positive and negative controls on each run.
Detection is dependent on the concentration of viral RNA in the specimen and the stage of disease. Quality of the specimen collection can affect the test results.
This test was developed, and its performance characteristics determined by, Co-Diagnostics Inc. It has received EUA approval by the Philippine and U.S. FDA. This test is used
for clinical purposes and should not be regarded as investigational or for research. This test has been validated in accordance with the FDA's Guidance Document ,Policy for
Diagnostics Testing in Laboratories Certified to Perform High Complexity Testing under CLIA prior to Emergency Use Authorization for Coronavirus Disease-2019 during the
Public Health Emergency, issued on February 29th, 2020. Safeguard DNA Diagnostics Inc. laboratory has been inspected by WHO, DOH and RITM and licensed by the
Philippine Department of Health as a COVID-19 testing laboratory.
This report has been electronically validated. No additional signature is required.
Disclaimer: This laboratory result should be interpreted together with the available clinical and epidemiological information. Please see SDDI.com.Ph for more information.
If Final Result is: Interpretation is:
SARS-CoV-2 viral RNA - DETECTED Positive for SARS-CoV-2 (causative agent of COVID-19)
SARS-CoV-2 viral RNA - NOT DETECTED Negative for SARS-CoV-2 (causative agent of COVID-19)
SARS-CoV-2 viral RNA - INCONCLUSIVE Negative for test internal control (most likely poor specimen quality)
This test was performed by Safeguard DNA Diagnostics, #3 Iñigo St., Barrio Obrero, Davao City, Philippines Phone:(+63) 968 854 2621
Patient - IRVIN BALTAZAR SAMANIEGO Accession - COV−40977 Page 1 of 1
You might also like
- Red Light Camera Effectiveness EvaluationDocument33 pagesRed Light Camera Effectiveness EvaluationRochester Democrat and ChronicleNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Kelli Belli100% (1)
- Philippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationDocument1 pagePhilippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationFrancis SevillenoNo ratings yet
- Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedDocument1 pageCovid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedRodel OrtegaNo ratings yet
- Philippine Red Cross Molecular Laboratory Result Form: Date: NameDocument1 pagePhilippine Red Cross Molecular Laboratory Result Form: Date: NameJohn De VillaNo ratings yet
- Tiny Tweaks James WedmoreDocument31 pagesTiny Tweaks James WedmoreErise Global100% (1)
- Muet 2006 To 2019 Past PapersDocument148 pagesMuet 2006 To 2019 Past PapersUmaid Ali Keerio100% (2)
- Biocredit COVID 19 Antigen Test Result Summary: NegativeDocument1 pageBiocredit COVID 19 Antigen Test Result Summary: NegativeJoan Ano CaneteNo ratings yet
- Bruce Scott Harvey Kwong TanDocument1 pageBruce Scott Harvey Kwong TanSean Kirby Kwong Tan 陈传信No ratings yet
- Check Out This File: COV-350851-1-SARS-CoV-2 - 2019-nCoV-1632039566Document1 pageCheck Out This File: COV-350851-1-SARS-CoV-2 - 2019-nCoV-1632039566Joana Marie DomingoNo ratings yet
- Biocredit COVID 19 Antigen Test Result Summary: NegativeDocument1 pageBiocredit COVID 19 Antigen Test Result Summary: NegativeYuuki zednanreFNo ratings yet
- Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedDocument1 pageCovid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not Detectedpogito ramosNo ratings yet
- Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedDocument1 pageCovid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedGEr JrvillaruElNo ratings yet
- LabResultTempPDF CJ0304865Document2 pagesLabResultTempPDF CJ0304865Jahred EstebanNo ratings yet
- Velasco, Crestita VelosoDocument1 pageVelasco, Crestita VelosoAdan NunungNo ratings yet
- CGH202106011819 Lab-2021-0279065 Laboratory Covid-Pcr-TestDocument2 pagesCGH202106011819 Lab-2021-0279065 Laboratory Covid-Pcr-TestAaron David SubaNo ratings yet
- This Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureDocument1 pageThis Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureIssa LlamasNo ratings yet
- Molecular Laboratory Test Result: de Loreto, San Isidro, City of Parañaque, NCR, Fourth District (Not A Province)Document2 pagesMolecular Laboratory Test Result: de Loreto, San Isidro, City of Parañaque, NCR, Fourth District (Not A Province)JJS INTERNATIONAL PLACEMENT AGENCY COMPANYNo ratings yet
- Sars-Cov-2 Reverse Transcription PCR (RT-PCR) ReportDocument1 pageSars-Cov-2 Reverse Transcription PCR (RT-PCR) ReportJanice AbasNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- Sta. Ana Hospital: Covid-19 Testing LaboratoryDocument1 pageSta. Ana Hospital: Covid-19 Testing LaboratoryNestor Limos Alfaro Jr.No ratings yet
- RESULTDocument1 pageRESULTjenifer bongaoNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?PerlaNo ratings yet
- Test Result Mary Hibet Torres VelezDocument1 pageTest Result Mary Hibet Torres Velezmarytorres8793No ratings yet
- Alcantara, Charmine Swab PDFDocument1 pageAlcantara, Charmine Swab PDFJohnmar AquinoNo ratings yet
- Philippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationDocument1 pagePhilippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationChris-Goldie LorezoNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not Detected (-)Document1 pageSars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not Detected (-)jeffry billanNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- This Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureDocument1 pageThis Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureIssa LlamasNo ratings yet
- Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedDocument2 pagesCovid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedFlordeliza VillarNo ratings yet
- Philippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationDocument1 pagePhilippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationAldrin TevesNo ratings yet
- (Full Last Name, First Name, Middle Name) : Lrd-Div-Spe-Fm-005Document2 pages(Full Last Name, First Name, Middle Name) : Lrd-Div-Spe-Fm-005RGC Calamba FacilityNo ratings yet
- Philippine Red Cross Molecular LaboratoryDocument2 pagesPhilippine Red Cross Molecular LaboratoryAngel Lou VillamonteNo ratings yet
- Sars-Cov-2/Covid-19 Test Report: Amendments / CorrectionsDocument1 pageSars-Cov-2/Covid-19 Test Report: Amendments / CorrectionsSebastian PradaNo ratings yet
- CGH202107021415 Lab-2021-0338689 Laboratory Covid-Pcr-TestDocument2 pagesCGH202107021415 Lab-2021-0338689 Laboratory Covid-Pcr-Testmichellene queNo ratings yet
- Covid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedDocument2 pagesCovid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedRonni PriceNo ratings yet
- This Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureDocument1 pageThis Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureRyan FernandezNo ratings yet
- Test Result Yarelis Alana Casillas SantiagoDocument1 pageTest Result Yarelis Alana Casillas SantiagoYarelis A.No ratings yet
- Philippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationDocument1 pagePhilippine Red Cross Molecular Laboratory: Covid-19 RT-PCR Test Report & CertificationCornelio J. FernandezNo ratings yet
- QCMDL 21 51393 Relata Leonardo NacionalDocument1 pageQCMDL 21 51393 Relata Leonardo NacionalAngel DetablanNo ratings yet
- Negative: Patient Information Specimen Information Provider InformationDocument1 pageNegative: Patient Information Specimen Information Provider InformationMelissa LopezNo ratings yet
- Patients Profile: Not Detected NegativeDocument2 pagesPatients Profile: Not Detected NegativeELLIE JAMES PLACIONo ratings yet
- ARNEL HERMIDA_SARS_COV_2_RT_PCR_BEST_DIAGNOSTICSDocument2 pagesARNEL HERMIDA_SARS_COV_2_RT_PCR_BEST_DIAGNOSTICSgezillemayespinozaNo ratings yet
- PCR COVID-19: Negative: If You Have Any Questions Regarding This Report Please Contact Your ProviderDocument1 pagePCR COVID-19: Negative: If You Have Any Questions Regarding This Report Please Contact Your ProviderLuis ReyesNo ratings yet
- Sta. Ana Hospital: Covid-19 Testing LaboratoryDocument1 pageSta. Ana Hospital: Covid-19 Testing LaboratoryRamel Yen CerantesNo ratings yet
- CGH202011011832 Lab-2020-0356025 Laboratory Covid-Pcr-TestDocument2 pagesCGH202011011832 Lab-2020-0356025 Laboratory Covid-Pcr-TestJhon Rosete ParicoNo ratings yet
- Nabin Tala Khanal ChaliseDocument1 pageNabin Tala Khanal ChaliseChalise SupremeNo ratings yet
- CGH202008000915 - Lab A2 2020 2231 - Laboratory - Covid PCR Test PDFDocument2 pagesCGH202008000915 - Lab A2 2020 2231 - Laboratory - Covid PCR Test PDFMichael JonasanNo ratings yet
- Clinical Laboratory Report Supreme Chalise: Test Name Result Flag Unit Reference RangeDocument1 pageClinical Laboratory Report Supreme Chalise: Test Name Result Flag Unit Reference RangeChalise SupremeNo ratings yet
- Rgat08967-Borromeo, Reynard PaglinawanDocument1 pageRgat08967-Borromeo, Reynard Paglinawanzanello grullaNo ratings yet
- Swab TestDocument2 pagesSwab TestGrey Del PilarNo ratings yet
- CGH202011008542 Lab-2020-0351447 Laboratory Covid-Pcr-Test PDFDocument2 pagesCGH202011008542 Lab-2020-0351447 Laboratory Covid-Pcr-Test PDFMae SampangNo ratings yet
- QCMDL 21 57987 Beltran Karen Villavicensio 1Document1 pageQCMDL 21 57987 Beltran Karen Villavicensio 1lemuel clausNo ratings yet
- Molecular Pathology ResultDocument2 pagesMolecular Pathology Resultsalima saripNo ratings yet
- Philippine Red Cross Molecular Laboratory Result Form: Date: NameDocument1 pagePhilippine Red Cross Molecular Laboratory Result Form: Date: NamePatrick John Estrada GayoNo ratings yet
- CGH202012022479 Lab-2020-0411918 Laboratory Covid-Pcr-TestDocument2 pagesCGH202012022479 Lab-2020-0411918 Laboratory Covid-Pcr-TestJosa Camille BungayNo ratings yet
- Aragaw 206714-1 364272Document1 pageAragaw 206714-1 364272zeine omerNo ratings yet
- KenDocument2 pagesKenRosemarie RomeroNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodPritam JanaNo ratings yet
- Result 14164 00251Document2 pagesResult 14164 00251Ministerio Sobrenatural GlobalNo ratings yet
- Covid-19 RT-PCR: Test Results PanelDocument1 pageCovid-19 RT-PCR: Test Results PanelPatricia Cottle-SalyerNo ratings yet
- This Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureDocument1 pageThis Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureIssa LlamasNo ratings yet
- SARS-CoV-2 Viral Outbreak Investigation: Laboratory Perspective: Clinical Updates in COVID-19From EverandSARS-CoV-2 Viral Outbreak Investigation: Laboratory Perspective: Clinical Updates in COVID-19Rating: 3 out of 5 stars3/5 (1)
- Splitter DimensionDocument2 pagesSplitter DimensionIrvin SamaniegoNo ratings yet
- Disturbed Area Monitoring - 26dec21Document9 pagesDisturbed Area Monitoring - 26dec21Irvin SamaniegoNo ratings yet
- Table of Organization 2020: Title: Department: Mine Department YearDocument1 pageTable of Organization 2020: Title: Department: Mine Department YearIrvin SamaniegoNo ratings yet
- Geological Logging Look Up Tables: Gansu Longjin Mining Resources CompanyDocument4 pagesGeological Logging Look Up Tables: Gansu Longjin Mining Resources CompanyIrvin SamaniegoNo ratings yet
- SimonSezIT MovieRentalActorImportDocument1 pageSimonSezIT MovieRentalActorImportIrvin SamaniegoNo ratings yet
- Database Exercise: Primary KEY Field Name Data TypeDocument3 pagesDatabase Exercise: Primary KEY Field Name Data TypeIrvin SamaniegoNo ratings yet
- DAX Cheat Sheet: Basic Aggregate and Math FunctionsDocument5 pagesDAX Cheat Sheet: Basic Aggregate and Math FunctionsIrvin SamaniegoNo ratings yet
- SurpacDocument2 pagesSurpacIrvin SamaniegoNo ratings yet
- Q4 PaperDeni IJEEDocument8 pagesQ4 PaperDeni IJEEIrvin SamaniegoNo ratings yet
- Ore Dilution Estimation Model in Tanzania Southern Highland Zone: A Case of New Luika Gold MineDocument6 pagesOre Dilution Estimation Model in Tanzania Southern Highland Zone: A Case of New Luika Gold MineIrvin SamaniegoNo ratings yet
- Mine Value Chain Reconciliation - Demonstrating VaDocument15 pagesMine Value Chain Reconciliation - Demonstrating VaIrvin SamaniegoNo ratings yet
- Esprit de Tour Trip ImportDocument6 pagesEsprit de Tour Trip ImportIrvin SamaniegoNo ratings yet
- International Journal of Mining Science and TechnologyDocument6 pagesInternational Journal of Mining Science and TechnologyIrvin SamaniegoNo ratings yet
- SimonSezIT NightMoviesMovieImportDocument1 pageSimonSezIT NightMoviesMovieImportIrvin SamaniegoNo ratings yet
- Esprit de Tour - Our Current Tours: Monday, May 30, 2016 Page 1 of 14Document14 pagesEsprit de Tour - Our Current Tours: Monday, May 30, 2016 Page 1 of 14Irvin SamaniegoNo ratings yet
- KCM Nchanga Monthly Geological Report To GSD - July 2018Document2 pagesKCM Nchanga Monthly Geological Report To GSD - July 2018Irvin Samaniego0% (1)
- Zero Based Budget WorksheetDocument13 pagesZero Based Budget WorksheetIrvin Samaniego100% (2)
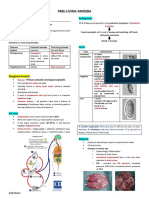
- Free-Living Amoeba: General CharacteristicsDocument3 pagesFree-Living Amoeba: General CharacteristicsIrvin SamaniegoNo ratings yet
- Technical Drawings of PlasticwareDocument69 pagesTechnical Drawings of PlasticwareGuldu KhanNo ratings yet
- Air Cooled Flooded Screw ChillersDocument50 pagesAir Cooled Flooded Screw ChillersAhmed Sofa100% (1)
- Practice Questions: Musculoskeletal SystemDocument4 pagesPractice Questions: Musculoskeletal SystemSali IqraNo ratings yet
- Analysis of StructureDocument4 pagesAnalysis of StructureCarlson CaliwanNo ratings yet
- A Human Orrery: Materials PreparationDocument4 pagesA Human Orrery: Materials Preparationari sudrajatNo ratings yet
- Statement of PurposeDocument3 pagesStatement of PurposeehsanNo ratings yet
- 12 Abm Bangelyns Group1Document64 pages12 Abm Bangelyns Group1G-VALDEZ, SHELAHNo ratings yet
- Sd10 Forecasting Free CashflowsDocument8 pagesSd10 Forecasting Free CashflowsDemi Tugano BernardinoNo ratings yet
- (Shuangzhu Jia Et Al 2020) Study On The Preparing and Mechanism of Chitosan-Based Nanomesoporous Carbons by Hydrothermal MethodDocument21 pages(Shuangzhu Jia Et Al 2020) Study On The Preparing and Mechanism of Chitosan-Based Nanomesoporous Carbons by Hydrothermal MethodSilvia Devi Eka PutriNo ratings yet
- Vol-37 Let's Eat! MagazineDocument80 pagesVol-37 Let's Eat! MagazineLetseatmagNo ratings yet
- Genuine Eaton Vicker HidrauDocument28 pagesGenuine Eaton Vicker HidrauJenner Volnney Quispe ChataNo ratings yet
- The First Vertebrates, Jawless Fishes, The Agnathans: 2.1 OstracodermsDocument22 pagesThe First Vertebrates, Jawless Fishes, The Agnathans: 2.1 OstracodermsAlejandro Tepoz TelloNo ratings yet
- Rope PDFDocument19 pagesRope PDFBenjamin van DierenNo ratings yet
- Akira - GA - Intro-To-Coding-Html-CssDocument46 pagesAkira - GA - Intro-To-Coding-Html-CssVinoth JayaprakasamNo ratings yet
- Christmas Vigil MassDocument106 pagesChristmas Vigil MassMary JosephNo ratings yet
- SAILOR 6081 Power Supply Unit and Charger: Installation ManualDocument72 pagesSAILOR 6081 Power Supply Unit and Charger: Installation ManualMariosNo ratings yet
- Jambajuicelv-Application-0618 1Document2 pagesJambajuicelv-Application-0618 1api-526082107No ratings yet
- 10 Science NcertSolutions Chapter 8 ExercisesDocument4 pages10 Science NcertSolutions Chapter 8 ExercisesAnita GargNo ratings yet
- BNVD Eaufrance Metadonnees Vente 20230130Document16 pagesBNVD Eaufrance Metadonnees Vente 20230130moussaouiNo ratings yet
- Gcrouch@wsu - Edu Rmancini@wsu - Edu Andreakl@wsu - Edu: Groups/chem.345Document5 pagesGcrouch@wsu - Edu Rmancini@wsu - Edu Andreakl@wsu - Edu: Groups/chem.345Daniel McDermottNo ratings yet
- Labour Law and Employment in Slovakia - 2019 GuideDocument13 pagesLabour Law and Employment in Slovakia - 2019 GuideAccaceNo ratings yet
- (00 Cari) - Iso - 8466 1 1990Document5 pages(00 Cari) - Iso - 8466 1 1990faridsidikNo ratings yet
- KISI USP INGGRIS Kelas 12Document39 pagesKISI USP INGGRIS Kelas 12Deny Cahyo SaputroNo ratings yet
- Power Electronics: Thyristor Controlled Power For Electric MotorsDocument10 pagesPower Electronics: Thyristor Controlled Power For Electric Motorsshahab moinNo ratings yet
- 3I Grading Rubric For Output PresentationDocument2 pages3I Grading Rubric For Output PresentationBinibining Michelle CenizaNo ratings yet
- Chapter 6Document18 pagesChapter 6Lydelle Mae CabaltejaNo ratings yet
- Lienard EquationDocument9 pagesLienard EquationmenguemengueNo ratings yet