Professional Documents
Culture Documents
Molecular Geometry
Molecular Geometry
Uploaded by
IsraClarke0 ratings0% found this document useful (0 votes)
78 views1 pageThe document outlines molecular geometry rules including:
1) The number of electron domains around an atom determines its geometry, such as tetrahedral for 4 domains and trigonal bipyramidal for 5 domains.
2) Bond angles are affected by the number of lone pairs on the central atom, with lone pairs causing greater repulsion and smaller bond angles compared to single bonds.
3) Molecular polarity depends on whether bond dipoles cancel out, with molecules containing unequal bonds or lone pairs usually being polar.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document outlines molecular geometry rules including:
1) The number of electron domains around an atom determines its geometry, such as tetrahedral for 4 domains and trigonal bipyramidal for 5 domains.
2) Bond angles are affected by the number of lone pairs on the central atom, with lone pairs causing greater repulsion and smaller bond angles compared to single bonds.
3) Molecular polarity depends on whether bond dipoles cancel out, with molecules containing unequal bonds or lone pairs usually being polar.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
78 views1 pageMolecular Geometry
Molecular Geometry
Uploaded by
IsraClarkeThe document outlines molecular geometry rules including:
1) The number of electron domains around an atom determines its geometry, such as tetrahedral for 4 domains and trigonal bipyramidal for 5 domains.
2) Bond angles are affected by the number of lone pairs on the central atom, with lone pairs causing greater repulsion and smaller bond angles compared to single bonds.
3) Molecular polarity depends on whether bond dipoles cancel out, with molecules containing unequal bonds or lone pairs usually being polar.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
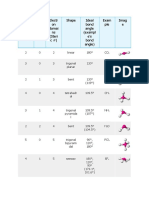
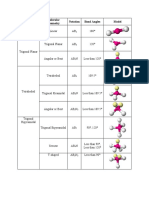
# of e- e- domain # of # of non- Molecular Ideal Bond General Example
domains geometry bonding bonding geometry Angles formula
domains domains
2 Linear 2 0 Linear 180° AX2 CO2
3 Trigonal 3 0 Trigonal 120° AX3 CO32-
Planar Planar
2 1 Bent 120° AX2E NO2-
4 Tetrahedral 4 0 Tetrahedral 109.5° AX4 CH4
3 1 Trigonal 109.5° AX3E NH3
Pyramidal
2 2 Bent 109.5° AX2E2 H2O
5 Trigonal 5 0 Trigonal 120°, 90° AX5 SbF5
Bipyrimidal Bipyramidal
4 1 See-saw 120°, 90° AX4E SF4
3 2 T-shaped 90° AX3E2 ClF3
2 3 Linear 180° AX2E3 XeF2
6 Octahedral 6 0 Octahedral 90° AX6 SF6
5 1 Square 90° AX5E IF5
Pyramidal
4 2 Square Planar 90° AX4E2 XeF4
Bond Angle Adjustments: Lone pairs repel other e- domains more than bonds do ( ≈ 2.5° per L.P. )
Example:
CH4 vs. NH3 vs. H2O
∠HCH = 109.5° ∠HNH = 107° ∠ HOH = 104.5°
Polarity Trends: A molecule will be non-polar if all dipoles cancel out, otherwise, it will be polar.
• Formula: AXnE0 with all the X's the same → Non-Polar Molecule
• Formula: AXnE0 with not all A-X bonds identically polar → Polar Molecule
• Formula: AXnEn≥1 → Usually Polar
◦ Exception : XeF4
Hypervalent Repulsion Energy:
LonePair-LonePair > LonePair-BondingPair > BondingPair-BondingPair
You might also like
- ASTM D6751 - Specification For Biodiesel PDFDocument8 pagesASTM D6751 - Specification For Biodiesel PDFDwi Wahyu Ramadhan100% (1)
- Molarity - Worksheet 1 Ans KeyDocument4 pagesMolarity - Worksheet 1 Ans KeyThentamilselvi MNo ratings yet
- Vsepr Theory Summary ChartDocument2 pagesVsepr Theory Summary ChartLittle One100% (2)
- Shapes of Molecules & Ions: Name . . FormDocument2 pagesShapes of Molecules & Ions: Name . . FormjnfjngsdjNo ratings yet
- Si and Ni As Alloying Elements To Vary Carbon Equivalent of Austenitic Ductile Cast Iron - Microstructure and Mechanical Properties-2Document9 pagesSi and Ni As Alloying Elements To Vary Carbon Equivalent of Austenitic Ductile Cast Iron - Microstructure and Mechanical Properties-2Chun-Yi LinNo ratings yet
- Denka Evolmer Bro 1Document5 pagesDenka Evolmer Bro 1mouds22100% (1)
- 001 QC 1 Lecture by LPB EditedDocument70 pages001 QC 1 Lecture by LPB EditedQuina PerezNo ratings yet
- Geometry of Molecules ChartDocument6 pagesGeometry of Molecules ChartShamsiNo ratings yet
- General Chemistry 1 Qt. 2 Week 4Document12 pagesGeneral Chemistry 1 Qt. 2 Week 4Nina Reca OmisolNo ratings yet
- LAS Physical-Science Week2Document11 pagesLAS Physical-Science Week2Shekaina Faith Cuizon LozadaNo ratings yet
- Molecular ShapeDocument1 pageMolecular ShapeNUR DEENA KHALID KM-PensyarahNo ratings yet
- Shapes of Covalent MoleculesDocument5 pagesShapes of Covalent MoleculesSiya ChiniahNo ratings yet
- Electron Domains and Molecular Geometry IBDP ChemistryDocument1 pageElectron Domains and Molecular Geometry IBDP Chemistryyasmeen alkhaterNo ratings yet
- VSEPR TableDocument1 pageVSEPR TableAudrey HizonNo ratings yet
- Introduction To MolecularDocument1 pageIntroduction To Molecularclairole quilantangNo ratings yet
- Formula 1Document3 pagesFormula 1ahmad90616No ratings yet
- VSEPRDocument1 pageVSEPRĐan KhanhNo ratings yet
- Molecular Geometry Unit 02Document26 pagesMolecular Geometry Unit 02Iqra BaigNo ratings yet
- Las 7Document3 pagesLas 7Carl DoriaNo ratings yet
- Bondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eDocument3 pagesBondi NG Electr On Pairs Lon e Pair S Electr On Domai Ns (Steri C#) Shape Ideal Bond Angle (Exampl E's Bond Angle) Exam Ple Imag eaadhyaNo ratings yet
- Vsepr HandoutDocument2 pagesVsepr Handout20718 LAY BUFFON FERNANDO GROSSONo ratings yet
- Vsepr HandoutDocument2 pagesVsepr HandoutAdrianne Jericho ValdezNo ratings yet
- VSEPR Handout PDFDocument2 pagesVSEPR Handout PDFLittle One0% (1)
- Vsepr-HlDocument25 pagesVsepr-HlRyan BoukaaNo ratings yet
- Electron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageDocument2 pagesElectron Domains (Steric Number) Atoms Bonded To Central Atom Lone Pairs Shape Bond Angle Example ImageBianca GuillermoNo ratings yet
- Lecture B2Document72 pagesLecture B2Nárēsh Yadav GäddēNo ratings yet
- Worktable For Shape and PolarityDocument2 pagesWorktable For Shape and PolarityDestinee LegendsNo ratings yet
- Bond Angle ChartDocument1 pageBond Angle ChartxwenhanNo ratings yet
- Steric No. Form Shape Angle HybridizationDocument1 pageSteric No. Form Shape Angle Hybridizationmica_tsukadaNo ratings yet
- Molecular Shapes: Course Outcome 3Document13 pagesMolecular Shapes: Course Outcome 3tin canNo ratings yet
- VSEPR and Molecular Geometries (Summery)Document2 pagesVSEPR and Molecular Geometries (Summery)MihadNo ratings yet
- Chapter 5: Chemical BondingDocument36 pagesChapter 5: Chemical BondingCt Sophie PheaNo ratings yet
- Vsepr Table PDFDocument1 pageVsepr Table PDFlucasNo ratings yet
- Chemical Bonding Double Prahaar - V1.3-ARCHIDocument60 pagesChemical Bonding Double Prahaar - V1.3-ARCHINeha esaralNo ratings yet
- Worksheet Vsepr Theory Using The Knowledge of VSEPR Theory, Complete The Table Given BelowDocument2 pagesWorksheet Vsepr Theory Using The Knowledge of VSEPR Theory, Complete The Table Given Belownidhi1478No ratings yet
- Molecular Geometry Chart (VSEPR Shapes)Document1 pageMolecular Geometry Chart (VSEPR Shapes)laila SheashaNo ratings yet
- 3.7 Geometry and Dipole MomentDocument9 pages3.7 Geometry and Dipole Momentelbadry mohamedNo ratings yet
- Vsepr ChartDocument1 pageVsepr ChartpankajNo ratings yet
- Electron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleDocument2 pagesElectron Groups Bonding Groups Lone Pairs Electronic Geometry Molecular Geometry Approximate Bond Angles ExampleRichamille Ann RicaforteNo ratings yet
- Nota VSEPR PDFDocument1 pageNota VSEPR PDFMarlene GazconNo ratings yet
- VSEPR GeometriesDocument1 pageVSEPR GeometriesJason JacksonNo ratings yet
- Chemical Bonding (F Only)Document28 pagesChemical Bonding (F Only)Raju SinghNo ratings yet
- 3VSEPR Theory 41-48Document8 pages3VSEPR Theory 41-48Raj KishoreNo ratings yet
- Compound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclDocument1 pageCompound Hybridization Lone Pair Bond Angle (°) Shape Becl Co BF CH NH H O PCL SF Xef Xef Xef NH BF CoclSakib KhanNo ratings yet
- UntitledDocument53 pagesUntitledchandrakanth maheshNo ratings yet
- CHM01 CO3 LESSON2 Molecular-ShapesDocument14 pagesCHM01 CO3 LESSON2 Molecular-ShapesErica MamauagNo ratings yet
- CHM01 CO3 LESSON2 Molecular-ShapesDocument14 pagesCHM01 CO3 LESSON2 Molecular-ShapesErica Mamauag0% (1)
- 3 1 3 As Shapes of Molecules ChemsheetsDocument27 pages3 1 3 As Shapes of Molecules ChemsheetsAkshar PatelNo ratings yet
- 3 AB Trigonal Planar Trigonal Planar 120 Between All BondsDocument5 pages3 AB Trigonal Planar Trigonal Planar 120 Between All BondsVedantNo ratings yet
- Lewis StructureDocument1 pageLewis Structureits aryamNo ratings yet
- Chemical BondingDocument28 pagesChemical BondingPrince DigvijayNo ratings yet
- Che2060 Vsepr Geometry Ws KeyDocument5 pagesChe2060 Vsepr Geometry Ws Keyqvcws4h5spNo ratings yet
- Chemistry-Molecular GeometryDocument2 pagesChemistry-Molecular GeometryBubbles Bubbles100% (1)
- Shapes of MoleculesDocument25 pagesShapes of MoleculesAsaph AharoniNo ratings yet
- Molecular GeometryDocument1 pageMolecular GeometryDean Joyce Alboroto100% (1)
- ACTIVITY SHEET Geometry of Simple CompoundsDocument4 pagesACTIVITY SHEET Geometry of Simple CompoundsUy, Jhavelaine Cassandra F.No ratings yet
- Vsepr TheoryDocument13 pagesVsepr TheorySana AjmalNo ratings yet
- 08 HybridizationPolarity PDFDocument22 pages08 HybridizationPolarity PDFROSEMARIE ONGNo ratings yet
- Geometry of MoleculesDocument16 pagesGeometry of MoleculesArwind RoyNo ratings yet
- Hybridisation and Bond AngleDocument13 pagesHybridisation and Bond Angleskye sueNo ratings yet
- MolGeom and IMFsDocument21 pagesMolGeom and IMFsJune Dela CruzNo ratings yet
- Chem 16 2nd Long Exam Reviewer 2 (Answer Key)Document2 pagesChem 16 2nd Long Exam Reviewer 2 (Answer Key)ben_aldaveNo ratings yet
- CHM 201 2019-2020 Note1Document38 pagesCHM 201 2019-2020 Note1Adams TemitopeNo ratings yet
- Vsper WorksheetDocument5 pagesVsper WorksheetAtiq AqilahNo ratings yet
- Asfier N-480LDocument2 pagesAsfier N-480Lherry prasetyoNo ratings yet
- Pharmaceutical Suspensions - PDFDocument59 pagesPharmaceutical Suspensions - PDFGopalaKrishnan Sivaraman100% (2)
- Calcine Methods Standard and FlashDocument19 pagesCalcine Methods Standard and FlashKemoy JohnsonNo ratings yet
- CHEM I 17 Acid Base Titrations OpenDocument4 pagesCHEM I 17 Acid Base Titrations OpenAchinthya PereraNo ratings yet
- Lectuer 8Document7 pagesLectuer 8Chandra MynNo ratings yet
- Arrow MechanismDocument49 pagesArrow MechanismtakomolyentinNo ratings yet
- ACTIVITY 5 Pchem 1Document3 pagesACTIVITY 5 Pchem 1Shopifyy ClothingNo ratings yet
- Physical Pharmaceutics-Ii: B. Pharm (IV Sem) Physical Pharmacy-II BP 403T Unit - III Coarse DispersionsDocument10 pagesPhysical Pharmaceutics-Ii: B. Pharm (IV Sem) Physical Pharmacy-II BP 403T Unit - III Coarse Dispersionsdipti_srivNo ratings yet
- AISC Certification Search - DomesticDocument43 pagesAISC Certification Search - DomesticLuis PlateroNo ratings yet
- SSP FertilizerDocument12 pagesSSP FertilizerSo NicNo ratings yet
- Viva Master SlideDocument36 pagesViva Master Slidethivya keasavanNo ratings yet
- 257 - Basic Manufacturing Processes-Ilovepdf-Compressed PDFDocument112 pages257 - Basic Manufacturing Processes-Ilovepdf-Compressed PDFsoul tunesNo ratings yet
- Cocamidopropyl BetaineDocument4 pagesCocamidopropyl BetaineNgeke KekeNo ratings yet
- 03 - Ipp 07 JM 2019 17 - RevDocument8 pages03 - Ipp 07 JM 2019 17 - RevTrần Duy TânNo ratings yet
- Piping Cleaning ProcedureDocument13 pagesPiping Cleaning ProcedureKandang SawanganNo ratings yet
- Biodiesel and Other Chemicals From Vegetable Oils and Animal FatsDocument108 pagesBiodiesel and Other Chemicals From Vegetable Oils and Animal FatsYulianto KurniawanNo ratings yet
- Aisi 1008 Carbon Steel (Uns g10080)Document3 pagesAisi 1008 Carbon Steel (Uns g10080)parasite01100% (1)
- Saep 348 PDFDocument33 pagesSaep 348 PDFRami ElloumiNo ratings yet
- U5L3 Classifying Reactions Portfolio WordDocument4 pagesU5L3 Classifying Reactions Portfolio Wordskulls993No ratings yet
- Release Agent ComparisonDocument1 pageRelease Agent ComparisonsulingNo ratings yet
- Lecture 23-25 Mechanism and Applications of Polymerization in Aromatic CompoundsDocument35 pagesLecture 23-25 Mechanism and Applications of Polymerization in Aromatic CompoundsFalak SherNo ratings yet
- 2015 - Effects of Two-Step Homogenization On Precipitation Behavior of Al3Zr Dispersoids and Recrystallization Resistance in 7150 Aluminum AlloyDocument9 pages2015 - Effects of Two-Step Homogenization On Precipitation Behavior of Al3Zr Dispersoids and Recrystallization Resistance in 7150 Aluminum AlloyzhaomingbaoNo ratings yet
- Best Practices For Aromatics Extractive Distillation in Integrated ComplexesDocument8 pagesBest Practices For Aromatics Extractive Distillation in Integrated ComplexesNaiduJagarapuNo ratings yet
- Sour Water Treatment UnitDocument16 pagesSour Water Treatment Unitpkgarg_iitkgp100% (2)
- Chapter 1 IntroductionDocument41 pagesChapter 1 IntroductionertNo ratings yet