Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
2 viewsOH6
OH6
Uploaded by
Ian Vergel CuevasCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5823)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Brain Death in The Adult Patient: Kathrin Husmann, MD Assistant Professor Medical Director, NEICUDocument63 pagesBrain Death in The Adult Patient: Kathrin Husmann, MD Assistant Professor Medical Director, NEICUAiman ButtNo ratings yet
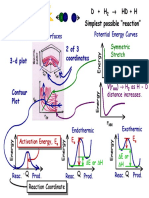
- 3-d Plot 2 of 3 Coordinates: Potential Energy Surfaces Potential Energy CurvesDocument9 pages3-d Plot 2 of 3 Coordinates: Potential Energy Surfaces Potential Energy CurvesAiman ButtNo ratings yet
- World AIDS Day Assembly 2019 - PowerPoint SlidesDocument17 pagesWorld AIDS Day Assembly 2019 - PowerPoint SlidesAiman ButtNo ratings yet
- Sample Powerpoint Presentations: 1. The Essentials of Hiv and AidsDocument27 pagesSample Powerpoint Presentations: 1. The Essentials of Hiv and AidsAiman ButtNo ratings yet
OH6
OH6
Uploaded by
Ian Vergel Cuevas0 ratings0% found this document useful (0 votes)
2 views5 pagesCopyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
0 ratings0% found this document useful (0 votes)
2 views5 pagesOH6
OH6
Uploaded by
Ian Vergel CuevasCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
You are on page 1of 5
MO APPROACH:
1, YOU ALWAYS END UP WITH AS
MANY ORBITALS AS YOu STARTED
WITH
2. BONDING CAN BE ACCOUNTED FOR
BY PUOITING ELECTRONS INTO
THE MOS BY HOUNDS ROLE ANO
PALLI EXCLUSION PRINCIPLE
3. BOND_ORDER:
ff BONDING — y ANTI-BONDING
22” ELECTRONS ELECTRONS
4. MOLECULAR CONFIGURATION:
ti, lot
N, 107 20%? §9 307°
lot 20%? 3o% int 2ne4
MOLECULAR ORBITALS AND BONDING
jp Ly “stk is
“4b ee :
ATOMIC ELECTRONIC STRUCTURE
Is? 2g? Zp* 35? see SG OF ELECTRONS
ATOMIC ORBITAL
MOLECULAR ELECTRONIC STRUCTURE
log 2og30, Youn, dh Sa or Evecrrons
Sime yy “TH —____— norecurae
lo? Jot? 3q74o%2 W!... ORBITAL
A, we Mo BO. Ran B.S,
dH; \ Q;' 08 100A 255 Se
Hy 2 o; 1 Off 432
Het 3 Igid! oS 108 322
He, 4 pis Oo °
oo eel
_ yh 1
ao tt ava
ap AL. 2p ja 4" Fp
kW ara
Aa___te Mo CONFIG. BS
i 3 7 B 105 ney
iz 6 Isa'lsotzsey | 267A Yon
Be, g asog 2s o. Oo
2 Ls 239
B, «10 290g 2so 2PM \
gq bey Oe
2
7 4,2 1.09% 94
N, 1 2PM PhO oo
2 1.20% 449%
oO le
FL oo wn Bp Zp Zp! \ 1.430 1S
HETERONUCLEAR DIATOMICS
H a x
‘ede. A
—L— “oF Op
Yo ? Cn is, + Celp t Cp 2p +Cr 2p,
Don's INTERACT
WITH H1sS
Yo = -33ls, + 4 2p, POLAR, oO ORBITAL
%, = any ' 2Pey NON- BONDING
You? .Wisy -332p, PocarR o* ORBITAL
DIPOLE MOMENT
ot aoe = 2
oo ter BL Gae
DISTRIBUTION OF SEPARATION OF CENTER
CHARGE OF Positive ANDO
NEGATIVE CHARGE
ON AVERAGE THE ELECTRON
> —sCDENSITY 16 ON F
St &-
DIPOLE HOMENT OPERATOR:
—
Ea NN
dure [ Wuvae = [etzeit bar
wf , ™
Pox s2,%Ya2,o") INTEGRATE OVER ALL ELECTRONS,
”
be $07) OG OG) -- Te (X,Y 8 XaYasteo )
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5823)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (898)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Brain Death in The Adult Patient: Kathrin Husmann, MD Assistant Professor Medical Director, NEICUDocument63 pagesBrain Death in The Adult Patient: Kathrin Husmann, MD Assistant Professor Medical Director, NEICUAiman ButtNo ratings yet
- 3-d Plot 2 of 3 Coordinates: Potential Energy Surfaces Potential Energy CurvesDocument9 pages3-d Plot 2 of 3 Coordinates: Potential Energy Surfaces Potential Energy CurvesAiman ButtNo ratings yet
- World AIDS Day Assembly 2019 - PowerPoint SlidesDocument17 pagesWorld AIDS Day Assembly 2019 - PowerPoint SlidesAiman ButtNo ratings yet
- Sample Powerpoint Presentations: 1. The Essentials of Hiv and AidsDocument27 pagesSample Powerpoint Presentations: 1. The Essentials of Hiv and AidsAiman ButtNo ratings yet