Professional Documents
Culture Documents
T/min 0 2 4 8 (A) / (M) 0.1 0.09 0.08 0.07 LN (A) - 2.3 - 2.4 - 2.5 - 2.7 LN (A) THDT
T/min 0 2 4 8 (A) / (M) 0.1 0.09 0.08 0.07 LN (A) - 2.3 - 2.4 - 2.5 - 2.7 LN (A) THDT
Uploaded by
Nuril AzmiCopyright:
Available Formats
You might also like
- Kuru TmaDocument3 pagesKuru Tmanahla6312No ratings yet
- IsotermasDocument14 pagesIsotermasDaniella HidalgoNo ratings yet
- Kinetics Lab DataDocument3 pagesKinetics Lab DataDianna NomanimNo ratings yet
- Tugas 5 MetNum 1 (Pengganti Kuis 2) RevisiDocument2 pagesTugas 5 MetNum 1 (Pengganti Kuis 2) RevisiFatih ArifNo ratings yet
- Model Eqn - D.PDocument57 pagesModel Eqn - D.PMd TosifNo ratings yet
- Chee4362 HWDocument8 pagesChee4362 HWHa Eun KimNo ratings yet
- Lab AssignmentDocument7 pagesLab AssignmentJoebel CristalesNo ratings yet
- 9 10 F (X) - 0.5x + 9.2 R 0.2840909091: Axis TitleDocument6 pages9 10 F (X) - 0.5x + 9.2 R 0.2840909091: Axis Titleregina pramuditaNo ratings yet
- Waktu (T) Pembacaan (ML) LN (A) 1/ (A) 1/ (A) 2 V - VT (A) : Orde 1Document5 pagesWaktu (T) Pembacaan (ML) LN (A) 1/ (A) 1/ (A) 2 V - VT (A) : Orde 1INDAHNo ratings yet
- Laboratorio 1Document7 pagesLaboratorio 1Karen RodriguezNo ratings yet
- Efr Vs Ema Fvsefr: P (Psia) GP (SCF) BG (BBL/SCF) BW (BBL/STB) F CW Cma CFR Swi Time (Days)Document3 pagesEfr Vs Ema Fvsefr: P (Psia) GP (SCF) BG (BBL/SCF) BW (BBL/STB) F CW Cma CFR Swi Time (Days)NandiniNo ratings yet
- Temperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Document8 pagesTemperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Melissa QuinteroNo ratings yet
- Tarea 1Document11 pagesTarea 1Diego Hdez AvilaNo ratings yet
- (LN (LN (1/ (1FT) ) ) : X y I Ti F (T) LN (Ti) (LN (LN (1/ (1FT) ) )Document6 pages(LN (LN (1/ (1FT) ) ) : X y I Ti F (T) LN (Ti) (LN (LN (1/ (1FT) ) )Macs Condori SalazarNo ratings yet
- Taller Corte 2 Reacciones ExcelDocument58 pagesTaller Corte 2 Reacciones ExcelEmanuel PataquivaNo ratings yet
- Taller ReaccioneDocument28 pagesTaller ReaccioneEmanuel PataquivaNo ratings yet
- TareaDocument12 pagesTareajajajaNo ratings yet
- Cálculos Cinética Método IntegralDocument3 pagesCálculos Cinética Método IntegralMaryin Paulina Bailon ArcentalesNo ratings yet
- Ecuación de Van LaarDocument5 pagesEcuación de Van LaarLjubenko GagliuffiNo ratings yet
- CalculosDocument4 pagesCalculosAlejandra SalasNo ratings yet
- LinealizacionDocument10 pagesLinealizacionDaniel Camilo SalamancaNo ratings yet
- 0 Repaso UltimoDocument37 pages0 Repaso UltimoLeonardo ReyesNo ratings yet
- Group 4 1-To-5 DilutionDocument4 pagesGroup 4 1-To-5 DilutionAngelica Mae Dela CruzNo ratings yet
- Ejerc Pract SeiceDocument5 pagesEjerc Pract SeiceAngel Toro ContrerasNo ratings yet
- RAAADocument25 pagesRAAARandyNo ratings yet
- X Y X XY X Y X X 4 T(S) K: Column C Linear (Column C) Polynomial (Column C)Document3 pagesX Y X XY X Y X X 4 T(S) K: Column C Linear (Column C) Polynomial (Column C)Yomar GuillenNo ratings yet
- Concentración Vs TiempoDocument4 pagesConcentración Vs TiempoSamie ReyNo ratings yet
- Grafica de Orden Cero: TemperaturaDocument3 pagesGrafica de Orden Cero: TemperaturaFernandita SalazarNo ratings yet
- Libro 6Document4 pagesLibro 6Raul Quispe PedrazaNo ratings yet
- Libro 6Document4 pagesLibro 6Raul Quispe PedrazaNo ratings yet
- Fvseo+Ef, W Graph (Example 11: W Wi F HCDocument4 pagesFvseo+Ef, W Graph (Example 11: W Wi F HCusmanNo ratings yet
- Pep 1 Cinetica CalculosDocument6 pagesPep 1 Cinetica CalculosYanina Molina CastilloNo ratings yet
- Solucià N EjerciciosDocument4 pagesSolucià N Ejercicioskotee87No ratings yet
- Grafik KinetikaDocument6 pagesGrafik KinetikaMelly FebrianaNo ratings yet
- Ambar Cantiikk TRKDocument13 pagesAmbar Cantiikk TRKACHMAD RUDY CAHYONONo ratings yet
- E StudioDocument16 pagesE StudioCristianNo ratings yet
- Tabla 3 Laboratorio #2)Document1 pageTabla 3 Laboratorio #2)Maday CrakerNo ratings yet
- BM Yaci. Sobre SaturadoDocument7 pagesBM Yaci. Sobre SaturadoAlejandro RivasNo ratings yet
- Cinetica Tarea 1Document10 pagesCinetica Tarea 1Karla Castelan MuñozNo ratings yet
- Thermodynamics Exercise 2Document7 pagesThermodynamics Exercise 2Julia SatrianiNo ratings yet
- Tugas2 Kimia MaterialDocument6 pagesTugas2 Kimia MaterialDuma AgintaNo ratings yet
- Vapor Pressure / Torr 25 55.9 30 70 35 97 40 117.5 45 154.1 50 190.7 55 241.9 Temperature/ CDocument4 pagesVapor Pressure / Torr 25 55.9 30 70 35 97 40 117.5 45 154.1 50 190.7 55 241.9 Temperature/ CIndi MagineNo ratings yet
- Tugas Pemod 3Document23 pagesTugas Pemod 3ayNo ratings yet
- Calculos SerieDocument6 pagesCalculos SerieDiego InfanteNo ratings yet
- Latihan TRK OrderDocument4 pagesLatihan TRK OrderReno NalendraNo ratings yet
- Solution 3.35Document1 pageSolution 3.35ALWAYNE BUCKNORNo ratings yet
- (KG) (M) A (M/S) (M/S) (S) (N)Document2 pages(KG) (M) A (M/S) (M/S) (S) (N)Katakuri CaceresNo ratings yet
- Tp6 Samba FayeDocument10 pagesTp6 Samba Fayekerimakdin50No ratings yet
- Book 2Document4 pagesBook 2Hasni KakaNo ratings yet
- Excel IsotermasDocument8 pagesExcel IsotermasJacob Dominguez GomezNo ratings yet
- DESDFSFSDFDocument3 pagesDESDFSFSDFNicolas Santiago Tejedor DominguezNo ratings yet
- Kurva Absorbsi 3 Titik 0,10,20: Axis TitleDocument6 pagesKurva Absorbsi 3 Titik 0,10,20: Axis Titlesahrul fauziNo ratings yet
- Taller II Weibull ParámetrosDocument20 pagesTaller II Weibull ParámetrosMayckNo ratings yet
- Analisis de Datos - LeyInversoCuadradoDistanciaDocument5 pagesAnalisis de Datos - LeyInversoCuadradoDistanciaFernando CheuquepilNo ratings yet
- kfDocument2 pageskfLukman El-lathifNo ratings yet
- Book 111Document4 pagesBook 111ArlieNo ratings yet
- El Tiempo Propuesto Es El Indicado, Aunque Lo Recomendable Seria Hacerlo Aun Más Tempranamente para Evitar Posibles Fallas. (96%)Document4 pagesEl Tiempo Propuesto Es El Indicado, Aunque Lo Recomendable Seria Hacerlo Aun Más Tempranamente para Evitar Posibles Fallas. (96%)Angel Eiltz Cespedes CayllahuaNo ratings yet
- Tarea 2Document8 pagesTarea 2Conde BonelessNo ratings yet
- 1 Model Cu Timp de ExecutieDocument3 pages1 Model Cu Timp de ExecutieSzabo LenkeNo ratings yet
T/min 0 2 4 8 (A) / (M) 0.1 0.09 0.08 0.07 LN (A) - 2.3 - 2.4 - 2.5 - 2.7 LN (A) THDT
T/min 0 2 4 8 (A) / (M) 0.1 0.09 0.08 0.07 LN (A) - 2.3 - 2.4 - 2.5 - 2.7 LN (A) THDT
Uploaded by
Nuril AzmiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
T/min 0 2 4 8 (A) / (M) 0.1 0.09 0.08 0.07 LN (A) - 2.3 - 2.4 - 2.5 - 2.7 LN (A) THDT
T/min 0 2 4 8 (A) / (M) 0.1 0.09 0.08 0.07 LN (A) - 2.3 - 2.4 - 2.5 - 2.7 LN (A) THDT
Uploaded by
Nuril AzmiCopyright:
Available Formats
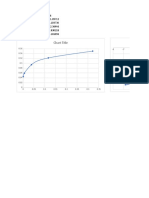
t/min 0 2 4 8
[A]/[M] 0.1 0.09 0.08 0.07
ln[A] -2.3 -2.4 -2.5 -2.7
ln[A]thdt
ln [A] vs t
-2.1
0 1 2 3 4 5 6 7 8 9
-2.2
-2.3
f(x) = − 0.044530521425562 x − 2.31802302073227
ln[A]
-2.4 R² = 0.981065744155468
-2.5
-2.6
-2.7
t (min)
k = -slope=-(-0,04453025)= 0,04453025 s-1
t1/2 = 0,693/k 15.5623603766585
[A]=[A]0 e^{-kt}
ln[A] = ln [A]0-kt
t= (ln[A]-ln[A]0)/-k
8 9
Time (s)
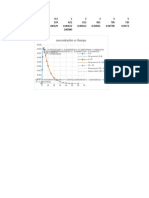
[PP] (M) ln[PP] t/[PP]
0.005 -5.29831736655 200 0
0.0045 -5.40367788221 222.2222222222 10.5
0.004 -5.52146091786 250 22.3
0.0035 -5.65499231049 285.7142857143 35.7
0.003 -5.80914299031 333.3333333333 51.1
0.0025 -5.99146454711 400 69.3
0.002 -6.21460809842 500 91.6
0.0015 -6.50229017087 666.6666666667 120.4
0.001 -6.90775527898 1000 160.9
0.0005 -7.60090245954 2000 230.3
0.00025 -8.2940496401 4000 299.6
0.00015 -8.80487526387 6666.666666667 350.7
0.0001 -9.21034037198 10000 391.2
ln[PP] vs t
[PP] vs t 1/[PP] vs t
0
0.006 0 50 100 150 200 250 300 350 400 450 12000
-1
0.005 -2 10000
-3
0.004 -4 8000
f(x) = − 1.17047981796982E-05 x + 0.003804762918638
[PP]
R² = 0.829812439591053 -5 f(x) = 20.6969679493051 x − 878.873635172511
0.003 6000
[PP]
[PP]
f(x) = − 0.009999100784582 x − 5.29842508459088 R² = 0.830946324059669
-6
R² = 0.999999961330541
0.002 -7 4000
-8
0.001 2000
-9
0 -10 0
0 50 100 150 200 250 300 350 400 450 0 50 100 150 200 250 300 350 400 450
t(s)
t(s) t(s)
jadi reaksi antara PP dan basa berlebih mengikuti kinetik reaksi orde 1 semu.
karena basanya excess
k=-slope=-(-0,01) = 0,01 s-1
rate law = 0,01 s-1 [PP]
t1/2=0,693/k
69.3
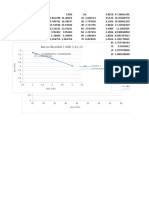
1/[PP] vs t
) = 20.6969679493051 x − 878.873635172511
= 0.830946324059669
100 150 200 250 300 350 400 450
t(s)
You might also like
- Kuru TmaDocument3 pagesKuru Tmanahla6312No ratings yet
- IsotermasDocument14 pagesIsotermasDaniella HidalgoNo ratings yet
- Kinetics Lab DataDocument3 pagesKinetics Lab DataDianna NomanimNo ratings yet
- Tugas 5 MetNum 1 (Pengganti Kuis 2) RevisiDocument2 pagesTugas 5 MetNum 1 (Pengganti Kuis 2) RevisiFatih ArifNo ratings yet
- Model Eqn - D.PDocument57 pagesModel Eqn - D.PMd TosifNo ratings yet
- Chee4362 HWDocument8 pagesChee4362 HWHa Eun KimNo ratings yet
- Lab AssignmentDocument7 pagesLab AssignmentJoebel CristalesNo ratings yet
- 9 10 F (X) - 0.5x + 9.2 R 0.2840909091: Axis TitleDocument6 pages9 10 F (X) - 0.5x + 9.2 R 0.2840909091: Axis Titleregina pramuditaNo ratings yet
- Waktu (T) Pembacaan (ML) LN (A) 1/ (A) 1/ (A) 2 V - VT (A) : Orde 1Document5 pagesWaktu (T) Pembacaan (ML) LN (A) 1/ (A) 1/ (A) 2 V - VT (A) : Orde 1INDAHNo ratings yet
- Laboratorio 1Document7 pagesLaboratorio 1Karen RodriguezNo ratings yet
- Efr Vs Ema Fvsefr: P (Psia) GP (SCF) BG (BBL/SCF) BW (BBL/STB) F CW Cma CFR Swi Time (Days)Document3 pagesEfr Vs Ema Fvsefr: P (Psia) GP (SCF) BG (BBL/SCF) BW (BBL/STB) F CW Cma CFR Swi Time (Days)NandiniNo ratings yet
- Temperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Document8 pagesTemperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Melissa QuinteroNo ratings yet
- Tarea 1Document11 pagesTarea 1Diego Hdez AvilaNo ratings yet
- (LN (LN (1/ (1FT) ) ) : X y I Ti F (T) LN (Ti) (LN (LN (1/ (1FT) ) )Document6 pages(LN (LN (1/ (1FT) ) ) : X y I Ti F (T) LN (Ti) (LN (LN (1/ (1FT) ) )Macs Condori SalazarNo ratings yet
- Taller Corte 2 Reacciones ExcelDocument58 pagesTaller Corte 2 Reacciones ExcelEmanuel PataquivaNo ratings yet
- Taller ReaccioneDocument28 pagesTaller ReaccioneEmanuel PataquivaNo ratings yet
- TareaDocument12 pagesTareajajajaNo ratings yet
- Cálculos Cinética Método IntegralDocument3 pagesCálculos Cinética Método IntegralMaryin Paulina Bailon ArcentalesNo ratings yet
- Ecuación de Van LaarDocument5 pagesEcuación de Van LaarLjubenko GagliuffiNo ratings yet
- CalculosDocument4 pagesCalculosAlejandra SalasNo ratings yet
- LinealizacionDocument10 pagesLinealizacionDaniel Camilo SalamancaNo ratings yet
- 0 Repaso UltimoDocument37 pages0 Repaso UltimoLeonardo ReyesNo ratings yet
- Group 4 1-To-5 DilutionDocument4 pagesGroup 4 1-To-5 DilutionAngelica Mae Dela CruzNo ratings yet
- Ejerc Pract SeiceDocument5 pagesEjerc Pract SeiceAngel Toro ContrerasNo ratings yet
- RAAADocument25 pagesRAAARandyNo ratings yet
- X Y X XY X Y X X 4 T(S) K: Column C Linear (Column C) Polynomial (Column C)Document3 pagesX Y X XY X Y X X 4 T(S) K: Column C Linear (Column C) Polynomial (Column C)Yomar GuillenNo ratings yet
- Concentración Vs TiempoDocument4 pagesConcentración Vs TiempoSamie ReyNo ratings yet
- Grafica de Orden Cero: TemperaturaDocument3 pagesGrafica de Orden Cero: TemperaturaFernandita SalazarNo ratings yet
- Libro 6Document4 pagesLibro 6Raul Quispe PedrazaNo ratings yet
- Libro 6Document4 pagesLibro 6Raul Quispe PedrazaNo ratings yet
- Fvseo+Ef, W Graph (Example 11: W Wi F HCDocument4 pagesFvseo+Ef, W Graph (Example 11: W Wi F HCusmanNo ratings yet
- Pep 1 Cinetica CalculosDocument6 pagesPep 1 Cinetica CalculosYanina Molina CastilloNo ratings yet
- Solucià N EjerciciosDocument4 pagesSolucià N Ejercicioskotee87No ratings yet
- Grafik KinetikaDocument6 pagesGrafik KinetikaMelly FebrianaNo ratings yet
- Ambar Cantiikk TRKDocument13 pagesAmbar Cantiikk TRKACHMAD RUDY CAHYONONo ratings yet
- E StudioDocument16 pagesE StudioCristianNo ratings yet
- Tabla 3 Laboratorio #2)Document1 pageTabla 3 Laboratorio #2)Maday CrakerNo ratings yet
- BM Yaci. Sobre SaturadoDocument7 pagesBM Yaci. Sobre SaturadoAlejandro RivasNo ratings yet
- Cinetica Tarea 1Document10 pagesCinetica Tarea 1Karla Castelan MuñozNo ratings yet
- Thermodynamics Exercise 2Document7 pagesThermodynamics Exercise 2Julia SatrianiNo ratings yet
- Tugas2 Kimia MaterialDocument6 pagesTugas2 Kimia MaterialDuma AgintaNo ratings yet
- Vapor Pressure / Torr 25 55.9 30 70 35 97 40 117.5 45 154.1 50 190.7 55 241.9 Temperature/ CDocument4 pagesVapor Pressure / Torr 25 55.9 30 70 35 97 40 117.5 45 154.1 50 190.7 55 241.9 Temperature/ CIndi MagineNo ratings yet
- Tugas Pemod 3Document23 pagesTugas Pemod 3ayNo ratings yet
- Calculos SerieDocument6 pagesCalculos SerieDiego InfanteNo ratings yet
- Latihan TRK OrderDocument4 pagesLatihan TRK OrderReno NalendraNo ratings yet
- Solution 3.35Document1 pageSolution 3.35ALWAYNE BUCKNORNo ratings yet
- (KG) (M) A (M/S) (M/S) (S) (N)Document2 pages(KG) (M) A (M/S) (M/S) (S) (N)Katakuri CaceresNo ratings yet
- Tp6 Samba FayeDocument10 pagesTp6 Samba Fayekerimakdin50No ratings yet
- Book 2Document4 pagesBook 2Hasni KakaNo ratings yet
- Excel IsotermasDocument8 pagesExcel IsotermasJacob Dominguez GomezNo ratings yet
- DESDFSFSDFDocument3 pagesDESDFSFSDFNicolas Santiago Tejedor DominguezNo ratings yet
- Kurva Absorbsi 3 Titik 0,10,20: Axis TitleDocument6 pagesKurva Absorbsi 3 Titik 0,10,20: Axis Titlesahrul fauziNo ratings yet
- Taller II Weibull ParámetrosDocument20 pagesTaller II Weibull ParámetrosMayckNo ratings yet
- Analisis de Datos - LeyInversoCuadradoDistanciaDocument5 pagesAnalisis de Datos - LeyInversoCuadradoDistanciaFernando CheuquepilNo ratings yet
- kfDocument2 pageskfLukman El-lathifNo ratings yet
- Book 111Document4 pagesBook 111ArlieNo ratings yet
- El Tiempo Propuesto Es El Indicado, Aunque Lo Recomendable Seria Hacerlo Aun Más Tempranamente para Evitar Posibles Fallas. (96%)Document4 pagesEl Tiempo Propuesto Es El Indicado, Aunque Lo Recomendable Seria Hacerlo Aun Más Tempranamente para Evitar Posibles Fallas. (96%)Angel Eiltz Cespedes CayllahuaNo ratings yet
- Tarea 2Document8 pagesTarea 2Conde BonelessNo ratings yet
- 1 Model Cu Timp de ExecutieDocument3 pages1 Model Cu Timp de ExecutieSzabo LenkeNo ratings yet