Professional Documents
Culture Documents
CDT综述 tang2018
CDT综述 tang2018
Uploaded by
13Copyright:
Available Formats
You might also like
- Varied Papers On MEMS - 5Document12 pagesVaried Papers On MEMS - 5Tiago PestanaNo ratings yet
- 10 1002@adfm 202006216Document14 pages10 1002@adfm 202006216Abhijeet MohantyNo ratings yet
- Facile Synthesis of Er3 Tm3 Co Doped Magnetic Luminescent - 2022 - Journal ofDocument9 pagesFacile Synthesis of Er3 Tm3 Co Doped Magnetic Luminescent - 2022 - Journal ofShuNo ratings yet
- 1 s2.0 S0142961219301528 MainDocument14 pages1 s2.0 S0142961219301528 MainHarshithNo ratings yet
- Deoliveira2014 PDT PDFDocument7 pagesDeoliveira2014 PDT PDFShriya ShahuNo ratings yet
- Angew Chem Int Ed - 2019 - Imberti - New Designs For Phototherapeutic Transition Metal ComplexesDocument13 pagesAngew Chem Int Ed - 2019 - Imberti - New Designs For Phototherapeutic Transition Metal ComplexesNohemiNo ratings yet
- Journal of Environmental Chemical Engineering: SciencedirectDocument27 pagesJournal of Environmental Chemical Engineering: SciencedirectHoàng KhôiNo ratings yet
- Titanium Dioxide Photocatalysis: Fundamentals and Application On PhotoinactivationDocument39 pagesTitanium Dioxide Photocatalysis: Fundamentals and Application On PhotoinactivationNikhi Maria RajuNo ratings yet
- 01 25117 MagalhaesDocument39 pages01 25117 Magalhaeskeveho9261No ratings yet
- Photodynamic TherapyDocument5 pagesPhotodynamic TherapyPranav UpadhyayaNo ratings yet
- Recent Progress in Photodynamic Immunotherapy With Metal-Based PhotosensitizersDocument13 pagesRecent Progress in Photodynamic Immunotherapy With Metal-Based PhotosensitizersWang JianweiNo ratings yet
- 326 831 1 PBDocument7 pages326 831 1 PBAmar AmarNo ratings yet
- Photodynamic Therapy in Endodontics: ReviewDocument15 pagesPhotodynamic Therapy in Endodontics: ReviewFabro HMNo ratings yet
- 1 - DOUGHERTY, T.J. Et Al. Photodynamic Therapy. J. Natl. Cancer Inst., Cary, V. 90, N. 12, P. 889 - 905, 1998.Document17 pages1 - DOUGHERTY, T.J. Et Al. Photodynamic Therapy. J. Natl. Cancer Inst., Cary, V. 90, N. 12, P. 889 - 905, 1998.Hylze ChavesNo ratings yet
- Novel Removal of Diazinon Pesticide by AdsorptionDocument7 pagesNovel Removal of Diazinon Pesticide by AdsorptionAlissom GomesNo ratings yet
- 10 1002@anie 202010714Document12 pages10 1002@anie 202010714Abhijeet MohantyNo ratings yet
- Boluki Et Al 2020 - The Combination of Antimicrobial Photocatalysis and Antimicrobial Photodynamic TherapyDocument6 pagesBoluki Et Al 2020 - The Combination of Antimicrobial Photocatalysis and Antimicrobial Photodynamic Therapysamusm.smNo ratings yet
- 1 s2.0 S1878535220301593 MainDocument18 pages1 s2.0 S1878535220301593 MainAbdul Wahab Assya RoniNo ratings yet
- 10 1016@j Cej 2020 125574Document32 pages10 1016@j Cej 2020 125574Abhijeet MohantyNo ratings yet
- Preparation and Applications of Titanium Dioxide and Zinc Oxide NanoparticlesDocument4 pagesPreparation and Applications of Titanium Dioxide and Zinc Oxide NanoparticlesNuzulul RahmahNo ratings yet
- Ijms 24 00410Document18 pagesIjms 24 00410Robert StryjakNo ratings yet
- Development of Iron/ethylcellulose (Core/shell) Nanoparticles Loaded With Diclofenac Sodium For Arthritis TreatmentDocument7 pagesDevelopment of Iron/ethylcellulose (Core/shell) Nanoparticles Loaded With Diclofenac Sodium For Arthritis Treatmentزياد سلمانNo ratings yet
- Unveiling Cutting-Edge Progress in The Fundamentals of MXene - Synthesis Strategies, Energy and Bio-Environmental ApplicationsDocument46 pagesUnveiling Cutting-Edge Progress in The Fundamentals of MXene - Synthesis Strategies, Energy and Bio-Environmental ApplicationsBurak KursNo ratings yet
- Photodynamic Therapy in Dentistry: A Literature ReviewDocument13 pagesPhotodynamic Therapy in Dentistry: A Literature ReviewShriya ShahuNo ratings yet
- Photodynamic Therapy in Endodontics: A Literature ReviewDocument8 pagesPhotodynamic Therapy in Endodontics: A Literature ReviewShriya ShahuNo ratings yet
- Effectiveness of PhotochemicalDocument7 pagesEffectiveness of PhotochemicalGHZNo ratings yet
- Crystals: A Literature Review On High-Performance Photocatalysts For Sustainable Cancer TherapyDocument13 pagesCrystals: A Literature Review On High-Performance Photocatalysts For Sustainable Cancer TherapyPol RecasensNo ratings yet
- Ternary TiO2-WO3-CQDs Nanocomposites For Enhanced Photocatalytic Mineralization of Aqueous Cephalexin Degradation Mechanism and Toxicity EvaluationDocument13 pagesTernary TiO2-WO3-CQDs Nanocomposites For Enhanced Photocatalytic Mineralization of Aqueous Cephalexin Degradation Mechanism and Toxicity EvaluationAtif sialNo ratings yet
- 2019 - Photodynamic Antimicrobial Therapy - Comparison of Thiocyanate and Selenocyanate For Potentiation ofDocument22 pages2019 - Photodynamic Antimicrobial Therapy - Comparison of Thiocyanate and Selenocyanate For Potentiation ofragod2No ratings yet
- Applicationof Porphyrinsin Antibacterial Photodynamic Therapy MoleculesDocument29 pagesApplicationof Porphyrinsin Antibacterial Photodynamic Therapy Moleculesisma drNo ratings yet
- NanozymesDocument10 pagesNanozymesdd donNo ratings yet
- Fotooxid, Cu Tian, 2018Document14 pagesFotooxid, Cu Tian, 2018Larisa MocanuNo ratings yet
- Photocatalytic Degradation of Organic Pollutants by MOFsDocument10 pagesPhotocatalytic Degradation of Organic Pollutants by MOFsHenry LalchhuankimaNo ratings yet
- Photo or Solar Ferrioxalate Disinfection Technology Without External Hydrogen Peroxide SupplyDocument6 pagesPhoto or Solar Ferrioxalate Disinfection Technology Without External Hydrogen Peroxide SupplyBEA FRANCINE DELOS SANTOSNo ratings yet
- Magnet 9Document7 pagesMagnet 9a.mahdieh90No ratings yet
- Advanced Science - 2021 - Fang - Thermally Activated Delayed Fluorescence Material An Emerging Class of Metal FreeDocument19 pagesAdvanced Science - 2021 - Fang - Thermally Activated Delayed Fluorescence Material An Emerging Class of Metal Freerajendrk1No ratings yet
- Adhikari 2016Document8 pagesAdhikari 2016Christian .Quijia QuezadaNo ratings yet
- Oxidaxion Us H2ho2 PDFDocument12 pagesOxidaxion Us H2ho2 PDFEstefany MartinezNo ratings yet
- 1 s2.0 S2405844020300074 MainDocument6 pages1 s2.0 S2405844020300074 MainAlvin Wahyu Puspita SariNo ratings yet
- Controlling Solar Hydrogen Production by Organizing PorphyrinsDocument11 pagesControlling Solar Hydrogen Production by Organizing PorphyrinsBeauty MoonNo ratings yet
- Ce3+ Ce4+ Doping Photocatalyst Degredation Metylene BlueDocument14 pagesCe3+ Ce4+ Doping Photocatalyst Degredation Metylene BlueHuanNo ratings yet
- Chemical Engineering JournalDocument10 pagesChemical Engineering Journalpedro cuellar proNo ratings yet
- Longo 2011Document10 pagesLongo 2011Luciana BetzlerNo ratings yet
- Applied Catalysis B: EnvironmentalDocument6 pagesApplied Catalysis B: EnvironmentalMinh TrầnNo ratings yet
- 2022 Romero AngewChemIntEd 61 E2022007831 SIDocument49 pages2022 Romero AngewChemIntEd 61 E2022007831 SIshamshel43No ratings yet
- Heterogeneous Fenton Catalytic Oxidation For Water TreatmentDocument11 pagesHeterogeneous Fenton Catalytic Oxidation For Water Treatmentanthonychoong9No ratings yet
- Wu 2017Document11 pagesWu 2017Kapas FernandoNo ratings yet
- Anti-Biofilm Property of Bioactive Upconversion NaDocument17 pagesAnti-Biofilm Property of Bioactive Upconversion NaMihaela TuculinaNo ratings yet
- Photodynamic Therapy For COVID-19: CorrespondenceDocument2 pagesPhotodynamic Therapy For COVID-19: CorrespondenceJuan Pablo Rodríguez CoronaNo ratings yet
- Journal of Industrial and Engineering Chemistry: Ines Nitoi, Tatiana Oncescu, Petruta OanceaDocument5 pagesJournal of Industrial and Engineering Chemistry: Ines Nitoi, Tatiana Oncescu, Petruta OanceaAndrea SilvaNo ratings yet
- The Binding Characteristics of Isoniazid With CoppDocument9 pagesThe Binding Characteristics of Isoniazid With CoppVOOGLS PUBLICATIONNo ratings yet
- GemifloxacinDocument8 pagesGemifloxacinSHERLY KIMBERLY RAMOS JESUSNo ratings yet
- Photocatalysis of B-Blockers - An Overview: Arabian Journal of ChemistryDocument8 pagesPhotocatalysis of B-Blockers - An Overview: Arabian Journal of Chemistrypetru apopeiNo ratings yet
- Abraham 2013Document15 pagesAbraham 2013priya panaleNo ratings yet
- Sciencedirect: Journal of Photochemistry & Photobiology, B: Biology 202 (2020) 111676Document8 pagesSciencedirect: Journal of Photochemistry & Photobiology, B: Biology 202 (2020) 111676Anonymous DyytrQGxNo ratings yet
- Fotodegradare OxidativaDocument7 pagesFotodegradare OxidativaAlexandra BanutaNo ratings yet
- Peróxido de HidrógenoDocument4 pagesPeróxido de Hidrógenojuanjosoto821No ratings yet
- Investigation of Photocatalytic Degradation of Phenol byDocument6 pagesInvestigation of Photocatalytic Degradation of Phenol byMokhtaria ReguigNo ratings yet
- Green Tio2 as Nanocarriers for Targeting Cervical Cancer Cell LinesFrom EverandGreen Tio2 as Nanocarriers for Targeting Cervical Cancer Cell LinesNo ratings yet
- Key Questions in Environmental Toxicology: A Study and Revision GuideFrom EverandKey Questions in Environmental Toxicology: A Study and Revision GuideNo ratings yet
- TechnicalDocument1 pageTechnicalUbac recrutementNo ratings yet
- Grade 8 Science Worksheets CbseDocument3 pagesGrade 8 Science Worksheets CbseHeidiNo ratings yet
- Tile Adhesive Heavy-Duty - Allgemeine Bau-Chemie Phil., IncDocument4 pagesTile Adhesive Heavy-Duty - Allgemeine Bau-Chemie Phil., IncRoland CepedaNo ratings yet
- Resources, Conservation & RecyclingDocument8 pagesResources, Conservation & RecyclingMiftahur RahmiNo ratings yet
- Manzil Coordination Compound: Please Fill in Your JEE Application Details by Clicking On Link BelowDocument4 pagesManzil Coordination Compound: Please Fill in Your JEE Application Details by Clicking On Link BelowAbhi kumarNo ratings yet
- Structural ConnectionsDocument205 pagesStructural ConnectionsbsitlerNo ratings yet
- P Block Elements 3Document28 pagesP Block Elements 3Shruti GaurNo ratings yet
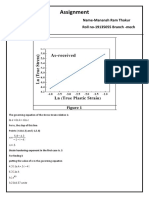
- Assignment: Name-Manansh Ram Thakur Roll No-19135055 Branch - MechDocument3 pagesAssignment: Name-Manansh Ram Thakur Roll No-19135055 Branch - MechManansh ThakurNo ratings yet
- RCD Slides?Document139 pagesRCD Slides?Nsb JejsNo ratings yet
- Mid Sem Question Bank-FFODocument2 pagesMid Sem Question Bank-FFOMihir KhuntNo ratings yet
- 5TH Ra Bill CCDocument64 pages5TH Ra Bill CCMd. Mizanur RahamanNo ratings yet
- Production of Maleic AnhydrideDocument8 pagesProduction of Maleic AnhydrideZafran AliNo ratings yet
- Goldium Steel Lamination CRNGO CRGODocument1 pageGoldium Steel Lamination CRNGO CRGOdisse_detiNo ratings yet
- Chemical Engineering Journal: A B C D e e CDocument10 pagesChemical Engineering Journal: A B C D e e CHemanth Peddavenkatappa GariNo ratings yet
- Centering and FormworkDocument50 pagesCentering and FormworkSakshi RawatNo ratings yet
- Vulcan XC68 Conductive Carbon BlackDocument2 pagesVulcan XC68 Conductive Carbon BlackBandana DholeNo ratings yet
- ZDHC MRSL v3.1 Aug 2023Document55 pagesZDHC MRSL v3.1 Aug 2023y-tsugeNo ratings yet
- FeasibilityDocument145 pagesFeasibilityOpteron K.No ratings yet
- Organic Chem With An Emphasis On BiologyDocument713 pagesOrganic Chem With An Emphasis On Biologymedranobarraza.manuel2021No ratings yet
- OPI LEGRIT 110 0 8 0 4 ISO enDocument3 pagesOPI LEGRIT 110 0 8 0 4 ISO ensonalisabirNo ratings yet
- PHD Thesis Development and Investigation of High-Performance Fire Retardant Polypropylene Nanocomposites Via High Energy ElectronsDocument141 pagesPHD Thesis Development and Investigation of High-Performance Fire Retardant Polypropylene Nanocomposites Via High Energy Electronskaplan mNo ratings yet
- Synthesis, Structure and Spectroscopic Characterization of Ni (II), Co (II), Cu (II) and ZN (II) Complexes With Saccharinate and PyrazoleDocument9 pagesSynthesis, Structure and Spectroscopic Characterization of Ni (II), Co (II), Cu (II) and ZN (II) Complexes With Saccharinate and PyrazoleLucía BolañosNo ratings yet
- Wrinkle Free FinishingDocument4 pagesWrinkle Free FinishingkreeshnuNo ratings yet
- TDS Auracast 100 IndiaDocument2 pagesTDS Auracast 100 IndiaSuresh BabuNo ratings yet
- Pricelist Katalog Holymed - Rev JanDocument23 pagesPricelist Katalog Holymed - Rev JanPT.KARYASURYA MULIANo ratings yet
- Ms ProofDocument4 pagesMs Proofrizky efrinaldoNo ratings yet
- 415 120 DB 0001Document30 pages415 120 DB 0001Mustafa AhsanNo ratings yet
- Four Golden RuleDocument6 pagesFour Golden RulerundyudaNo ratings yet
- Introduction To Materials Science For Engineers Global Edition 9Th Edition James F Shackelford Full Chapter PDFDocument69 pagesIntroduction To Materials Science For Engineers Global Edition 9Th Edition James F Shackelford Full Chapter PDFirenoradiet100% (7)
CDT综述 tang2018
CDT综述 tang2018
Uploaded by
13Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CDT综述 tang2018
CDT综述 tang2018
Uploaded by
13Copyright:
Available Formats
Angewandte
A Journal of the Gesellschaft Deutscher Chemiker
International Edition Chemie www.angewandte.org
Accepted Article
Title: Chemodynamic Therapy: Tumour Microenvironment-Mediated
Fenton and Fenton-like Reaction
Authors: Zhongmin Tang, Yanyan Liu, Mingyuan He, and Wenbo Bu
This manuscript has been accepted after peer review and appears as an
Accepted Article online prior to editing, proofing, and formal publication
of the final Version of Record (VoR). This work is currently citable by
using the Digital Object Identifier (DOI) given below. The VoR will be
published online in Early View as soon as possible and may be different
to this Accepted Article as a result of editing. Readers should obtain
the VoR from the journal website shown below when it is published
to ensure accuracy of information. The authors are responsible for the
content of this Accepted Article.
To be cited as: Angew. Chem. Int. Ed. 10.1002/anie.201805664
Angew. Chem. 10.1002/ange.201805664
Link to VoR: http://dx.doi.org/10.1002/anie.201805664
http://dx.doi.org/10.1002/ange.201805664
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Chemodynamic Therapy: Tumour Microenvironment-Mediated
Fenton and Fenton-like Reaction
Zhongmin Tang,[a,b] Yanyan Liu,[c] Mingyuan He,[c] Wenbo Bu*[a,c]
Accepted Manuscript
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Abstract: Tailored to the specific tumour microenvironment, explore more suitable new therapies. For example, radiotherapy
which involves acidity and the overproduction of hydrogen generates reactive oxygen species (ROS)[19] in the tumour area
peroxide, advanced nanotechnology has been introduced to through X-rays to destroy the tumour. Certain chemotherapeutic
generate the hydroxyl radical (•OH) primarily for tumour drugs, such as tirapazamine (TPZ)[20,21] and doxorubicin
chemodynamic therapy (CDT) via the Fenton reaction and (DOX),[22] also generate ROS to fight tumours. In addition, the
Fenton-like reaction. Numerous studies have investigated the drug artemisinin is a highly effective treatment for malaria. One
enhancement of CDT efficiency, primarily the increase in the of the principles underlying the effects of this drug is that the iron
amount of •OH generated. Notably, various strategies based on in the residual haeme observed in patients with malaria induces
the Fenton reaction have been employed to enhance •OH the decomposition of artemisinin by catalysing the formation of a
generation, including nanomaterials selection, modulation of the peroxide bridge to produce hydroxy radicals,[23,24] which are
reaction environment and external energy fields stimulation, highly active and lethal, subsequently eliminating the malaria
which are discussed systematically in this minireview. parasite. Chemists quickly linked this mechanism to the classical
Furthermore, the potential challenges and the methods used to Fenton reaction.
Accepted Manuscript
facilitate CDT effectiveness are also presented to support this After considering these different fields, chemodynamic therapy
cutting-edge research area. (CDT), an emerging therapeutic strategy, was recently proposed
by our group[25] and defined as in situ treatments using the
Fenton reaction or Fenton-like reaction to generate ·OH in
tumour sites. Briefly, iron-based nanomaterials dissolve ferrous
1. Introduction
ions under the mildly acidic conditions of the TME and initiate
the Fenton reaction to overproduce H2O2, generating •OH to
The Fenton reaction, which is simply defined as the
trigger apoptosis and inhibit the tumour. Most importantly, this
generation of highly oxidative hydroxyl radicals (•OH) from
approach ensures normal tissue safety in some degree,
hydrogen peroxide (H2O2), is catalysed by ferrous ion (Fe2+) and
because the Fenton reaction is substantially suppressed under
has been widely used to remove refractory organics.[1-3] After
the slight alkaline conditions and in the presence of insufficient
extensive research, advanced nanotechnology has created a
H2O2 in a normal microenvironment.[26] Even so, the potential
broad stage for the further development and extension of the
toxicities of nanomaterials should be taken into consideration for
Fenton reaction, such as iron-based nanomaterials for the
further applications. This strategy not only broadens the
Fenton reaction and Fenton-like reaction,[4,5] other metal-based
applications of the Fenton reaction but also simplifies its
nanomaterials, and graphene oxide for the Fenton-like
potential for clinical translation. Compared with chemotherapy,
reaction.[6-8] However, a major issue encountered by scientists is
radiotherapy, photothermal therapy and photodynamic therapy,
how to broaden the applications of the Fenton reaction and
CDT has the following advantages: i) highly logical and selective,
Fenton-like reaction, which would also provide possibilities and
ii) and activated by endogenous stimulus. Meanwhile, we could
has prompted many studies in other fields in addition to the
also find the connections between CDT and current following
ecological environmental field. Characterized by mild acidity,[9]
clinical cases except for the malaria treatment using artemisinin:
H2O2 overproduction,[10] low catalase activity[11] and hypoxia,[12]
The antitumor principle of bleomycin[27a, 27b] is that the bleomycin
the tumour microenvironment (TME) not only provides a suitable
is firstly embedded in DNA, and then the complex of bleomycin
environment and nutrition for tumour development[13] and
with iron would generate superoxide and hydroxyl radicals to
metastasis[14,15] but also furnishes the "gate" for selective and
break DNA strand. At the same time, the cardiotoxicity of
efficient tumour treatments. In this case, numerous TME-
anthracycline drugs is mainly from the reactive oxygen species,
responsive nanomaterials have been developed in recent
especially hydroxyl radicals, generated by the binding iron ions
decades, the majority of which are TME-responsive drug
with H2O2,[27c] which also provides enlightment for CDT.
delivery nanocarriers[16-18] that have deficiencies, such as
Therefore, studies aimed at the further development of CDT
insufficient drug loading and easy leakage to damage normal
have flourished, which supports the potential utility of CDT for
tissues. Moreover, studies focusing on current clinical
clinical translation.
treatments would provide researchers with effective guidance to
Since the development of CDT, the selection of suitable
nanomaterials, the modulation of the reaction environment
(reduced pH levels, increased amounts of reactants and
[a] Dr. Z. Tang and Prof. W. Bu decreased amounts of glutathione) and the assistance of an
State Key Laboratory of High Performance Ceramics and Superfine
exogenous energy field have been used to optimize the effect of
Microstructure, Shanghai Institute of Ceramics, Chinese Academy of
Sciences, Shanghai 200050, P.R. China CDT, which relies on the guidance of the Fenton or Fenton-like
E-mail: wbbu@mail.sic.ac.cn reactions and basic chemical principles. Although the majority of
[b] Dr. Z. Tang the existing CDT agents are iron-based inorganic nanomaterials,
University of Chinese Academy of Sciences, Beijing 100049, P.R.
other inorganic nanomaterials and organic nanomaterials have
China.
[c] Dr. Y. Liu, Prof. M. He, and Prof. W. Bu also been used to enrich the library. Moreover, nanomaterials
Shanghai Key Laboratory of Green Chemistry and Chemical used for CDT without low pH dependence are also satisfactory
Processes, School of Chemistry and Molecular Engineering, East choices. Nanomaterials have the ability to exclusively produce
China Normal University, Shanghai 200062, P.R. China
protons or H2O2 in tumours should also be taken into
E-mail: wbbu@chem.ecnu.edu.cn;
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
consideration. Above all, the selection of nanomaterials should 2. Nanomaterials selection
consider the following issues: more functionality and easy
synthesis procedures, which are crucial for the application in the Due to the irreplaceable role of the catalyst in the Fenton
real world.[28] In addition, the efficiency of CDT could also be reaction and Fenton-like reaction, the selection of nanomaterials
improved by the consumption of reducing substances in the (iron-based inorganic materials, other metal-based inorganic
TME, such as glutathione (GSH), or the modulation of H+ and materials or metal-organic framework nanomaterials) is very
H2O2 levels by regulating gene expression. Fortunately, several important.
other external energy fields, such as light, heat, sonification,
electricity or magnetic fields, are desirable to promote the
Fenton-like reaction. In summary, the purpose of this minireview
is not merely to concentrate on the recent important findings 2.1. Iron-based inorganic nanomaterials
related to CDT, but also to provide rational instructions to exploit
other tumour treatments. The first step in increasing the CDT efficiency is to increase
Accepted Manuscript
the catalyst ion amount. The method should closely linked with
the TME, such as the acidity level, which is created by the
generation of large amounts of lactic acid resulting from the
Zhong Min Tang graduated from Shandong
upregulation of glycolytic metabolism.[29] For instance, our group
University with a B.E. in Materials Science
synthesized amorphous Fe0 nanoparticles (AFeNPs) (Figure
and Engineering in 2014. He is now pursing
1a),[25] which were rapidly ionized in an acidic TME to release
his Ph.D. under the supervision of Prof.
more Fe2+ ions than Fe0 nanocrystals (FeNCs) for CDT, because
Wenbo Bu at Shanghai Institute of Ceramics,
it had the reactive nature of metallic glasses. As presented in
Chinese Academy of Sciences (SICCAS).
Figure 1b and Figure 1c, the release rate of ferrous ions from
Currently, his research is focused on tumour
the AFeNPs reached 57% at a pH of 6.5 and 100% at a pH of
microenvironment-sensitive nanoplatforms
5.4 within 6 h, which were much higher than those observed for
for diagnosis/therapeutic systems.
the FeNCs. Moreover, both AFeNPs and FeNCs tended to
slowly release ferrous ions at a neutral pH. These results
Yan Yan Liu received her Ph.D. degree from confirmed the capacity of AFeNPs for selective ferrous ion
SICCAS. She is now a lecturer at East China release, thus ensuring the efficiency of CDT. In addition, many
Normal University (ECNU). Her research is other iron-based nanomaterials, including Fe oxides,[30-32]
focused on the design and synthesis [FeO(OH)n][33] and M(Sn,Mn)Fe2O4[34,35] have also been
multifunctional nanomaterials for biomedical introduced as CDT agents, but none have focused on increasing
applications the release of the catalytic Fe2+ ions.
Ming Yuan He, Chinese Academician of
Sciences, is a chemistry professor at ECNU.
His research areas are catalytic materials
and petrochemical catalysts. He has
published over 200 scientific papers and has
submitted more than 200 domestic and
foreign patents, over 150 of which have been
authorized.
Wen Bo Bu received his Ph.D. degree from
Nanjing University of Technology. He is a full
professor at ECNU and an adjunct professor
at SICCAS. His current research interests
include the design and synthesis of
multifunctional nanomaterials for further
cancer imaging and therapeutic applications.
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Accepted Manuscript
Figure 1. (a) Preparation of AFeNPs. (b and c) Time-dependent ion release from FeNCs and AFeNPs at different pH values. Reproduced with permission
from ref. [25]. Copyright 2016. Wiley-VCH. (d) The mechanism underlying the effects of MnO2 as an enhanced CDT agent for cancer. (e) UV/Vis absorption
spectra and photo (inset) of methylene blue (MB) after degradation by the Mn2+-mediated Fenton-like reaction in different solutions. (f) MB degradation of
different groups. (g) Bar graph showing the percentage of MB degradation in various groups. Reproduced with permission from ref. [38]. Copyright 2018,
Wiley-VCH.
2.2. Other metal-based inorganic nanomaterials
Although the TME exhibits the characteristics of a low pH and
hydrogen peroxide overproduction, it also has a higher
concentration of GSH than normal tissues,[36] which consumes
the generated hydroxyl radicals, thereby limiting the CDT effect.
In addition to the catalytic effect of ferrous ions, other transition
metal ions, including Mn2+, Ti3+, Cu2+ and Co2+ ions, could also
act as catalytic ions.[37] From this perspective, the combination of
the GSH consumption with increasing catalyst ion generation in
one nanomaterial is both a challenge and a potential application.
For example, Lin et al. prepared a CDT agent, MnO2-coated MS
NPs (MS@MnO2 NPs), which combined the ·OH generation and
GSH depletion.[38] As depicted in Figure 1d, the MnO2 shell
reacted with GSH to produce Mn2+ and glutathione disulphide
(GSSG) and subsequently triggers ·OH generation from H2O2 in
the presence of HCO3-/CO2. The efficiency of ·OH generation
was investigated by monitoring methylene blue (MB)
degradation. Compared with the group treated with H2O2 and Figure 2. (a) Schematic illustrating the preparation of MON-p53. (b)
Schematic illustrating MON-p53-mediated anticancer therapy (c) Viability of
MnCl2 in aqueous solution, apparent degradation of MB was
HT1080 cells treated using different metal−organic networks coated with
detected after a 30 min incubation in NaHCO3/CO2 buffer, which PEI/p53 and erastin with metal ions. (d) Curves showing HT1080 tumour
confirmed the indispensable role of HCO3-/CO2 (Figure 1e). In volumes in mice during the first 25 days of treatment. Reproduced with
addition, MS@MnO2 NPs still exhibited sufficient MB permission from ref. [40]. Copyright 2016, American Chemical Society.
degradation in the presence of a high concentration of GSH of nanoparticles (the assembly of small molecules and does not
10 mM (Figure 1f and 1g), which was approximately 1.3 times have the periodic network structure) also have the potential to
higher than the concentration of free Mn2+, suggesting that be utilized for CDT. Compared with stable inorganic
MS@MnO2 NPs increased the efficiency of CDT. Therefore, the nanomaterials, MOF and macromolecular nanomaterials have
combination of other metal ions with the TME is a promising been designed for therapies or imaging[39] because of their
approach for improving CDT efficiency. flexibility and better responsiveness. Zheng et al. synthesized a
metal-organic network (MON)-p53 with a core-shell structure
2.3. Metal-organic framework nanomaterials based on a MOF, and the resulting network resulted from the
coordination between Fe3+ and tannic acid (Figure 2a).[40] In the
In addition to inorganic nanomaterials, nanomaterials with a system, ferric ions generated ·OH, and the expression of p53
metal-organic framework (MOF) and macromolecular
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Accepted Manuscript
Figure 3. (a) Schematic illustrating the synthetic procedure used to prepare rMOF-FA. (b) Schematic showing the mechanism underlying the therapeutic
effects of rMOF-FA nanoparticles on cancer cells. (c) Iron ion release from rMOF-FA in buffers with different pH values (5 and 7.4). Reproduced with
permission from ref. [41]. Copyright 2017, American Chemical Society
protein inhibited the expression of the SLC7A11 protein The Fenton reaction is affected not only by the catalytic ions
expression, thereby inducing GSH depletion to further enhance amount but also by the reaction environment, including the pH
the ·OH yield (Figure 2b). Various metal ions (Fe2+, Fe3+, Al3+, and the amount of hydrogen peroxide or GSH. Recently, several
Co2+, Ni2+, Cu2+, Mn2+ and Ca2+) had been introduced to evaluate studies focused on modulating the reaction environment
the cytotoxicity towards HT1080 cells, and significant (reducing the pH, generating more H2O2 and decreasing the
cytotoxicities were detected in cells co-incubated with MON-p53 GSH amount) for CDT have emerged and presented potential
and Fe2+ and Fe3+, confirming that the MON-p53-induced cell applications, which promotes researchers to develop additional
death was mediated by an iron-dependent mechanism (Figure methods to optimize the treatment effect.
2c). Furthermore, the shell p53 protein exhibited the ability to
regulate the SLC7A11 level and block GSH synthesis, which 3.1. Reducing pH levels
ultimately enhanced the CDT efficiency in vivo, as evidenced by
the changes in the tumour volumes of different groups (Figure For the Fenton reaction, the optimal reaction pH ranges from
2d). In addition, Burachaloo et al. fabricated a reduced iron MOF 2-4[42] because of the following reasons: i) it inhibits the
conjugated with folic acid (rMOF-FA) as the CDT agent[41] precipitation of ferrous and iron ions in a low pH environment;
(Figure 3a). The MOF was unstable in an acidic environment and ii) in addition, hydrogen peroxide would be degraded under
and rapidly released iron ions for CDT, while it was stable at extremely low pH conditions. For tumours, the pH of the TME
neutral pH (Figure 3b). As shown in Figure 3c, the rMOF-FA predominantly ranges from 6.5 to 7, the endosomes of tumour
released approximately 100% of the iron ions at pH 5.0 cells have a pH of approximately 5.0 and lysosomes have a pH
compared with the negligible release observed at neutral pH, of approximately 4.5.[43] For most materials, the number of
consistent with the well responsiveness and specificity of catalytic ions released is limited primarily by the requirement for
amorphous iron nanomaterials. Other advantages of MOF, such a weakly acidic environment, and the Fenton reaction is also
as the high porosity and large surface area could also been inhibited in a weakly acidic environment. Therefore, reducing the
utilized for promoting CDT efficiency. pH of TME or delivering the nanomaterials to the nucleus or
lysosomes might also enhance the CDT efficiency.
3. Modulation of the reaction environment
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Accepted Manuscript
Figure 4. (a) Illustration of the synthesis and (b) multiple functions of AIO RNAi NPs. (c) Tissue penetration of AIO NPs (upper panel) and polymer NPs
(lower panel) loaded with Cy5.5-siRNA in a 3D spheroid model after a 4 h incubation. (d) Western blot and (e) immunofluorescence staining for MCT4 in PC3
cells treated with NP (siControl) or NP-(siMCT4). (f) ROS production in PC3 cells treated with or without the MCT4 siRNA. (g) Representative photos of
tumour-bearing mice and tumour tissues from various groups. Reproduced with permission from ref. [44]. Copyright 2018. Wiley-VCH.
Liu et al. developed an amorphous iron oxide (AIO) RNAi NP concentration of ascorbic acid, the nanoparticles promoted H2O2
nanoplatform (Figure 4a),[44] The siRNA was encapsulated generation, and the Fc groups subsequently suppressed tumour
within NPs to limit enzymatic contact, degradation and burst growth (Figure 5b). The hydroxyl radicals were then monitored
release during circulation in the blood, and the small size of the by detecting the fluorescence intensity at a wavelength of 425
NPs permitted it to deeply penetrate tumour tissues. This novel nm of 2-hydroxyterephthalic disodium, which was the product of
strategy was summarized as an initial blockade of intracellular the reaction between disodium terephthalate and hydroxyl
lactate/H+ production via MCT4 silencing to induce cell acidosis radicals. Excitingly, Figure 5c showed the large increase in the
and the subsequent release of large amounts of iron ions to fluorescence intensity of PA/Fc-micelles after 3 h compared with
react with more H2O2 molecules, which was also stimulated by the nearly unchanged fluorescence intensity of PA and DFc
the intracellular lactate efflux block, as depicted in Figure 4b. As alone, which confirmed the sufficient generation of ·OH.
shown in Figure 4c, AIO NPs penetrated greater depths of the Meanwhile, comet assays of different groups (Figure 5d) were
tumour than polymer NPs loaded with Cy5.5-siRNA, thus conducted to reveal the degree of DNA damage, which also
potentially extending the treatment area to enhance CDT revealed the efficient treatment effect of PA/Fc-Micelles (Figure
efficiency. Moreover, the expression of MCT4 analysed by 5e). In addition to the use of MnO2,[38] the strategy of introducing
western blotting and immunofluorescence staining (Figure 4d RNA to modulate the amount of H2O2 production is also suitable
and 4e) confirmed the silencing effect of AIO NPs. The for decreasing the amount of GSH.
intracellular H2O2 concentration was substantially increased after Huo et al. fused natural glucose oxidase (GOD) with
silencing in PC3 cells, which resulted from intracellular lactate biodegradable dendritic mesoporous silica (DMS) nanoparticles
accumulation (Figure 4f). Similarly, MCT4 expression was also and ultrasmall Fe3O4 nanoparticles into one system for tumour
inhibited, and the AIO NPs exhibited sufficient inhibition on ablation.[46] GOD served as the enzyme that reacted with
tumour growth in vivo (Figure 4g). glucose in the tumour area to generate H2O2 and DMS released
Fe3O4 to initiate a Fenton-like reaction, generating amounts
3.2. Increasing the H2O2 level and decreasing the GSH level of ·OH (Figure 6a). Furthermore, the anticancer ability of GFD
NCs at the cellular level was evaluated using a calcein-AM/PI
The concentration of H2O2 in tumour cells is up to 100 µM, probe, and the confocal laser scanning microscope (CLSM)
while it is just 20 nM in normal tissues.[26] However, the amount images showed well CDT effect on 4T1 cells (Figure 6b). The
in tumour cells is still low to serve as an efficient CDT. The blood glucose levels decreased 30 min after the injection of the
strategy of inhibiting MCT4 expression using the (AIO) RNAi NP nanoplatform and recovered 1 h after the injection (Figure 6c),
nanoplatform not only reduces the pH but also increases H2O2 which indicated glucose consumption by GOD. Nevertheless,
generation. Additionally, Wang et al. also constructed integrated several issues remain to be resolved. First, the glucose
multifunctional polymeric nanoparticles for tumour therapy.[45] consumption process also requires oxygen, but the TME is
The micelles had cores formed by L-ascorbyl palmitate (PA) and relatively hypoxic, which would inhibit the generation of H2O2. In
ferrocenecarboxylic acid hexadecyl ester (DFc) (Figure 5a) and addition, a lack of GOD targeting to the TME would also enable
poly(ethylene glycol) (PEG) shells, which were linked via the this enzyme to react with glucose in normal tissues, reducing the
host-guest interaction. Responding to the pharmacological selectivity of the reaction.
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Accepted Manuscript
Figure 5. (a) Synthesis of PA/Fc-micelles. (b) Schematic illustrating the application of polymeric micelles for cancer therapy using Fenton reactions. (c)
Changes in the fluorescence intensity of 2-hydroxyterephthalic acid recorded at a wavelength of 425 nm over time after an incubation with three groups in 10%
serum-containing medium. Mean ± s.d. (n = 3). (d) Results of the comet assay using 4T1 cells and MCF-7 cells treated with various agents. Scale bars: 100
μm. (e) DNA damage in different groups, as determined from the ratio of the comet tail. Mean ± s.d. (n = 10), **p < 0.01, ***p < 0.001 (t-test). Reproduced with
permission from ref. [45]. Copyright 2018, American Chemical Society.
as well as an adjustable spot area. Moreover, the relatively low
cost, availability and noninvasiveness also make light stimulation
more appealing. Many studies have examined tumour imaging
and therapies using light-triggered nanomaterials, such as
4. Stimulation by external energy fields
photoacoustic imaging, upconversion fluorescence imaging,
photothermal therapy (PTT), photodynamic therapy (PDT) and
Despite the limitations of external energy fields, such as an
light-responsive drug release for chemotherapy.[47] Ultraviolet
insufficient penetration depth of light and heat radiation in
(UV) light promotes the Fenton reaction, which is recognized as
normal tissues, external energy fields (e.g., light, heat,
a photo Fenton reaction. Thus, the participation of UV light
ultrasound, magnetic fields and electric fields) are more feasible
would also enhance CDT efficiency. Due to the damage to
for development as auxiliary treatments to speed up Fenton
normal tissues and the extremely low penetration depth of UV
reaction and Fenton-like reaction to improve CDT effects.
light, near-infrared light (NIR) is typically combined with
4.1. Light stimulation to speed up Fenton reaction upconversion nanomaterials (UCNPs) as UCNPs could convert
NIR into UV/Vis,[48] which has also been applied frequently in
As a commonly used external stimulus, light possesses the nanomedicine.
unique characteristics of a controllable wavelength and intensity,
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Accepted Manuscript
Figure 6. (a) Procedures used to synthesize Fe3O4@DMSN nanocatalysts and GOD-Fe3O4@DMSN nanocatalysts. Schematic showing the sequential
catalytic-therapeutic mechanism by which GFD NCs generate hydroxyl radicals for cancer therapy. (b) CLSM images of the distributions of viable and dead
cells stained with calcein-AM/PI after a co-incubation with various concentrations of GFD NCs at pH 7.4 and pH 6.0 for 4 h. (c) Blood glucose level in mice
at different times of day. Mean ± s.d. (n = 3). Reproduced with permission from ref. [46]. Copyright 2017, Nature.
Based on the above considerations, our group developed a UCSRF. Compared with the phosphate buffer saline (PBS)
nanoplatform, NaYF4:Yb3+,Tm3+@NaYF4@dSiO2@mSiO2- control, PBS with NIR irradiation, UCS-Ru2+/Fe2+ alone, UCS-
2+ 2+
Ru /Fe (UCSRF), featuring mitochondrial DNA targeting that TPP/Fe2+ with NIR irradiation and UCS-Ru2+/Fe2+ with UV
generates ROS via an NIR-triggered photo Fenton reaction.[49] irradiation groups, the UCS-Ru2+/Fe2+ with NIR irradiation group
As shown in Figure 7a, the UCNP cores converted NIR to exhibited the strongest green fluorescence, indicating the
UV/Vis, and the mesoporous silica shell loaded and delivered highest level of ·OH generation (Figure 7d), consistent with the
the Fenton agent and Ru2+ complex on the surface to bind the MTT results (Figure 7e). Moreover, tumour growth was inhibited
mitochondrial DNA. When irradiated with NIR, the in the UCSRF-treated group (Figure 7f and 7g). Although the
generated ·OH would destroy the mitochondrial DNA. The bio- study did not directly utilize NIR light, it provided the foundation
TEM image with the corresponding energy dispersive spectrum of introducing NIR to enhance the CDT efficiency .
(EDS) (Figure 7b) and the CLSM (Figure 7c) images all clearly
proved that the mitochondria were the preferred cellular target of 4.2 Heat stimulation to speed up Fenton reaction
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Accepted Manuscript
Figure 7. (a) Schematic illustrating the procedures used to synthesize UCSRF and a mitochondrial DNA-targeted and NIR-initiated photochemical therapy
(PCT) for cancer. (b) Bio-TEM image of UCSRF in cellular mitochondria. (c) Confocal images and the quantification of fluorescent intensity of the line
scanning profiles of HepG2 cells treated with different nanomaterials. (d) CLSM images of HepG2 cells from various groups reveal the generation of ·OH
using DCFH-DA as the probe. (e) In vitro viability of HepG2 cells undergoing apoptosis in response to different treatments (**p < 0.01 and ***P < 0.001). (f)
Relative tumour volumes in groups receiving different treatments for 12 days. (g) H&E staining of the tumour tissue sections from different groups of mice 3
days after NIR irradiation. Reproduced with permission from ref. [49]. Copyright 2017, Elsevier.
As another external energy field, heat has also been reported researchers have focused on combining these treatments with
to promote CDT using FeS2-PEG nanoparticles (Figure 8a).[50] nanotechnology and developed novel treatment methods. Under
The synthesized FeS2-PEG nanoparticles generated localized the direction of this pursuit, CDT has emerged as a new “green”
heat in a photothermal conversion process using an NIR laser. treatment displaying selectivity and specificity, and represents a
Subsequently, the heat accelerated and enhanced the Fenton further development of the mechanism of chemotherapy and
reaction for amplified CDT. Figure 8b showed the electron radiotherapy. More importantly, CDT combines the TME and
paramagnetic resonance (EPR) spectra and the results classical Fenton reaction or Fenton-like reaction and then
obviously revealed the ability of heat to promote the Fenton destroys the tumour in situ, holding potential for clinical
reaction. Moreover, the FeS2-PEG nanoparticles exhibited translation. To date, many studies have reported new
sufficient performance in MB decolourization (Figure 8c), as the nanomaterials that regulate the Fenton reaction in a particular
degradation ratio improved when heat was introduced. The environment and use external energy fields, all of which have
imaged captured after the administration of different treatments promoted the development of CDT nanoagents in biomedicine.
shown in Figure 8d revealed that the FeS2-PEG nanoparticles This minireview summarizes the current development of the
substantially suppressed tumour growth after NIR irradiation, field of CDT (in situ treatments using the Fenton reaction or
which was ascribed to the synergistic effect of PTT and heat- Fenton-like reactions to generate ·OH in tumour sites), including
enhanced CDT. Although heat was generated indirectly via NIR the selection of nanomaterials, the regulation of the reaction
laser radiation, the results still provided evidence for the ability of environment, and the introduction of an external energy fields as
heat to enhance CDT and propel interests of developing other stimulus (including light, heat, etc.) to improve the CDT
heat-enhanced CDT nanomaterials. efficiency. These strategies are all based on the principle of CDT
and are discussed in depth, with an emphasis on the design and
5. Conclusions and outlook implementation of the ideas. Nevertheless, CDT is still in its
infancy. Additional studies are required before CDT is ready for
The side effects of traditional chemotherapy and radiotherapy clinical translation, as some important scientific issues must be
technology on patients cannot be ignored. Therefore, many considered. Representative issues are listed below.
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Accepted Manuscript
Figure 8. (a) Schematic of the FeS2-PEG-mediated self-enhanced MRI and CDT/PTT. (b) EPR spectra obtained using the 5,5-dimethyl-L-pyrroline N-oxide
(DMPO) as the spin trap after different treatments. (c) Decolourized MB data from different groups. (d) Representative images of Balb/c mice with bilateral
4T1 tumour xenografts captured at different times after treatment. Reproduced with permission from ref. [50]. Copyright 2017, Wiley-VCH.
First, in-depth explorations of the anticancer pathways of be considered to substantially increase the amount of H2O2 for
CDT using genetic and molecular methodologies are required efficient CDT. With the introduction of an external light field,
for further CDT optimization. Second, the specificity or UCNPs will facilitate the generation of ·OH of CDT agents, as it
biocompatibility of CDT must be improved to prevent potential could convert NIR to UV to promote Fenton-like reactions.
damage to normal cells, because the pH of lysosomes in normal Moreover, MOFs could also be used for assistant
cells is also low and would promote ·OH generation, even if the phototherapy[55] when act as CDT agents under light irradiation.
concentration of hydrogen peroxide is very low. Third, the A magnetic field has been shown to stimulate heat production
targeting efficiency of CDT agents for tumour tissues should be from magnetic nanomaterials and indirectly enhance CDT
improved and it is a common challenge for all nanoagents. efficiency. Some unstable molecules containing peroxy bridges
Moreover, unified diagnostic and treatment guidelines for also produce H2O2 under sonication to enhance the ·OH
different diseases should be developed. [51] Thus, it is urgent to production during CDT. CDT would also adequately exert its
introduce advanced diagnostic methods (such as computed potential when applied in combination with clinical
tomography imaging, magnetic resonance imaging or positron radiofrequency ablation technology, which utilizes an electric
emission computed tomography imaging) to achieve real-time field to produce hyperthermia. In summary, CDT is a flourishing
monitoring and an assessment of CDT effect. Finally, the most research frontier that requires further exploitation, despite the
crucial issue is to increase the ·OH amount in situ in the tumour presence of several unresolved issues. Finally, our hope is to
area during the CDT process. Several feasible strategies have pursue and design optimal nanomedicines with on-demand CDT
been proposed as responses to these challenges and the details efficiency combined with advanced diagnostic methods to
are presented below. Last but not the least, the development of provide specific, efficient and safe protocols for treating cancer
nanomaterials should considers both the functionality and easy or other diseases and improving patient health.
synthesis procedures as we want to apply them in the real world.
The fields of catalysis and environmental protection might be
the source of guidelines for the selection of nanomaterials, as Acknowledgements
certain polyoxometalates (POMs)[52] serve as pH-independent
agents that induce Fenton-like reactions. In addition, MOFs We gratefully acknowledge support from the National Funds for
provide extensive choices for CDT such as selecting suitable
Distinguished Young Scientists (Grant No. 51725202), the
Fe3+ MOFs for magnetic resonance contrast agents[53] when
National Science Foundation for the Young Scientists of China
playing roles in CDT and the surface of iron-based MOFs could
be easily functionalized for demand CDT.[54] (Grant No. 51702211), and the Shanghai Excellent Academic
Second, we may increase lactic acid accumulation by Leaders Program (Grant No. 16XD1404000).
inhibiting aerobic glycolysis using strategies that reduce the
oxygen content in tumour to reduce the TME pH. Furthermore,
metal-based peroxides, which are acid-sensitive agents, should
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Keywords: Fenton reaction • chemodynamic therapy • [32] L. Wang, M. Huo, Y. Chen, J. Shi. Biomaterials. 2018, 163, 1-13.
[33] D. Cioloboc, C. Kennedy, E. N. Boice, E. R. Clark, D. M. Kurtz Jr,
nanomaterials selection • reaction environment • external energy
Biomacromolecules. 2017, 19, 178-187.
fields [34] K. T. Lee, Y. J. Lu, F. L. Mi, T. Burnouf, Y. T. Wei, S. C. Chiu, E. Y.
Chuang, S. Y. Lu. ACS Appl. Mater. Inter. 2017, 9, 1273-1279.
[1] J. J. Pignatello, E. Oliveros, A. MacKay. Crit. Rev. Env. Sci. Tec. 2006, [35] H. Bi, Y. Dai, P. Yang, J. Xu, D. Yang, S. Gai, F. He, B. Liu, C. Zhong,
36, 1-84. G. An, J. Lin. Small. 2018, 14, 1703809
[2] E. Chamarro , A. Marco, S. Esplugas. Water Res. 2001, 35,1047-1051. [36] A. N. Koo, K. H. Min, H. J. Lee, S.-U. Lee, K. Kim, I. Chan Kwon, S. H.
[3] Perez, F. Torrades, J. A. Garcıa ́ -Hortal, X. Domènech, J. Peral. Appl. Cho, S. Y. Jeong, S. C. Lee. Biomaterials. 2012, 33, 1489-1499.
Catal. B-Envir. 2002, 36, 63-74. [37] A. D. Bokare, W. Choi. J. Hazard. Mater. 2014, 275, 121-135.
[4] R. Li, X. Jin, M. Megharaj, R. Naidu, Z. Chen. Chem. Eng. J. 2015, 264, [38] L. S. Lin, J. Song, L. Song, K. Ke, Y. Liu, Z. Zhou, Z. Shen, J. Li, Z.
587-594. Yang, W. Tang, G. Niu, H. H. Yang, X. Chen. Angew. Chem. 2018, 130,
[5] X. Yang, X. Xu, J. Xu, Y. Han. J. Am. Chem. Soc. 2013, 135, 16058- 4996-5000.
16061. [39] a) W. Chen, C. Wu. Dalton Trans. 2018, 47, 2114-2133; b) S. Wuttke,
[6] E. Saputra, S. Muhammad, H. Sun, H. M. Ang, M. O. Tade, S. M. Lismont, A. Escudero, B. Rungtaweevoranit, W. J. Parak.
Accepted Manuscript
Wang. Environ. Sci. Technol. 2013, 47, 5882-5887. Biomaterials 2017, 123, 172-183; c) M. Gimenez-Margues, M. Hidalgo,
[7] T. Rhadfi, J. Y. Piquemal, L. Sicard, F. Herbst, E. Briot, M. Benedetti, A. T. Serre, P. Horcajada. Coord. Chem. Rev. 2015, 307, 342-360; d) S.
Atlamsani. Appl. Catal. A-Gen. 2010, 386, 132-139. Furukawa, J. Reboul, S. Diring, K. Sumida, S. Kitagawa. Chem. Soc.
[8] X. Zhou, Y. Zhang, C. Wang, X. Wu, Y. Yang, B. Zheng, H. Wu, S. Guo, Rev. 2014, 43, 5700-5734.
J. Zhang.. ACS Nano. 2012, 6, 6592-6599. [40] D. W. Zheng, Q. Lei, J. Y. Zhu, J. X. Fan, C. X. Li, C. Li, Z. Xu, S. X.
[9] R. A. Gatenby, R. J. Gillies. Nat. Rev. Cancer. 2004, 4, 891-899. Cheng, X. Z. Zhang. Nano. Lett. 2016, 17, 284-291.
[10] Q. Chen, C. Liang, X. Sun, J. Chen, Z. Yang, H. Zhao, L. Feng, Z. Liu, [41] H. Ranji-Burachaloo, F. Karimi, K. Xie, Q. Fu, P. A. Gurr, D. E.
Proc. Natl. Acad. Sci. USA 2017, 114, 5343-5348. Dunstan, G. G. Qiao. ACS Appl. Mater. Inter. 2017, 9, 33599-33608.
[11] M. Nishikawa, A. Tamada, H. Kumai, F. Yamashita, M. Hashida. Int. J. [42] Y. W. Kang, K. Y. Hwang. Water Res. 2000, 34, 2786-2790.
Cancer. 2002, 99, 474-479. [43] M. Breunig, S. Bauer, A. Goepferich, Eur. J. Pharm. Biopharm. 2008,
[12] T. Y. Reynolds, S. Rockwell, P. M. Glazer. Cancer. Res. 1996, 56, 68, 112-128.
5754-5757. [44] Y. Liu, X. Ji, W. W. Tong, D. Askhatova, T. Yang, H. Cheng, Y. Wang, J.
[13] T. L. Whiteside. Oncogene, 2008, 27, 5904-5912. Shi. Angew. Chem. Int. Edit. 2018, 130, 1526-1529.
[14] H. A. Goubran, R. R. Kotb, J. Stakiw, M. E. Emara, T. Burnouf. Cancer [45] Y. Wang, W. Yin, W. Ke, W. Chen, C. He, Z. Ge. Biomacromolecules
growth and metastasis, 2014, 7, CGM-S11285. DOI: 10.1021/acs.biomac.7b01777
[15] C. Kahlert, Kalluri, R. J. Mol. Med. 2013, 91, 431-437. [46] M. Huo, L. Wang, Y. Chen, J. Shi. Nat. Comm. 2017, 8, 357.
[16] Chen, Q., Feng, L., Liu, J., Zhu, W., Dong, Z., Wu, Y., & Liu, Z. Adv. [47] a) D. Y. Lee, J. Y. Kim, Y. Lee, S. Lee, W. Miao, H. S. Kim, J. J. Min, S.
Mater. 2016, 28, 7129-7136. Jon. Angew. Chem. Inter. Edit. 2017, 56, 13684-13688; b) G. Chen, R.
[17] Q. Yang, S. Wang, P. Fan, L. Wang, Y. Di, K. Lin, F. Xiao. Chem. Jaskula‐Sztul, C. R. Esquibel, I. Lou, Q. Zheng, A. Dammalapati, A.
Mater. 2005, 17, 5999-6003. Harrison, K. W. Eliceiri, W. Tang, H. Chen, S. Gong. Adv. Funct.
[18] R. M. Sawant, J. P. Hurley, S. Salmaso, A. Kale, E. Tolcheva, T. S. Mater. 2017, 27, 1604671: c) W. Tao, X. Ji, X. Xu, M. A. Islam, Z. Li, S.
Levchenko, V. P. Torchilin. Bioconjugate Chem. 2006, 17, 943-949. Chen, P. E. Saw, H. Zhang, Z. Bharwani, Z. Guo, J. Shi, O.. C.
[19] G. H. Fletcher.. Textbook of radiotherapy. 1973, Lea & Febiger. Farokhzad. Angew. Chem. Inter. Edit. 2017, 56, 11656-11656.; d) X. Li,
[20] J. M. Brown. Brit. J. Cancer. 1993, 67, 1163-1170. S. Kolemen, J. Yoon, E. U. Akkaya. Adv. Funct. Mater. 2017, 27,
[21] K. B. Peters, J. M. Brown. Cancer Res. 2002, 62, 5248-5253. 1604053; e) K. M. Park, K. Baek, Y. H. Ko, A. Shrinidhi, J. Murray, W.
[22] H. Mizutani, S. Tada-Oikawa, Y. Hiraku, M. Kojima, S. Kawanishi. Life H. Jang, K. H. Kim, J. S. Lee, J. Yoo, S. Kim, K. Kim. Angew. Chem.
Sci. 2005, 76, 1439-1453. Inter. Edit. 2018, 57, 3132-3136.
[23] J. Golenser, J. H. Waknine, M. Krugliak, N. H. Hunt, G. E. Grau. Int. J [48] Y. Yang, Q. Shao, R. Deng, C. Wang, X. Teng, K. Cheng, Z. Cheng, L.
Parasitol. 2006, 36, 1427-1441. Huang, Z. Liu, X. Liu, B.Xing. Angew. Chem. Int. Ed. 2012, 51, 3125 –
[24] S. Krishna, A. C. Uhlemann, R. K. Haynes. Drug Resis. Update. 2004, 3129
7, 233-244. [49] P. Hu, T. Wu, W. Fan, L. Chen, Y. Liu, D. Ni, W. Bu, J.
[25] C. Zhang, W. Bu, D. Ni, S. Zhang, Q. Li, Z. Yao, J. Zhang, H. Yao, Z. Shi. Biomaterials 2017, 141, 86-95.
Wang, J. Shi. Angew. Chem. Int. Edit. 2016, 55, 2101-2106. [50] Z. Tang, H. Zhang, Y. Liu, D. Ni, H. Zhang, J. Zhang, Z. Yao, M. He, J.
[26] a) T. P. Szatrowski, C. F. Nathan. Cancer. Res. 1991, 51, 794-798; b) Shi, W. Bu. Adv. Mater. 2017, 29, 1701683.
M. P. Murohy. Biochem. J. 2009, 417, 1-13; c) S. Zhai, X. Hu, Y, Hu, B. [51] a) Angew. Chem. Int. Ed. Doi:10.1002/anie.201800594; b) N. Oliva, J.
Wu, D. Xing, Biomaterials 2017, 121, 41-54. Conda, K. Wang, N. Artzi. Acc. Chem. Res., 2017, 50 ,669–679
[27] a) A. D. D'Andrea, W. A. Haseltine. PNAS 1978, 75, 3608-3612; b) H. [52] C. Lee, D. L. Sedlak. J. Mol. Catal. A-Chem. 2009, 311, 1-6.
Kappus, H. Sies. Experientia 1981, 37, 1233-1241; c) H. Nohl, W. [53] A. Zimpel, T. Prei, R. Roder, H. Engelke, M. Ingrisch, M. Peller, J. O.
Jordan. Biochem. Bioph. Res. Commun. 1983, 114, 197-205. Radler, E. Wagner, T. Bein, U. Lachelt, S. Wuttke. Chem. Mater., 2016,
[28] R. Freund, U. Lachelt, T. Gruber, B. Ruhle, S. Wuttke. ACS Nano. 2018, 28, 3318-3326.
12, 2094-2105. [54] R. Roder, T. Prei, P. Hirschle, B. Steinborn, A. Zimpel, M. Hohn, J. O.
[29] R. A. Gatenby, R. J. Gillies, Nat. Rev. Cancer 2004, 4, 891-899. Radler, T. Bein, E. Wagner, S. Wuttke, U. Lachelt. J. Am. Chem. Soc.
[30] Q. An, C. Sun, D. Li, K. Xu, J. Gu, C. Wang. ACS Appl. Mater. 2017 139 , 2359-2368.
Inter. 2013, 5, 13248–13257 [55] M. Lismont, L. Dreesen, S. Wuttke. Adv. Funct. Mater. 2017, 27,
[31] D. Wang, J. Zhou, R. Chen, R. Shi, G. Xia, S. Zhou, Z. Liu, N. Zhang, 1606314.
H. Wang, Z. Guo, Q. Chen. Biomaterials. 2016, 107, 88-101.
This article is protected by copyright. All rights reserved.
Angewandte Chemie International Edition 10.1002/anie.201805664
MINIREVIEW
Entry for the Table of Contents (Please choose one layout)
REVIEW
Recent rapid developments in Zhongmin Tang, Yanyan Liu,
chemodynamic therapy using Mingyuan He, Wenbo Bu*
Fenton reactions or Fenton-like
reactions to generate ·OH at tumour Page No. – Page No.
sites are discussed in depth in this
Chemodynamic Therapy: Tumour
minireview, with an emphasis on the
Microenvironment-Mediated Fenton
strategies designed to enhance
Accepted Manuscript
and Fenton-Like Reaction
therapeutic efficiency, providing
guidelines for potential clinical
translation.
This article is protected by copyright. All rights reserved.
You might also like
- Varied Papers On MEMS - 5Document12 pagesVaried Papers On MEMS - 5Tiago PestanaNo ratings yet
- 10 1002@adfm 202006216Document14 pages10 1002@adfm 202006216Abhijeet MohantyNo ratings yet
- Facile Synthesis of Er3 Tm3 Co Doped Magnetic Luminescent - 2022 - Journal ofDocument9 pagesFacile Synthesis of Er3 Tm3 Co Doped Magnetic Luminescent - 2022 - Journal ofShuNo ratings yet
- 1 s2.0 S0142961219301528 MainDocument14 pages1 s2.0 S0142961219301528 MainHarshithNo ratings yet
- Deoliveira2014 PDT PDFDocument7 pagesDeoliveira2014 PDT PDFShriya ShahuNo ratings yet
- Angew Chem Int Ed - 2019 - Imberti - New Designs For Phototherapeutic Transition Metal ComplexesDocument13 pagesAngew Chem Int Ed - 2019 - Imberti - New Designs For Phototherapeutic Transition Metal ComplexesNohemiNo ratings yet
- Journal of Environmental Chemical Engineering: SciencedirectDocument27 pagesJournal of Environmental Chemical Engineering: SciencedirectHoàng KhôiNo ratings yet
- Titanium Dioxide Photocatalysis: Fundamentals and Application On PhotoinactivationDocument39 pagesTitanium Dioxide Photocatalysis: Fundamentals and Application On PhotoinactivationNikhi Maria RajuNo ratings yet
- 01 25117 MagalhaesDocument39 pages01 25117 Magalhaeskeveho9261No ratings yet
- Photodynamic TherapyDocument5 pagesPhotodynamic TherapyPranav UpadhyayaNo ratings yet
- Recent Progress in Photodynamic Immunotherapy With Metal-Based PhotosensitizersDocument13 pagesRecent Progress in Photodynamic Immunotherapy With Metal-Based PhotosensitizersWang JianweiNo ratings yet
- 326 831 1 PBDocument7 pages326 831 1 PBAmar AmarNo ratings yet
- Photodynamic Therapy in Endodontics: ReviewDocument15 pagesPhotodynamic Therapy in Endodontics: ReviewFabro HMNo ratings yet
- 1 - DOUGHERTY, T.J. Et Al. Photodynamic Therapy. J. Natl. Cancer Inst., Cary, V. 90, N. 12, P. 889 - 905, 1998.Document17 pages1 - DOUGHERTY, T.J. Et Al. Photodynamic Therapy. J. Natl. Cancer Inst., Cary, V. 90, N. 12, P. 889 - 905, 1998.Hylze ChavesNo ratings yet
- Novel Removal of Diazinon Pesticide by AdsorptionDocument7 pagesNovel Removal of Diazinon Pesticide by AdsorptionAlissom GomesNo ratings yet
- 10 1002@anie 202010714Document12 pages10 1002@anie 202010714Abhijeet MohantyNo ratings yet
- Boluki Et Al 2020 - The Combination of Antimicrobial Photocatalysis and Antimicrobial Photodynamic TherapyDocument6 pagesBoluki Et Al 2020 - The Combination of Antimicrobial Photocatalysis and Antimicrobial Photodynamic Therapysamusm.smNo ratings yet
- 1 s2.0 S1878535220301593 MainDocument18 pages1 s2.0 S1878535220301593 MainAbdul Wahab Assya RoniNo ratings yet
- 10 1016@j Cej 2020 125574Document32 pages10 1016@j Cej 2020 125574Abhijeet MohantyNo ratings yet
- Preparation and Applications of Titanium Dioxide and Zinc Oxide NanoparticlesDocument4 pagesPreparation and Applications of Titanium Dioxide and Zinc Oxide NanoparticlesNuzulul RahmahNo ratings yet
- Ijms 24 00410Document18 pagesIjms 24 00410Robert StryjakNo ratings yet
- Development of Iron/ethylcellulose (Core/shell) Nanoparticles Loaded With Diclofenac Sodium For Arthritis TreatmentDocument7 pagesDevelopment of Iron/ethylcellulose (Core/shell) Nanoparticles Loaded With Diclofenac Sodium For Arthritis Treatmentزياد سلمانNo ratings yet
- Unveiling Cutting-Edge Progress in The Fundamentals of MXene - Synthesis Strategies, Energy and Bio-Environmental ApplicationsDocument46 pagesUnveiling Cutting-Edge Progress in The Fundamentals of MXene - Synthesis Strategies, Energy and Bio-Environmental ApplicationsBurak KursNo ratings yet
- Photodynamic Therapy in Dentistry: A Literature ReviewDocument13 pagesPhotodynamic Therapy in Dentistry: A Literature ReviewShriya ShahuNo ratings yet
- Photodynamic Therapy in Endodontics: A Literature ReviewDocument8 pagesPhotodynamic Therapy in Endodontics: A Literature ReviewShriya ShahuNo ratings yet
- Effectiveness of PhotochemicalDocument7 pagesEffectiveness of PhotochemicalGHZNo ratings yet
- Crystals: A Literature Review On High-Performance Photocatalysts For Sustainable Cancer TherapyDocument13 pagesCrystals: A Literature Review On High-Performance Photocatalysts For Sustainable Cancer TherapyPol RecasensNo ratings yet
- Ternary TiO2-WO3-CQDs Nanocomposites For Enhanced Photocatalytic Mineralization of Aqueous Cephalexin Degradation Mechanism and Toxicity EvaluationDocument13 pagesTernary TiO2-WO3-CQDs Nanocomposites For Enhanced Photocatalytic Mineralization of Aqueous Cephalexin Degradation Mechanism and Toxicity EvaluationAtif sialNo ratings yet
- 2019 - Photodynamic Antimicrobial Therapy - Comparison of Thiocyanate and Selenocyanate For Potentiation ofDocument22 pages2019 - Photodynamic Antimicrobial Therapy - Comparison of Thiocyanate and Selenocyanate For Potentiation ofragod2No ratings yet
- Applicationof Porphyrinsin Antibacterial Photodynamic Therapy MoleculesDocument29 pagesApplicationof Porphyrinsin Antibacterial Photodynamic Therapy Moleculesisma drNo ratings yet
- NanozymesDocument10 pagesNanozymesdd donNo ratings yet
- Fotooxid, Cu Tian, 2018Document14 pagesFotooxid, Cu Tian, 2018Larisa MocanuNo ratings yet
- Photocatalytic Degradation of Organic Pollutants by MOFsDocument10 pagesPhotocatalytic Degradation of Organic Pollutants by MOFsHenry LalchhuankimaNo ratings yet
- Photo or Solar Ferrioxalate Disinfection Technology Without External Hydrogen Peroxide SupplyDocument6 pagesPhoto or Solar Ferrioxalate Disinfection Technology Without External Hydrogen Peroxide SupplyBEA FRANCINE DELOS SANTOSNo ratings yet
- Magnet 9Document7 pagesMagnet 9a.mahdieh90No ratings yet
- Advanced Science - 2021 - Fang - Thermally Activated Delayed Fluorescence Material An Emerging Class of Metal FreeDocument19 pagesAdvanced Science - 2021 - Fang - Thermally Activated Delayed Fluorescence Material An Emerging Class of Metal Freerajendrk1No ratings yet
- Adhikari 2016Document8 pagesAdhikari 2016Christian .Quijia QuezadaNo ratings yet
- Oxidaxion Us H2ho2 PDFDocument12 pagesOxidaxion Us H2ho2 PDFEstefany MartinezNo ratings yet
- 1 s2.0 S2405844020300074 MainDocument6 pages1 s2.0 S2405844020300074 MainAlvin Wahyu Puspita SariNo ratings yet
- Controlling Solar Hydrogen Production by Organizing PorphyrinsDocument11 pagesControlling Solar Hydrogen Production by Organizing PorphyrinsBeauty MoonNo ratings yet
- Ce3+ Ce4+ Doping Photocatalyst Degredation Metylene BlueDocument14 pagesCe3+ Ce4+ Doping Photocatalyst Degredation Metylene BlueHuanNo ratings yet
- Chemical Engineering JournalDocument10 pagesChemical Engineering Journalpedro cuellar proNo ratings yet
- Longo 2011Document10 pagesLongo 2011Luciana BetzlerNo ratings yet
- Applied Catalysis B: EnvironmentalDocument6 pagesApplied Catalysis B: EnvironmentalMinh TrầnNo ratings yet
- 2022 Romero AngewChemIntEd 61 E2022007831 SIDocument49 pages2022 Romero AngewChemIntEd 61 E2022007831 SIshamshel43No ratings yet
- Heterogeneous Fenton Catalytic Oxidation For Water TreatmentDocument11 pagesHeterogeneous Fenton Catalytic Oxidation For Water Treatmentanthonychoong9No ratings yet
- Wu 2017Document11 pagesWu 2017Kapas FernandoNo ratings yet
- Anti-Biofilm Property of Bioactive Upconversion NaDocument17 pagesAnti-Biofilm Property of Bioactive Upconversion NaMihaela TuculinaNo ratings yet
- Photodynamic Therapy For COVID-19: CorrespondenceDocument2 pagesPhotodynamic Therapy For COVID-19: CorrespondenceJuan Pablo Rodríguez CoronaNo ratings yet
- Journal of Industrial and Engineering Chemistry: Ines Nitoi, Tatiana Oncescu, Petruta OanceaDocument5 pagesJournal of Industrial and Engineering Chemistry: Ines Nitoi, Tatiana Oncescu, Petruta OanceaAndrea SilvaNo ratings yet
- The Binding Characteristics of Isoniazid With CoppDocument9 pagesThe Binding Characteristics of Isoniazid With CoppVOOGLS PUBLICATIONNo ratings yet
- GemifloxacinDocument8 pagesGemifloxacinSHERLY KIMBERLY RAMOS JESUSNo ratings yet
- Photocatalysis of B-Blockers - An Overview: Arabian Journal of ChemistryDocument8 pagesPhotocatalysis of B-Blockers - An Overview: Arabian Journal of Chemistrypetru apopeiNo ratings yet
- Abraham 2013Document15 pagesAbraham 2013priya panaleNo ratings yet
- Sciencedirect: Journal of Photochemistry & Photobiology, B: Biology 202 (2020) 111676Document8 pagesSciencedirect: Journal of Photochemistry & Photobiology, B: Biology 202 (2020) 111676Anonymous DyytrQGxNo ratings yet
- Fotodegradare OxidativaDocument7 pagesFotodegradare OxidativaAlexandra BanutaNo ratings yet
- Peróxido de HidrógenoDocument4 pagesPeróxido de Hidrógenojuanjosoto821No ratings yet
- Investigation of Photocatalytic Degradation of Phenol byDocument6 pagesInvestigation of Photocatalytic Degradation of Phenol byMokhtaria ReguigNo ratings yet
- Green Tio2 as Nanocarriers for Targeting Cervical Cancer Cell LinesFrom EverandGreen Tio2 as Nanocarriers for Targeting Cervical Cancer Cell LinesNo ratings yet
- Key Questions in Environmental Toxicology: A Study and Revision GuideFrom EverandKey Questions in Environmental Toxicology: A Study and Revision GuideNo ratings yet
- TechnicalDocument1 pageTechnicalUbac recrutementNo ratings yet
- Grade 8 Science Worksheets CbseDocument3 pagesGrade 8 Science Worksheets CbseHeidiNo ratings yet
- Tile Adhesive Heavy-Duty - Allgemeine Bau-Chemie Phil., IncDocument4 pagesTile Adhesive Heavy-Duty - Allgemeine Bau-Chemie Phil., IncRoland CepedaNo ratings yet
- Resources, Conservation & RecyclingDocument8 pagesResources, Conservation & RecyclingMiftahur RahmiNo ratings yet
- Manzil Coordination Compound: Please Fill in Your JEE Application Details by Clicking On Link BelowDocument4 pagesManzil Coordination Compound: Please Fill in Your JEE Application Details by Clicking On Link BelowAbhi kumarNo ratings yet
- Structural ConnectionsDocument205 pagesStructural ConnectionsbsitlerNo ratings yet
- P Block Elements 3Document28 pagesP Block Elements 3Shruti GaurNo ratings yet
- Assignment: Name-Manansh Ram Thakur Roll No-19135055 Branch - MechDocument3 pagesAssignment: Name-Manansh Ram Thakur Roll No-19135055 Branch - MechManansh ThakurNo ratings yet
- RCD Slides?Document139 pagesRCD Slides?Nsb JejsNo ratings yet
- Mid Sem Question Bank-FFODocument2 pagesMid Sem Question Bank-FFOMihir KhuntNo ratings yet
- 5TH Ra Bill CCDocument64 pages5TH Ra Bill CCMd. Mizanur RahamanNo ratings yet
- Production of Maleic AnhydrideDocument8 pagesProduction of Maleic AnhydrideZafran AliNo ratings yet
- Goldium Steel Lamination CRNGO CRGODocument1 pageGoldium Steel Lamination CRNGO CRGOdisse_detiNo ratings yet
- Chemical Engineering Journal: A B C D e e CDocument10 pagesChemical Engineering Journal: A B C D e e CHemanth Peddavenkatappa GariNo ratings yet
- Centering and FormworkDocument50 pagesCentering and FormworkSakshi RawatNo ratings yet
- Vulcan XC68 Conductive Carbon BlackDocument2 pagesVulcan XC68 Conductive Carbon BlackBandana DholeNo ratings yet
- ZDHC MRSL v3.1 Aug 2023Document55 pagesZDHC MRSL v3.1 Aug 2023y-tsugeNo ratings yet
- FeasibilityDocument145 pagesFeasibilityOpteron K.No ratings yet
- Organic Chem With An Emphasis On BiologyDocument713 pagesOrganic Chem With An Emphasis On Biologymedranobarraza.manuel2021No ratings yet
- OPI LEGRIT 110 0 8 0 4 ISO enDocument3 pagesOPI LEGRIT 110 0 8 0 4 ISO ensonalisabirNo ratings yet
- PHD Thesis Development and Investigation of High-Performance Fire Retardant Polypropylene Nanocomposites Via High Energy ElectronsDocument141 pagesPHD Thesis Development and Investigation of High-Performance Fire Retardant Polypropylene Nanocomposites Via High Energy Electronskaplan mNo ratings yet
- Synthesis, Structure and Spectroscopic Characterization of Ni (II), Co (II), Cu (II) and ZN (II) Complexes With Saccharinate and PyrazoleDocument9 pagesSynthesis, Structure and Spectroscopic Characterization of Ni (II), Co (II), Cu (II) and ZN (II) Complexes With Saccharinate and PyrazoleLucía BolañosNo ratings yet
- Wrinkle Free FinishingDocument4 pagesWrinkle Free FinishingkreeshnuNo ratings yet
- TDS Auracast 100 IndiaDocument2 pagesTDS Auracast 100 IndiaSuresh BabuNo ratings yet
- Pricelist Katalog Holymed - Rev JanDocument23 pagesPricelist Katalog Holymed - Rev JanPT.KARYASURYA MULIANo ratings yet
- Ms ProofDocument4 pagesMs Proofrizky efrinaldoNo ratings yet
- 415 120 DB 0001Document30 pages415 120 DB 0001Mustafa AhsanNo ratings yet
- Four Golden RuleDocument6 pagesFour Golden RulerundyudaNo ratings yet
- Introduction To Materials Science For Engineers Global Edition 9Th Edition James F Shackelford Full Chapter PDFDocument69 pagesIntroduction To Materials Science For Engineers Global Edition 9Th Edition James F Shackelford Full Chapter PDFirenoradiet100% (7)