Professional Documents
Culture Documents
Peri :: : ': - : - ' /., /: Energy Density and Classical Average Energy
Peri :: : ': - : - ' /., /: Energy Density and Classical Average Energy
Uploaded by
Ben WrightOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Peri :: : ': - : - ' /., /: Energy Density and Classical Average Energy
Peri :: : ': - : - ' /., /: Energy Density and Classical Average Energy
Uploaded by
Ben WrightCopyright:
Available Formats
Energy Density and Classical Average Energy
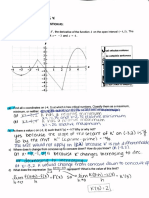
Classically, expectation values of a quantity are found by integrating the quantity times probabJJity
of the quantity ofbeing at that particular value over the entire range of values for that quantity.
You should have seen this in PH235 when finding the average speed of a molecule at a particular
temperature (see Chapter 18 Young and Freedman).
Classically, blackbody radiation was expected to behave the same way. The energy of the radiation
was thought to take on any value and to find the average (or expectation value) of the energy for
the radiation, f, the equation:
f = fef(c)dc
0
was used. Where f (c) was the classical Maxwell-Boltzmann distribution for a simple harmonic
oscillator:
Do the integral (note change variables x= ,/,,r, and use: J0 xe-x dx = 1).
~;tsJ:,d-,. : ~.;r
00
1.
d>
'f =f£ -t((.) d6
Ct>
x~ i4bT
o 6'1. = K,T -0 af_
j
0
(t.io;;) 1<;! & '· 1
/
-1-
()°)(=- i{~d~
2. Subsititu~_µ.(,l)..to_geLdas ical (Rayleigh-Jean) energy density of radiation:
'611' ( 1 61
~\
uea)-: ~ 1
3. Does this look like "experimental" blackbody curve? Where does it fail? What happens to it
~LIJ1)
1'1-i -
a.~,,,,l\ ,I --:v__ -
r '.'."' :_:•,peri~::~:;':•:_ • -' \.,;\
_r~ I ~,(L ,, ,l @ J.,j'~-
1'>' '•'< r= hl,, II"
,.,,,.hl•~ ,v,\ h,\s @
c,,-
S',..-\ .....,blj-1\l\,
SI Pa ge
You might also like
- Weld Consumable CalculatorDocument7 pagesWeld Consumable Calculatorjappozander86% (7)
- Thermo Accela 1250 - Pump - Service Training - ManualDocument90 pagesThermo Accela 1250 - Pump - Service Training - ManualOliver MüllerNo ratings yet
- Sakurai - Modern Quantum Mechanics - Solutions ManualDocument130 pagesSakurai - Modern Quantum Mechanics - Solutions Manualxiaoquanzz100% (1)
- Lug Analysis - MechaniCalcDocument26 pagesLug Analysis - MechaniCalcgbscribd73No ratings yet
- Math Major Exam 2Document5 pagesMath Major Exam 2Kurochan LozanoNo ratings yet
- CtaekrDocument10 pagesCtaekrayanda shwalaNo ratings yet
- Department of Mathematics National Institute of Technology, TiruchirappalliDocument6 pagesDepartment of Mathematics National Institute of Technology, TiruchirappalliprathikNo ratings yet
- Maths Practice 512Document7 pagesMaths Practice 512Vaishakh VarierNo ratings yet
- VibrationDocument7 pagesVibrationMat MatttNo ratings yet
- Adobe Scan 17 Oct 2022Document11 pagesAdobe Scan 17 Oct 2022Sangramjit SinghNo ratings yet
- Short Notes 4Document22 pagesShort Notes 4Ritan DasNo ratings yet
- I) /NC, N)Document1 pageI) /NC, N)rajendrasomkuwar35652No ratings yet
- Acrostic oDocument2 pagesAcrostic oAna Gaby AlvarezNo ratings yet
- Unit 6 PDFDocument16 pagesUnit 6 PDFDiego SerranoNo ratings yet
- Assignment 9 and 10 SolutionDocument22 pagesAssignment 9 and 10 Solutionkrrish ChoudharyNo ratings yet
- Exam 2 SolutionsDocument5 pagesExam 2 SolutionsJoe PiriaNo ratings yet
- Pert I Dak SamaanDocument5 pagesPert I Dak SamaanAllan Pradipta AndriantoNo ratings yet
- Adobe Scan 05 Oct 2023Document4 pagesAdobe Scan 05 Oct 2023neet2024.tdNo ratings yet
- ROBOTICS NotesDocument186 pagesROBOTICS NoteswtkovtuprNo ratings yet
- FourierTransfirm 2Document8 pagesFourierTransfirm 2Dipayan DasNo ratings yet
- FourierTransform 2Document8 pagesFourierTransform 2AnimeNo ratings yet
- Bri'an Barrow wk.6Document4 pagesBri'an Barrow wk.6brieNo ratings yet
- Taller 1 Cesar Gutierrez y Nathaly Marín 07 Sept 2023Document4 pagesTaller 1 Cesar Gutierrez y Nathaly Marín 07 Sept 2023César Augusto Gutierrez JaramilloNo ratings yet
- Quantu1n Optical Technologies: NameDocument13 pagesQuantu1n Optical Technologies: NameGuillermo Prol CasteloNo ratings yet
- Midterm 1 SolDocument3 pagesMidterm 1 Solshahmeer sultanNo ratings yet
- Special Theory of RelativityDocument21 pagesSpecial Theory of Relativityyipabi8864No ratings yet
- Mid 1 SolutionDocument8 pagesMid 1 SolutionARSLANNo ratings yet
- Saksham Dabas 051 PDFDocument5 pagesSaksham Dabas 051 PDFSaksham DabasNo ratings yet
- Cálculo Vectorial.: Instituto Tecnológico de Mérida. Cálculo Vectorial. Eduardo Ismael Canul CanDocument4 pagesCálculo Vectorial.: Instituto Tecnológico de Mérida. Cálculo Vectorial. Eduardo Ismael Canul CanEduarco ismael canul canNo ratings yet
- Tutorial 6 SolutionDocument3 pagesTutorial 6 SolutionSara 123No ratings yet
- JL6CO) ,: (V"lu H IDocument4 pagesJL6CO) ,: (V"lu H Ifw7jvnc8s4No ratings yet
- Unit Test Study Gulie: I Il-/ - ':: JDocument6 pagesUnit Test Study Gulie: I Il-/ - ':: JPaulina TorresNo ratings yet
- HDT CilDocument2 pagesHDT Cilerickdardon02No ratings yet
- MIT8 07F12 ln17Document44 pagesMIT8 07F12 ln17Sanagesi KomaNo ratings yet
- Calculus Practical Sem. 1Document1 pageCalculus Practical Sem. 1Lallu PalNo ratings yet
- Screenshot 2022-02-07 at 11.25.32 PMDocument13 pagesScreenshot 2022-02-07 at 11.25.32 PM020 D.M HASIBUR RAHMAN 18No ratings yet
- SHC Sem2 PDFDocument6 pagesSHC Sem2 PDFnoniNo ratings yet
- Ce102Document4 pagesCe102jaideepjdcNo ratings yet
- Adobe Scan Apr 09, 2023Document5 pagesAdobe Scan Apr 09, 2023Priyanka chitragarNo ratings yet
- Solutions Prep ADocument6 pagesSolutions Prep AWill MillerNo ratings yet
- NA Assignment IIDocument15 pagesNA Assignment IIPrajwal DupareNo ratings yet
- B 1method of Variation of Parameters To Solve ODEDocument19 pagesB 1method of Variation of Parameters To Solve ODEAnus AyanNo ratings yet
- Analysis of Spineless Geared FigjetDocument1 pageAnalysis of Spineless Geared Figjetsanket jadhavNo ratings yet
- Álgebra Lineal 3x3Document5 pagesÁlgebra Lineal 3x3andyNo ratings yet
- Lecture2:3 Calc1Document6 pagesLecture2:3 Calc1LodeNo ratings yet
- Class 11 Physics FormulaDocument4 pagesClass 11 Physics FormulaRahul Prasad KeshriNo ratings yet
- Potential Energy of A SpringDocument3 pagesPotential Energy of A SpringunknownrabulNo ratings yet
- Alcenos AromáticosDocument17 pagesAlcenos AromáticoswwNo ratings yet
- Adobe Scan 08 Oct 2021Document14 pagesAdobe Scan 08 Oct 2021Varatha RajNo ratings yet
- Robin Lewington T4 Trig FunctionsDocument2 pagesRobin Lewington T4 Trig FunctionsRobinNo ratings yet
- M:cl... L1e: JV, Ou1..llDocument7 pagesM:cl... L1e: JV, Ou1..llKrunal PatelNo ratings yet
- Tran Duc Tuong 20191653Document3 pagesTran Duc Tuong 20191653Thinh NgoNo ratings yet
- M1L5 Assign KadenceMariashDocument1 pageM1L5 Assign KadenceMariashkadencemariash404No ratings yet
- 2020 Student Sample 3 KEYDocument1 page2020 Student Sample 3 KEYStephen BailNo ratings yet
- Tarea 3 y 4 Modulo 1Document45 pagesTarea 3 y 4 Modulo 1ELISEO RODRIGUEZ SERRANONo ratings yet
- PF, Vi: Sen, 1eDocument2 pagesPF, Vi: Sen, 1edaljit97No ratings yet
- Ws 4Document3 pagesWs 4teflon.glueNo ratings yet
- DerivativeDocument143 pagesDerivativebbNo ratings yet
- Hardy Cross MethodDocument17 pagesHardy Cross MethodVEYINI RAMAMOORTHYNo ratings yet
- Electro ExamnDocument5 pagesElectro ExamnSusan Solange Puero SorianoNo ratings yet
- Last 2 emDocument9 pagesLast 2 emĐhřüv .ČNo ratings yet
- The Spectral Theory of Toeplitz Operators. (AM-99), Volume 99From EverandThe Spectral Theory of Toeplitz Operators. (AM-99), Volume 99No ratings yet
- Extraction of Keratin Protein From Chicken FeatherDocument10 pagesExtraction of Keratin Protein From Chicken FeatheralexNo ratings yet
- Alfa Laval - Heat Exchanger InstallationDocument66 pagesAlfa Laval - Heat Exchanger InstallationFabricio AlvarezNo ratings yet
- Linear Motion Power Transmission Design Guide 02Document274 pagesLinear Motion Power Transmission Design Guide 02Ravindra KirangeNo ratings yet
- Master SpecificationsDocument395 pagesMaster SpecificationsHaymanot BaynesagnNo ratings yet
- De Moivre's TheoremDocument3 pagesDe Moivre's TheoremDagim AleneNo ratings yet
- Data Sheet: RISH LM 1350 / LM 1360Document10 pagesData Sheet: RISH LM 1350 / LM 1360Erik Marcelo BarrenecheaNo ratings yet
- F Burner Instruction Manual: ModelDocument44 pagesF Burner Instruction Manual: ModelTurgay PolatNo ratings yet
- A MODIFIED PENG ROBINSON EQUATION OF STATE FOR ELV 20519 FTP PDFDocument16 pagesA MODIFIED PENG ROBINSON EQUATION OF STATE FOR ELV 20519 FTP PDFalex rene cardonaNo ratings yet
- Aakash Neet 2017 Code D SolutionDocument32 pagesAakash Neet 2017 Code D SolutionHari Vb100% (1)
- CM-PFS: Three-Phase Monitoring RelaysDocument10 pagesCM-PFS: Three-Phase Monitoring RelaysfirozNo ratings yet
- Chem (Day1) 7am 2019 JambDocument9 pagesChem (Day1) 7am 2019 JambUmar FaruqNo ratings yet
- Module 2 Laplace TransformsDocument36 pagesModule 2 Laplace TransformsErnie Mark Patosa MaratasNo ratings yet
- Ocean Engineering: Yingchun Xie, Jintong Huang, Xiangkun Li, Xiaojie Tian, Guijie Liu, Dingxin LengDocument15 pagesOcean Engineering: Yingchun Xie, Jintong Huang, Xiangkun Li, Xiaojie Tian, Guijie Liu, Dingxin Lengmohamad shahrakNo ratings yet
- Pamantasan NG Lungsod NG ValenzuelaDocument2 pagesPamantasan NG Lungsod NG ValenzuelaDiane de OcampoNo ratings yet
- Component Transformation MatrixDocument11 pagesComponent Transformation MatrixSurya BhartiNo ratings yet
- KSOU Distance Diploma in Mechanical Engineering SyllabusDocument64 pagesKSOU Distance Diploma in Mechanical Engineering SyllabusSunil JhaNo ratings yet
- Lecture 02Document19 pagesLecture 02Sajjad AhmadNo ratings yet
- (35621) F. Cabo 12 Fibras Autosust As120m-12f G652D SMDocument5 pages(35621) F. Cabo 12 Fibras Autosust As120m-12f G652D SMAbelMouraNo ratings yet
- 425ºC AHT: Ower EATDocument2 pages425ºC AHT: Ower EATSivagurunathan SpNo ratings yet
- MaestroDocument32 pagesMaestrodanielhoseaNo ratings yet
- Modules BP 3230Q enDocument2 pagesModules BP 3230Q enJoaquim AlvesNo ratings yet
- Jurnal 4Document11 pagesJurnal 4Raihan Syuhada 1607123510No ratings yet
- Design of Flexible Pavement - Irc MethodDocument9 pagesDesign of Flexible Pavement - Irc MethodOSIRIS EvilNo ratings yet
- QBR Operation ManualDocument8 pagesQBR Operation Manualmahmoudebrahim96745No ratings yet
- Manual de Serviço Dual 1219Document43 pagesManual de Serviço Dual 1219rogerioNo ratings yet
- Silicon Institute of Technology Department of Electrical & Electronics Engineering Quiz Test Electrical Machine-II Lab 5 Sem EEE-B, Gr.-I & IIDocument3 pagesSilicon Institute of Technology Department of Electrical & Electronics Engineering Quiz Test Electrical Machine-II Lab 5 Sem EEE-B, Gr.-I & IIT. VARMANo ratings yet
- CN-DT-006 - ENG - Geocells vs. Mattresses - Rev. 0Document1 pageCN-DT-006 - ENG - Geocells vs. Mattresses - Rev. 0szemianNo ratings yet