Professional Documents
Culture Documents
Immulite 2000 - PM Manual
Immulite 2000 - PM Manual
Uploaded by
роман жуковCopyright:
Available Formats
You might also like
- Mispa Count Service ManualDocument76 pagesMispa Count Service Manuallemanhcuong1503No ratings yet
- Kirschen2000 - The Royal London Space Planning - Part 1Document8 pagesKirschen2000 - The Royal London Space Planning - Part 1drgeorgejose7818100% (2)
- Manual Roller 20 PNDocument33 pagesManual Roller 20 PNSilvio Luiz SalotiNo ratings yet
- RP500 Interfaz LISDocument14 pagesRP500 Interfaz LISJavier Andres leon HigueraNo ratings yet
- VUM108 Abacus Vet5 User's Manual v1.01 PDFDocument135 pagesVUM108 Abacus Vet5 User's Manual v1.01 PDFdmantsioNo ratings yet
- KT-6200 Recommended Spare Parts List V1.2-20190718Document1 pageKT-6200 Recommended Spare Parts List V1.2-20190718Christian PintoNo ratings yet
- Opti Cca ts2 Interface Specifications PDFDocument104 pagesOpti Cca ts2 Interface Specifications PDFnejivan100% (1)
- XS-Series PM Standard Procedure (E)Document10 pagesXS-Series PM Standard Procedure (E)Armando VargasNo ratings yet
- Use of ForceDocument7 pagesUse of ForceNewsChannel 9100% (1)
- XS Series E 03 ElectronicsDocument15 pagesXS Series E 03 Electronicsjocund55No ratings yet
- XS Series E 02 HydraulicsDocument101 pagesXS Series E 02 Hydraulicsjocund55No ratings yet
- XS Series E 04 AdjustmentDocument41 pagesXS Series E 04 Adjustmentjuan miguelNo ratings yet
- XN-L E Chap04 AdjustmentDocument65 pagesXN-L E Chap04 Adjustmentsuraj adhikariNo ratings yet
- Section 1 Specifications: Edition: 175Document40 pagesSection 1 Specifications: Edition: 175Delzi Guindra AdriNo ratings yet
- Service Manual M18 - M18vet-Rev3Document144 pagesService Manual M18 - M18vet-Rev3Ivan ParedesNo ratings yet
- K-Litte5 Service ManualDocument25 pagesK-Litte5 Service ManualJackson Njaramba100% (1)
- Service Manual Update Record MODEL: XS-seriesDocument2 pagesService Manual Update Record MODEL: XS-seriesHuseyn aliyevNo ratings yet
- XS Series E 06 Error MessagesDocument72 pagesXS Series E 06 Error Messagesjocund55No ratings yet
- SUIT Protocol Description v8.0 PDFDocument59 pagesSUIT Protocol Description v8.0 PDFEzzudien Khames HamedNo ratings yet
- XN-L - Series - (MANTENIMIENTO PREVENTIVO)Document8 pagesXN-L - Series - (MANTENIMIENTO PREVENTIVO)Omar GómezNo ratings yet
- Appendix B Installation: XS Series S/M December 13, 2007Document51 pagesAppendix B Installation: XS Series S/M December 13, 2007Huseyn aliyevNo ratings yet
- KT-6200 V2.0 Spare Parts List V5.0eDocument8 pagesKT-6200 V2.0 Spare Parts List V5.0eChristian PintoNo ratings yet
- mek7222血球仪维修手册 PDFDocument185 pagesmek7222血球仪维修手册 PDFSurendra SharmaNo ratings yet
- Mispa Count Plus Service ManualDocument331 pagesMispa Count Plus Service Manuallemanhcuong1503No ratings yet
- Urit 5380 Protocolo BidirecionalDocument5 pagesUrit 5380 Protocolo BidirecionalManuais Interfaceamento 2No ratings yet
- Scil Vet ABC Plus Hematology Analyzer Operations Manual: Scil Animal Care CompanyDocument96 pagesScil Vet ABC Plus Hematology Analyzer Operations Manual: Scil Animal Care CompanyCharity Wesley100% (1)
- MEK-9100 Overview and Operating ProceduresDocument168 pagesMEK-9100 Overview and Operating ProceduresAn VyNo ratings yet
- HydraulicsDocument101 pagesHydraulicsHuseyn aliyevNo ratings yet
- Mek-6400 Parts ListDocument32 pagesMek-6400 Parts ListAsad IqbalNo ratings yet
- Xp100 Vs Micros Es60Document2 pagesXp100 Vs Micros Es60asgbalaji100% (1)
- Service ProgramDocument47 pagesService ProgramHuseyn aliyevNo ratings yet
- Cobas U 411 ST Analyzer: Installation and Training ManualDocument21 pagesCobas U 411 ST Analyzer: Installation and Training ManualFabiolaNo ratings yet
- Astm Protocol: Diagnostica Stago S.A.S - 9, Rue Des Frères Chausson - 92600 Asnières Sur Seine (France)Document48 pagesAstm Protocol: Diagnostica Stago S.A.S - 9, Rue Des Frères Chausson - 92600 Asnières Sur Seine (France)Carlos Alberto Mollinedo GilNo ratings yet
- Ves Matic Cube ASTM Specifications 1 - 1Document10 pagesVes Matic Cube ASTM Specifications 1 - 1Jason HullNo ratings yet
- Mispa I2 Service ManualDocument25 pagesMispa I2 Service Manuallemanhcuong1503No ratings yet
- Internal Technical Note 8.60 20130102 FV - enDocument82 pagesInternal Technical Note 8.60 20130102 FV - enVu Anh100% (1)
- CD 3200 Service Manual PDFDocument334 pagesCD 3200 Service Manual PDFPhan QuanNo ratings yet
- Mek8222 SM-DDocument249 pagesMek8222 SM-Dthanhtu987No ratings yet
- Troobelsooting WideDocument34 pagesTroobelsooting WideEduardo Ricardo SilvaNo ratings yet
- Section 3 Electronics: June 5, 2008Document12 pagesSection 3 Electronics: June 5, 2008Huseyn aliyevNo ratings yet
- Trinity Amelung KC4 - Service ManualDocument34 pagesTrinity Amelung KC4 - Service ManualDenis SinedNo ratings yet
- Mek8222 TRM BDocument50 pagesMek8222 TRM BSergioUrichNo ratings yet
- Bt3000plus Servicemanual Ing PDF Ver (1) .9Document263 pagesBt3000plus Servicemanual Ing PDF Ver (1) .9Alvaro Restrepo GarciaNo ratings yet
- Combostik R 300Document1 pageCombostik R 300Laboratorium Ansari SalehNo ratings yet
- 0614 904173J Mek7300k OmDocument424 pages0614 904173J Mek7300k OmMbonimana GasanaNo ratings yet
- Microlab300 2010 PDFDocument2 pagesMicrolab300 2010 PDFgemra lumban gaol0% (1)
- Counter XS 20 Service Manual (English)Document226 pagesCounter XS 20 Service Manual (English)Josesito SifuentesNo ratings yet
- Insight - X-Series and XN-Series Analyzers - QC Data Instructions Using SNCS - 1575-TS - Rev3Document12 pagesInsight - X-Series and XN-Series Analyzers - QC Data Instructions Using SNCS - 1575-TS - Rev3Julio AtahualpaNo ratings yet
- User Biolyzer 200Document268 pagesUser Biolyzer 200Tran Chi Tien100% (1)
- Parts List (Main)Document97 pagesParts List (Main)rodeoaNo ratings yet
- Sysmex XN 1000 BrochureDocument4 pagesSysmex XN 1000 BrochureJade Kanen100% (1)
- ST Art 4 Reference ManualDocument60 pagesST Art 4 Reference ManualHernan VinetNo ratings yet
- PDFDocument526 pagesPDFjairodiaz88No ratings yet
- Aquila Service Manual VaDocument138 pagesAquila Service Manual VaZakaria ZebbicheNo ratings yet
- Celltac MEK 6500Document3 pagesCelltac MEK 6500RiduanNo ratings yet
- Alcor Carryover Intersample TestDocument9 pagesAlcor Carryover Intersample TestAmine CHAHIDNo ratings yet
- Celldyn Emerald-Interface E22ALDocument36 pagesCelldyn Emerald-Interface E22ALgustavodlr100% (1)
- IQ200 Family Protocol 300-4941JBDocument90 pagesIQ200 Family Protocol 300-4941JBADI SUSENO100% (1)
- Abx Pentra400 ManualDocument24 pagesAbx Pentra400 ManualNurNo ratings yet
- BC5000¡ BC5150 Service Training Material1.0Document180 pagesBC5000¡ BC5150 Service Training Material1.0Jaime EspinosaNo ratings yet
- Dynajet 350 - MG - PlusDocument71 pagesDynajet 350 - MG - PlusJose Maria Castro PazosNo ratings yet
- POLYDOROS R and F Family, POLYDOROS R and F Family CSTD AXD3-500.805.01 AX65-120.842.01Document34 pagesPOLYDOROS R and F Family, POLYDOROS R and F Family CSTD AXD3-500.805.01 AX65-120.842.01Izzeldin ZakiNo ratings yet
- Siemens Advia 2400 Operator's GuideDocument200 pagesSiemens Advia 2400 Operator's Guideроман жуковNo ratings yet
- Instruction Manual For Table Top Refrigerated Centrifuge Z 513 KDocument34 pagesInstruction Manual For Table Top Refrigerated Centrifuge Z 513 Kроман жуковNo ratings yet
- Heraeus Megafuge 16 Heraeus Megafuge 16R: Instruction ManualDocument56 pagesHeraeus Megafuge 16 Heraeus Megafuge 16R: Instruction Manualроман жуковNo ratings yet
- инструкция на аллегра PDFDocument64 pagesинструкция на аллегра PDFроман жуковNo ratings yet
- Jacob Wolfe - Native American Land Use WebquestDocument3 pagesJacob Wolfe - Native American Land Use Webquestapi-550219419No ratings yet
- Organic Chemistry: WorksheetDocument50 pagesOrganic Chemistry: Worksheetshahed khayyatNo ratings yet
- 3300 Xl-8mm-Proximity-transducer-system Datasheet 141194 Cda 000 0Document35 pages3300 Xl-8mm-Proximity-transducer-system Datasheet 141194 Cda 000 0Jaime Andres Quijano CossioNo ratings yet
- Neck ExercisesDocument8 pagesNeck ExercisesAnthony Dinicolantonio100% (1)
- CoverDocument177 pagesCoverBernard OwusuNo ratings yet
- VIVA - TarrifDocument1 pageVIVA - TarrifSrinivas VadtheNo ratings yet
- Kemasan HNA NO Produk SyrupDocument2 pagesKemasan HNA NO Produk SyruprianNo ratings yet
- HIRA - COVID 19 Prevention and ControlsDocument17 pagesHIRA - COVID 19 Prevention and ControlsGyanendra Narayan NayakNo ratings yet
- Q.1 Write Short Answers of The Following Questions: Ghazali Guess Chemistry 1 10thDocument9 pagesQ.1 Write Short Answers of The Following Questions: Ghazali Guess Chemistry 1 10thAwais AliNo ratings yet
- Quality Management Principles For Excellence: by - Sacchidanand Gogawale, Zen International SystemsDocument5 pagesQuality Management Principles For Excellence: by - Sacchidanand Gogawale, Zen International SystemsSanjeevani GogawaleNo ratings yet
- Hydraulic Stacker - Model No. Mn397: Operating Instructions and Parts ListDocument9 pagesHydraulic Stacker - Model No. Mn397: Operating Instructions and Parts ListAJ MusicNo ratings yet
- The Importance of Shapes Fitting Together in Cells and OrganismsDocument4 pagesThe Importance of Shapes Fitting Together in Cells and Organisms17athermonNo ratings yet
- General Description: Plug Fan ER..C With Standard MotorDocument7 pagesGeneral Description: Plug Fan ER..C With Standard MotorwildanNo ratings yet
- Transposition: Shubhangi ShuklaDocument24 pagesTransposition: Shubhangi ShuklaShubhangi ShuklaNo ratings yet
- Sexuality Research in India An UpdateDocument5 pagesSexuality Research in India An UpdatekavalapparaNo ratings yet
- SDS Biplas PL 4 MMDocument3 pagesSDS Biplas PL 4 MMAkhilNo ratings yet
- New and Future Developments in Sustainable Agriculture Advances in Microbe Based Biostimulants Harikesh Bahadur Singh Download PDF ChapterDocument52 pagesNew and Future Developments in Sustainable Agriculture Advances in Microbe Based Biostimulants Harikesh Bahadur Singh Download PDF Chaptersamantha.lara160100% (6)
- The Role of Public Health Leadership (2) FinalDocument20 pagesThe Role of Public Health Leadership (2) FinalKing Mugitah18No ratings yet
- Algae Final InteractiveDocument8 pagesAlgae Final InteractivesuziethefatcatNo ratings yet
- Homeopathic Remedies For 'Flu - Flow ChartDocument1 pageHomeopathic Remedies For 'Flu - Flow Chartisadore97% (32)
- Swift 2018Document33 pagesSwift 2018Sam Steven Hernandez JañaNo ratings yet
- Router7031 NS-01-002 CNV AllAccessGuideDocument20 pagesRouter7031 NS-01-002 CNV AllAccessGuideAHMAD FUADNo ratings yet
- 10-12si Brush Holder ReplacementDocument1 page10-12si Brush Holder Replacementrogerc1894No ratings yet
- Quiz Chapter 2 3Document25 pagesQuiz Chapter 2 3THỊNH DƯƠNG GIANo ratings yet
- Elements, Compounds and Mixtures: Gen. ScienceDocument31 pagesElements, Compounds and Mixtures: Gen. ScienceMadhavi KapadiaNo ratings yet
- Technical Data Sheet: Hanson Construction AggregatesDocument9 pagesTechnical Data Sheet: Hanson Construction AggregatesShaiful ZamriNo ratings yet
- 1 Human Reaction On Different WordsDocument13 pages1 Human Reaction On Different Wordsshamim islam limonNo ratings yet
- Abdomen SEQDocument9 pagesAbdomen SEQcataztropher100% (1)
Immulite 2000 - PM Manual
Immulite 2000 - PM Manual
Uploaded by
роман жуковCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Immulite 2000 - PM Manual
Immulite 2000 - PM Manual
Uploaded by
роман жуковCopyright:
Available Formats
IMMULITE 2000/2000 XPi
Maintenance Instructions
System
Preventive Maintenance Instructions
The protocol DCIM-B02.832.01.08.XX is required for these instructions
2 Document Version / Disclaimer / Copyright
Document Version
Siemens Healthcare GmbH reserves the right to change its products and services at any
time.
In addition, manuals are subject to change without notice. The hardcopy documents cor‐
respond to the version at the time of system delivery and/or printout. Versions to hard‐
copy documentation are not automatically distributed.
Please contact your local Siemens Healthineers office to order a current version or refer to
our website http://www.siemens-healthineers.com.
Disclaimer
Siemens Healthcare GmbH provides this documentation “as is” without the assumption of
any liability under any theory of law.
The content described herein shall be used by qualified personnel who are employed by
Siemens Healthcare GmbH or one of its affiliates or who are otherwise authorized by Sie‐
mens Healthcare GmbH or its affiliates to use such documents.
Assemblers and other persons who are not employed by or otherwise directly affiliated
with or authorized by Siemens Healthcare GmbH or one of its affiliates are not entitled to
use this documentation without prior written authority.
Copyright
“© Siemens Healthineers, 2020” refers to the copyright of a Siemens Healthineers entity
such as:
Siemens Healthcare GmbH - Germany
Siemens Shenzhen Magnetic Resonance Ltd. - China
Siemens Shanghai Medical Equipment Ltd. - China
Siemens Healthcare Private Ltd. - India
Siemens Medical Solutions USA Inc. - USA
Siemens Healthcare Diagnostics Inc. - USA
Siemens Healthcare Diagnostics Products GmbH - Germany
Corindus Inc. - USA
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 2 of 34 © Siemens Healthineers, 2020
12.20 Restricted
Table of Contents 3
1 Overview 4
1.1 General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
1.2 Tools and Materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
2 PM Checklist 6
2.1 Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
2.2 Power Supply Voltages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
2.2.1 Checking the Voltages at the DC Distribution PCB . . . . . . . . . . . . 8
2.2.2 Checking the Voltage at the Sample Carousel PCB . . . . . . . . . . . . 8
2.2.3 Checking the Voltages at the PMT Power Supply . . . . . . . . . . . . . 8
2.3 Dual Resolution Diluter (DRD) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
2.3.1 Replacing the DRD Seals and Springs . . . . . . . . . . . . . . . . . . . . . . 9
2.3.2 Lubricating the DRD. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
2.4 Dual Pipettor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
2.4.1 Checking the Sample and Reagent Pipettor Probes . . . . . . . . . . . 11
2.4.2 Verifying Tube Bottom Position of Sample Arm Z . . . . . . . . . . . . . 11
2.5 Bead Carousel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
2.6 Tube Hopper Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
2.7 Reagent Carousel. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
2.8 Tube Processor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
2.9 Sample Carousel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
2.10 Solenoid and Linear Actuator Pumps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
2.10.1 Performing a Volume Check for Solenoid and Linear Actuator 19
Pumps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
2.10.2 Adjusting the Drawback of the Substrate and Water Pumps . . . . 19
2.11 Fluidics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
2.11.1 Replacing Filters and Tubing . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
2.11.2 Replacing the Vacuum Pump Trap . . . . . . . . . . . . . . . . . . . . . . . . 20
2.11.3 Verifying the Vacuum Pump Function . . . . . . . . . . . . . . . . . . . . . 21
2.11.4 Turning on Clot Detection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
2.11.5 Checking the Transducer Waveform . . . . . . . . . . . . . . . . . . . . . . . 22
2.12 Water Test PM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
2.13 Computer Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
2.13.1 General Software Check and Cleanup . . . . . . . . . . . . . . . . . . . . . . 27
2.13.2 Verifying Mirror Mode Functionality . . . . . . . . . . . . . . . . . . . . . . . 27
2.13.3 Scanning the Control-Side Hard Drive for Problems . . . . . . . . . . . 28
2.13.4 Defragmenting the User-Side Hard Drive . . . . . . . . . . . . . . . . . . . 29
2.14 System Temperatures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
2.15 Final Check . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
3 Changes to Previous Version 32
3.1 Version 08 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
4 List of Hazard IDs 33
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 3 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
4 1 Overview
1.1 General Information
This document describes the procedures to perform preventive maintenance on the IM‐
MULITE 2000/2000 XPi system. All procedures reflect the preventative maintenance proc‐
ess for both systems. In cases where a procedure differs for the system, the differences
are noted within the text of the document.
Preventive maintenance is performed on IMMULITE 2000/2000 XPi systems once annual‐
ly or after every 100,000 tests in high-volume laboratories, whichever represents the
shorter time period.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 4 of 34 © Siemens Healthineers, 2020
12.20 Restricted
Overview 1 5
1.2 Tools and Materials
Make sure you have the following tools and materials available when visiting a customer’s
site for a routine maintenance call.
n Preventive Maintenance (PM) Kit
n Fluke 75III multimeter or equivalent
n Adjustable pipette (10–1000 µL) Eppendorf or equivalent
n IMMULITE 2000/2000 XPi reaction tubes
n 13 x 100 mm sample tubes
n 12 x 75 mm sample tubes
n Rubbing alcohol and cotton swabs or lint-free cloth
n Gloves
n DRD Lubrication Kit (10291661)
n Forceps
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 5 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
6 2 PM Checklist
2.1 Preparation
WARNING
The instrument could be contaminated with human body fluids.
Wear personal protective equipment and use universal precautions.
1. Enter the Detailed View of the Event Log and look for any unusual activity. If there are
any recurring errors, closely inspect the affected module.
2. Discuss the instrument’s past performance with the customer.
3. Verify that the UPS sustains power when it is unplugged:
a) Remove the UPS power plug from the wall outlet.
b) Confirm that the backup battery light comes on and the IMMULITE 2000/2000 XPi
keeps running.
c) Plug the UPS back into the wall outlet.
CAUTION
Performing preventive maintenance while the user computer is logged in to Win‐
dows may damage the operating system and the IMMULITE software.
To prevent any software damage, make sure that Windows XP or Windows NT dis‐
plays the login screen.
Restart Windows, but do not log in.
4. With the system power off, make sure power connections are secure and free of de‐
fects and that there are no objects on or around the uninterruptible power supply
(UPS).
5. Visually inspect all areas of the system for any signs of potential problems.
- Inspect the pipetting area for any signs of fluidic leaks.
- Inspect the tube feeder area for any signs of dropped beads or dropped tubes
around the hopper.
6. Remove and clean the main fan filter.
- The main fan filter is located on the right side of the system next to the electronic
chassis.
- Clean the filter by holding it under running water and rubbing gently to remove
any dust.
- Make sure the filter is dry before reinstalling it.
7. Remove the dilution well insert and install the replacement included in the PM kit.
Make sure the insert is securely seated in the brass fitting by pressing down on it.
The dilution well sample pipettor positions must be verified using the procedures in the
Alignments section ( Alignments / DCIM-B02.842.01).
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 6 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 7
8. Clean the trackball with rubbing alcohol using cotton swabs or a lint-free cloth, and
make sure the area where the trackball sits is clean and free from debris.
Refer to the figure below for the areas on the Kensington and Logitech trackballs that
need to be cleaned.
Fig. 1: Trackball Cleaning Points
PM Discuss past performance with customer.
PM Check detailed event log.
PM Make necessary upgrades.
PM Check that the UPS sustains power when unplugged.
PM Clean the main fan filter.
PM Replace dilution well insert.
PM Verify the dilution well bottom position configuration.
PM Clean the trackball.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 7 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
8 2 PM Checklist
2.2 Power Supply Voltages
1. Restart the user-side computer and verify that Windows displays the login screen.
2. Remove the gold protective casing covering the power supply area.
2.2.1 Checking the Voltages at the DC Distribution PCB
PM Check the 24 VDC voltage at test points 1 and 2. The acceptable range is 23.76
to 24.24.
PM Check the 24 VAC voltage at connector J12. The acceptable range is ~28.
PM Check the 12 VDC voltage at test points 5 and 6. The acceptable range is 11.88
to 12.12.
PM Check the –12 VDC voltage at test points 7and 6. The acceptable range is –11.88
to –12.12.
2.2.2 Checking the Voltage at the Sample Carousel PCB
PM Check the 5 VDC voltage at connector J2, pins 1 and 2. The acceptable range is
5.00 to 5.20.
2.2.3 Checking the Voltages at the PMT Power Supply
PM Board SMN 10484630: Check the voltage at connectors J1, J3, J5A/B and pin 1
(–5 VDC). The acceptable range is 4.85 to 5.15.
PM Board SMN 10484630: Check the voltage at connectors J1, J3, J5A/B and pin 2
(Ground). The acceptable range is 4.85 to 5.15.
PM Board SMN 10484630: Check the voltage at connectors J1, J3, J5A/B and pin 3
(+5 VDC). The acceptable range is 4.85 to 5.15.
PM Board SMN 10389935: Check the voltage at connectors J2, J3, and J4 and pin 1
(-5 VDC). The acceptable range is 5.00 to 5.20.
PM Board SMN 10389935: Check the voltage at connectors J2, J3, and J4 and pin 2
(Ground). The acceptable range is 5.00 to 5.20.
PM Board SMN 10389935: Check the voltage at connectors J2, J3, and J4 and pin 3
(+5 VDC). The acceptable range is 5.00 to 5.20.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 8 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 9
2.3 Dual Resolution Diluter (DRD)
CAUTION
Contamination may occur when the DRD seals are being replaced.
Wear gloves to avoid contaminating the diluter assembly.
2.3.1 Replacing the DRD Seals and Springs
1. Log in to the system as FSE to gain access to the service level of the Diagnostics pro‐
gram:
a) At the Windows Desktop, double-click the Diagnostics icon.
b) In the Diagnostics Main Menu window, click Special Testing.
c) Enter the password 1ML2000 in the text field and press Enter.
2. Move the diluter to the bottom position.
3. Loosen the upper piston housing by turning it clockwise. If the housing is the alumi‐
num type, first loosen the housing lock nut.
4. Remove the two shoulder screws.
5. Gently remove the black "top hat" and the clear acrylic block.
6. Unscrew the upper piston housing from the black top piston support. Remove and dis‐
card the spring, and then replace the spring with the new one provided.
7. Carefully remove the upper seal and the lower seal.
8. Replace the seals with the new ones provided.
If you cannot determine the difference visually, use a micrometer to measure the size
of each seal. The figure below shows the inner and outer diameters in inches.
Fig. 2: Diluter Seal Measurements (in Inches)
CAUTION
Improper seal installation may cause leaking and poor priming.
When you insert a seal into its cavity in the DRD acrylic block, it should make a
snapping sound to indicate that it is seated correctly.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 9 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
10 2 PM Checklist
9. Hand-tighten the upper piston housing nut by turning it clockwise.
If the housing is aluminum, make sure the locking nut is tightened toward the black
piston support at the top to prevent leaks, which may cause poor priming.
PM Replace DRD seals.
PM Replace DRD springs.
2.3.2 Lubricating the DRD
This procedure requires the DRD Lubrication Kit.
1. Move the DRD to the bottom position.
2. Using the syringe, slowly inject 250 μL of oil into the lubrication access hole.
Fig. 3: DRD
(1) Lubrication Access Hole
Verify the positions using the procedures in ( Alignments / DCIM-B02.842.01).
PM Verify sample and reagent DRD positions.
PM Lubricate DRD (sample & reagent).
PM Verify DRD Delron is tightened to the left.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 10 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 11
2.4 Dual Pipettor
2.4.1 Checking the Sample and Reagent Pipettor Probes
Replace the probes if the dispense angle is greater than 3.5 degrees. To verify the dis‐
pense angle, run the following tests in the Diagnostics program:
n Sample Probe Dispense Angle
n Reagent Probe Dispense Angle
PM Check sample and reagent pipettor positions.
PM Verify sample and reagent probe angle dispense.
PM IMMULITE 2000 systems, serial numbers below E0920 only: Clean and lubri‐
cate the sample and reagent pipettor lead screw assemblies with a Teflon-based
lubricant.
Lubrication is not required for IMMULITE 2000 systems, serial number E0920 and
above and IMMULITE 2000 XPi systems.
2.4.2 Verifying Tube Bottom Position of Sample Arm Z
1. Insert a 12 x 75 mm tube containing 75 μL of diluent or control sample into position
A1.
2. Move the sample carousel to the A1@Pipettor position.
3. Make sure the sample arm and sample carousel are properly homed.
4. Move the sample arm to the outer sample position in the X direction.
The sample arm should be centered directly above position A1.
5. Begin moving the sample pipettor arm down until the level-sense LED flickers.
6. Compare the saved hexadecimal value with the value in the encoder position box.
The values should be within six steps of each other. If this is not true, repeat these
steps, making sure that the liquid was pipetted correctly and that the sample pipettor
arm is properly homed.
PM Verify "Tube Bottom" position.
PM Check pipettor level sense.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 11 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
12 2 PM Checklist
2.5 Bead Carousel
Verify the positions using the procedures in ( Alignments / DCIM-B02.842.01).
PM Check bead pack carousel positions.
PM Check bead dispense positions.
PM Check bead pack barcode reader positions.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 12 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 13
2.6 Tube Hopper Assembly
1. Run the Diagnostics program Sensor Test in condensed mode to check the operation
of sensors for the queue, bead, upper hopper, and lower hopper.
2. Follow the on-screen instructions, making sure the sensor changes from green to red,
until the program finishes.
3. Slide reaction cups down the queue to check for smooth movement to the tube index‐
er.
Verify the positions using the procedures in ( Alignments / DCIM-B02.842.01).
PM Check operation of tube full & low queue sensor.
PM Check operation of upper & lower hopper sensor.
PM Check tube in place indexer sensor.
PM Check tube indexer positions.
PM Check queue for a smooth reaction tube movement.
PM Check operation of bead sensors.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 13 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
14 2 PM Checklist
2.7 Reagent Carousel
Verify positions using the procedures in the Alignments section ( Align‐
ments / DCIM-B02.842.01).
Fig. 4: Reagent Carousel Positions
(1) Inner
(2) Middle
(3) Outer
PM Check reagent carousel positions.
PMA Check reagent barcode reader alignment.
PM Check pack lid opener positions.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 14 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 15
2.8 Tube Processor
1. Remove the top cover of the luminometer.
2. Remove the lubricated detent gasket and discard.
3. Install the new lubricated detent gasket provided.
Make sure that the gasket is seated correctly. If it is not, luminometer chain or high dark
count errors may occur.
4. Replace the top cover of the luminometer.
5. Remove the wash station and transfer cover plate.
6. Remove the waste manifold insert.
7. Install the new waste manifold insert provided.
8. Clean the wash station assembly, including the spindle gear and sump, but do not re‐
install it.
9. Verify the tube lifter positions and spring compression.
10. Verify that tube lifter releases reaction cups smoothly.
11. Check the Geneva drive functionality by homing the Geneva indexer four or five times
to make sure that there is no backlash of the transfer baffles when they start moving.
If there is, replace the Geneva drive assembly as soon as possible.
12. Verify that the incubator chain is aligned correctly.
Verify the positions using the procedures in the Alignments section ( Align‐
ments / DCIM-B02.842.01).
13. Remove and replace the solid waste exit chute provided in the kit.
a) Disconnect the waste chute sensor from J22 on the dual pipette PCB.
b) Remove all hardware from the waste chute assembly, and discard the original
waste chute.
c) Install the new waste chute.
d) Once the assembly is in place, adjust the chute so that the reaction cups fall
smoothly into the solid waste container.
If the assembly is not adjusted correctly, the used reaction cups may bounce off the
metal bracket and not fall into the solid waste container.
e) Reconnect the sensor to J22 on the dual pipette PCB and verify that the tube drop
sensor is operating correctly by running Tube Chute Test 2000 in the Diagnostics
program.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 15 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
16 2 PM Checklist
PM Replace detent disk gasket.
PM Replace solid waste exit chute.
PM Check operation of tube drop sensor.
PM Clean tube drop section of tube processor and replace waste manifold insert.
PM Check tube lifter tension spring compression (approximately 1/8 in.).
PM Check for bent transfer chain baffles.
PMA Verify incubator chain alignment.
PM Verify functionality of Geneva.
PM Check release of reaction cup from tube lifter.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 16 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 17
2.9 Sample Carousel
1. Clean the inside of the sample carousel to improve contact with the grounding brush.
Make sure the grounding brush makes contact with the sample carousel.
2. Remove the sample carousel ground brush and replace it with the one provided in the
kit.
3. As the sample carousel rotates, measure the resistance between any of the screws
holding the black sample rack guide blocks and ground. The reading should be less
than 6 ohms.
4. Check the bar code scan rate. The readings will be different depending whether the
instrument uses a Microscan or JADAK scanner.
a) In Windows Explorer, go to the C:\DPC\C\ folder.
b) Double-click Filetransfer.exe.
c) Click Control Utilities.
d) Click Bar Code Scanner, then click Bar Code Scan Rate.
The sample bar code scanner starts, and the Scan Rate window opens.
e) Verify the scan rate for the following: sample rack IDs, the reagent carousel posi‐
tions, and the inner and outer sample carousel positions. (You will need to insert a
bar-coded tube in the sample carousel to verify the sample carousel positions.)
Microscan Scanner: For each position the bar code reader should be 300–500 de‐
codes per second.
JADAK Scanner: For each position the bar code reader should be 20–200 millisec‐
onds for the time to read (TTR).
If the customer uses its own printed labels, make sure they fall within the appropriate
ranges.
Verify the positions using the procedures in ( Alignments / DCIM-B02.842.01).
5. Replace the foam roller.
a) If necessary, remove the sample racks to facilitate the replacement of the foam
roller
b) Find the brown V-roller that is a few inches from the dilution well assembly.
c) Remove the foam roller from the foam roller bushing.
d) Gently install the new foam roller onto the foam roller bushing.
e) If necessary, reinstall the sample racks.
f) Rotate the sample carousel to ensure proper operation.
Verify the positions using the procedures in ( Alignments / DCIM-B02.842.01).
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 17 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
18 2 PM Checklist
PM Replace sample carousel ground brush.
PM Check carousel positions.
PM Check tube in place sensors.
PM Check the scan rate for the Microscan scanner. The expected scan rate is 300 to
500 decodes per second.
PM Check the scan rate for the JADAK scanner. The expected scan rate is 20 to 200
milliseconds for the TTR.
PM Replace the foam roller.
PM IMMULITE 2000 XPi only: Check rack loader sensors and operation.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 18 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 19
2.10 Solenoid and Linear Actuator Pumps
2.10.1 Performing a Volume Check for Solenoid and Linear Actuator Pumps
To verify the dispense volume of the substrate or water pump, you’ll need a beaker and
several reaction cups. They should be set up so that they are in easy reach.
n The substrate pump should dispense a consistent 200 μL (±10 μL).
n The water pump should dispense a consistent 400 μL (±20 μL).
1. Click Run IMMULITE, and allow the initialization to finish.
2. Remove the probe you are checking and begin dispensing into the beaker by pressing
the green prime button and holding it for four full primes.
3. Keeping the green button pressed through the fourth prime, and then dispense liquid
into several reaction cups.
If you stop pressing the green prime button before this step is completed, the dispensed
volumes may be inconsistent.
4. Stop pressing the green button, and return the probe to its holder.
5. Measure the volume of each reaction cup to make sure that the dispensed volumes
are consistent. If they are not, replace the pump.
PM Check volume dispense of substrate (200 μL) and water (400 μL) pumps.
2.10.2 Adjusting the Drawback of the Substrate and Water Pumps
1. For each pump, unscrew the tubing from the associated probe and verify that the air
slug at the end of the tubing is 1/4 inch, ±1/8 inch.
2. Hold the tubing over a beaker, and then press and hold the green prime button.
3. After the fourth prime, continue pressing the green prime button, and verify the
drawback.
4. If necessary, use the potentiometers on top of the digital fluidics PCB to adjust the sol‐
enoid pump drawback:
- Adjust RP3 for the substrate pump drawback.
- Adjust RP4 for the water pump drawback.
5. If necessary, use the sixth and seventh switches on the bank of switches on the bot‐
tom of this pump to control the drawback.
- Turn each switch to the down position to increase drawback.
- Turn each switch to the up position to decrease the drawback.
PM Check that drawback of pumps is 1/4 in. (± 1/8 in.).
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 19 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
20 2 PM Checklist
2.11 Fluidics
2.11.1 Replacing Filters and Tubing
1. Remove and discard the in-line filters for the water and probe wash bottles.
2. Install the in-line filters provided, making sure that the arrow on the filter point in the
direction that the liquid flows.
3. Remove and discard the liquid waste drain tubing and the waste bottle valve, and
then install the replacement parts provided.
4. Lubricate the O-ring using a silicon-based lubricant to ensure a good seal with the liq‐
uid waste bottle cap.
5. Remove and discard the dilution well drain tubing, drain elbow, and clamps, and then
install the replacement parts provided.
6. Inspect all tubing, and replace any tubing that is discolored or has occlusions or kinks.
PM Replace water and probe wash in-line filters.
PM Replace waste bottle tubing assembly.
PM Lubricate waste bottle valve O-ring.
PM Replace dilution well drain tubing and elbow fitting.
PM Check all tubes for kinks and tighten all fittings.
PM Check all tubing within component deck for discoloration and possible occlusion.
Replace if necessary.
2.11.2 Replacing the Vacuum Pump Trap
1. Remove the existing liquid waste tubing from the reagent wash well drain, the wash
sump drain, the KNF vacuum pump inlet, and the waste manifold assembly for the re‐
agent wash well connection only.
2. Loosen the nut on the component deck standoff support.
3. Install the trap assembly and tighten the nut to secure the trap bracket.
4. Connect the trap tubing to the reagent wash well drain, the wash sump drain, the
drain manifold assembly and reagent wash well connection, and to the KNF vacuum
pump inlet. Refer to the figure below.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 20 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 21
Fig. 5: Connecting the Trap Tubing
(1) Connect to Wash Sump Drain
(2) Connect to Vacuum Pump Inlet
(3) Connect to Waste Manifold
(4) Connect to Reagent Wash
PM Replace waste trap kit.
PM Check wash sump drain for leaks.
2.11.3 Verifying the Vacuum Pump Function
1. Open the left door and unlatch the panel to open the component deck.
2. In the Diagnostics program, click Vacuum Pump Check - 2000.
3. Use a set of forceps to gently pinch off the 1/2-inch tubing from the lower cavity of
the waste trap to the waste manifold.
4. Carefully pour 10 mL of water into the reagent probe wash well.
5. Click Run in the Diagnostics menu, which starts the vacuum pump. Watch carefully to
make sure that all the fluid is evacuated through the 1/4-inch tube in less than five
seconds.
6. Click Press to Turn Pump Off and exit the Diagnostics program.
PM Check operation of vacuum pump and waste tubing angle.
2.11.4 Turning on Clot Detection
1. In Windows Explorer, go to the C:\DPC\C\ folder.
2. Double-click Filetransfer.exe.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 21 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
22 2 PM Checklist
3. Click Control Utilities.
4. Click Control Utilities > Clot Detection > Clot Detection ON.
PM Confirm clot detection is ON.
2.11.5 Checking the Transducer Waveform
1. In the Diagnostics program, run Clot Prime and Prime DRD #2 to prime the transduc‐
er and the system to make sure they are well primed.
2. Place a sample tube with at least 250 μL of diluent or control material in position A1.
3. Click Clot Analyze on the right side of the screen.
4. Click Clot Test #1.
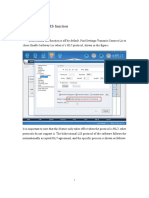
When the program starts, a white screen displays options on the top and bottom. At
the bottom left, in area labeled Acquire there are three color options.
5. Click Red.
The system then goes through the program and aspirates a sample of the material.
See ( Fig. 6 Page 22) for an example of an acquired waveform. During this process,
the system displays information about the memory, as shown in ( Fig. 7 Page 23).
If it displays all zeros, then there is an electrical problem.
Fig. 6: Acquired Waveform (Red)
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 22 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 23
Fig. 7: Acquired Memory Display
6. To finish the test, in the area where the word Load is displayed, click Blue from the
three color options, and then click No Clot. The system displays a saved blue wave‐
form, as shown in ( Fig. 8 Page 23).
Fig. 8: No Clot Waveform (Blue)
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 23 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
24 2 PM Checklist
7. Compare your acquired waveform with the blue waveform, which is represents a per‐
fect “no clot.” If the waveform is comparable, the transducer is performing as expec‐
ted.
For contrast, see ( Fig. 9 Page 24), which shows a waveform that represents a clot.
Fig. 9: Clot Waveform (Green)
8. If the transducer has been well primed and the waveform is not comparable, run
Transducer Decon in the Diagnostics program, and then repeat these steps.
If the waveform is still not comparable, the sample probe or transducer may need to
be replaced.
PM Check the transducer waveform.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 24 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 25
2.12 Water Test PM
Run the WatertestPM - 2000 or WatertestPM - 2000XPi diagnostic program to establish
the current condition of the system’s water supply.
The WatertestPM diagnostic tests for alkaline phosphatase contamination in the water.
1. If necessary, initialize diagnostics.
2. If necessary, prime the substrate probe.
3. Select Load Program to load the list of diagnostics.
4. In the Diagnostics Program window:
- For the IMMULITE 2000, select WatertestPM – 2000.
- For the IMMULITE 2000 XPi, select WatertestPM – 2000XPi.
5. Select Run.
The instrument automatically homes all motors.
6. When prompted, place a clean, empty 12 x 75 mm tube in position 1 of a sample rack.
7. Place the sample rack in position 1 of the sample carousel.
8. After the tube is loaded on the instrument, select Load tube and press to continue.
9. When prompted, remove the water probe from the wash station.
10. Place the water probe from the previous step into the tube.
11. Select Place Water Probe in tube 1-1.
12. Select Press to dispense from the Water Probe.
The message “Collecting Water from the Water Probe” is displayed.
If the water probe touches the water inside the sample tube, wipe the water probe with
a lint-free cloth before returning it to the wash station.
13. When prompted, remove the Water Probe from the tube and return it to the Wash Sta‐
tion.
14. Select Replace Water Probe.
The instrument primes the sample and reagent probes, pipettes water, transfers the
reaction tubes to the luminometer, and dispenses substrate. The photomultiplier tube
(PMT) then reads the reaction tubes. After the instrument reads the tubes, the mes‐
sage “Program Complete” is displayed and the instrument generates a report of the re‐
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 25 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
26 2 PM Checklist
sults. The figure below shows an example blank result report for a water test PM on
an IMMULITE 2000 or IMMULITE 2000 XPi system.
Tab. 1 Blank Watertest PM Result Report for the IMMULITE 2000 / 2000 XPi Systems
IMMULITE 2000 (XPi)
WATER TEST PM
mm-dd-yy hh:mm:ss
Serial Number: annnn
Operator: _______________________
NOTE: All results have been multiplied by the PMT factor.
Sample Probe CPS: xxx.x – xxx.x (Substrate Only) = xxx.x
Reagent Probe CPS: xxx.x – xxx.x (Substrate Only) = xxx.x
Water Probe CPS: xxx.x - xxx.x (Substrate Only) = xxx.x
Sample Probe CPS: xxx.x
Reagent Probe CPS: xxx.x
Water Probe CPS: xxx.x
Substrate Only CPS: xxx.x
15. Evaluate the results. If the Open CPS measurement of any reaction tube is > 1100 CPS,
the instrument may be contaminated.
In this case, consider performing a decontamination procedure, followed by a repeti‐
tion of the WatertestPM 2000 or WatertestPM 2000XPi procedure.
16. To stop running diagnostics, select Exit, then select Quit.
PM Confirm that the Watertest PM – 2000 or the Watertest PM – 2000XPi returns an
Open CPS measurement for each reaction tube of less than 1100 CPS.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 26 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 27
2.13 Computer Software
2.13.1 General Software Check and Cleanup
1. Verify that the system is running the correct version of the IMMULITE operational soft‐
ware.
2. Make sure that the CPU fans are operating on both the user- and control-side comput‐
ers.
3. Verify that the CD-ROM, DVD-ROM, and floppy drives are working.
4. Verify that the printer is working.
5. For IMMULITE 2000 systems running Windows NT only: Go to C:\Temp and delete all
files except those with the current date.
6. For all systems, go to C:\IMMULITE 2000\Databases and make sure that there are no
unnecessary copies of any system files, including zipped files and old copies of any da‐
tabase.
7. Verify that the databases have been backed up within the last 24 hours. The following
files should be present in the C:\Backup folder:
- Maindata.mdb
- Motorfig.mdb
- Errorlog.mdb
PM Check software version.
PM Delete unnecessary files using Windows Explorer.
PM Verify backup of database in last 24 hours.
PM IMMULITE 2000: Confirm both user-side and control-side CPU fans are spinning.
PM IMMULITE 2000 XPi: Confirm user-side CPU fan is spinning.
PM Verify functionality of floppy drive and CD-ROM drive.
2.13.2 Verifying Mirror Mode Functionality
Verify that the mirroring device is functioning correctly. The procedure varies depending
whether the system is equipped with a DupliDisk, promise card, or RadiSys RAID. Each
procedure is described in the following sections.
2.13.2.1 DupliDisk
Make sure that the three LEDs on the front of the DupliDisk are green. See the figure be‐
low, which shows the LEDs not illuminated. When the DupliDisk is saving data the status
LED is amber.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 27 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
28 2 PM Checklist
Fig. 10: DupliDisk (With LEDs Not Illuminated)
2.13.2.2 Promise Card
1. Restart the system and watch the messages displayed during the boot sequence.
Make sure that the following message appears: “Array is successful.”
2. Alternatively, you can enter the promise card setup by typing Ctrl+F when you are
prompted during the boot sequence.
2.13.2.3 RadiSys RAID
To verify RadiSys RAID functionality, you will need to view the Intel Matrix Storage Con‐
sole, as follows:
1. At the Windows desktop, right-click Start and then click Explore. Navigate to the
C:\Program\intel folder.
2. Double-click shell.exe.
The Intel Matrix Storage Console opens.
3. Verify that both hard drives (shown as Port 0 and Port 1) are green. If not, replace the
defective drive.
Refer to ( Computer and Accessories Replacement / DCIM-B02.841.03).
2.13.3 Scanning the Control-Side Hard Drive for Problems
This procedure does not apply to systems that use an IDE flash drive.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 28 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 29
1. Disconnect the keyboard and monitor cable from the user-side computer and recon‐
nect them to the control-side computer.
The monitor should display DOS software.
2. Press Esc.
3. At the prompt type C:\Bin, and press Enter.
4. Type scandisk and press Enter.
5. Click Yes to run a complete scan.
The scan could take 30 minutes or more. If scandisk stops running, or if the results of
the scan indicate that there are several bad sectors, the hard drive should be replaced.
6. When the scan is complete, exit scandisk.
7. Press Ctrl+Alt+Del to restart the control-side computer.
8. Disconnect the keyboard and monitor cable from the control-side computer and re‐
connect them to the user-side computer.
9. Close the Run IMMULITE window, and then double-click the Run IMMULITE icon on
the desktop to reestablish communication between the control-side and user-side
computers.
PM Perform scan disk on control-side hard drive.
2.13.4 Defragmenting the User-Side Hard Drive
Over time, the user-side hard drive may become fragmented, reducing the speed by
which it accesses data. Periodic defragmentation may help the hard drive to operate more
efficiently.
Computers running Windows NT cannot be defragmented. This procedures applies only
to systems running Windows XP.
1. On the Windows XP desktop, double-click the My Computer icon.
A Windows Explorer window opens.
2. Right-click the icon for the C drive and click Properties in the pop-up menu.
The System Properties window opens.
3. Click the Tools tab and click Defragment Now.
The Disk Defragmenter window opens.
4. Click Defragment, and allow the defragmentation to finish. This process could take
several minutes to an hour or more, depending on the extent of the disk fragmenta‐
tion.
PM Perform disk defragmenter.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 29 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
30 2 PM Checklist
2.14 System Temperatures
1. In the Run IMMULITE software, click Menu and then Temperatures to open the View
Temperatures window.
2. Verify that the temperatures are in range.
3. Record the temperatures.
PM Check reagent carousel temperature. Record temperature in degrees Celsius.
PM Check incubator temperature. Record temperature in degrees Celsius.
PM Check luminometer temperature. Record temperature in degrees Celsius.
PM Check ambient temperature. Record temperature in degrees Celsius.
PM Check substrate heater temperature. Record temperature in degrees Celsius.
PM Check bead chamber humidity. Record humidity as a percentage.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 30 of 34 © Siemens Healthineers, 2020
12.20 Restricted
PM Checklist 2 31
2.15 Final Check
1. As a final check of the integrity of the IMMULITE 2000/2000 XPi system, run available
assays at the site. If necessary, adjust each assay before running controls.
2. Run the customer calibrators and controls to verify system performance.
At a minimum, run a set of customer controls on at least one assay.
3. Attach the assay results to the PM protocol.
4. Double-click the Maintenance PM Tracker icon on the desktop, or go to C:\DPC\Util‐
ties and double-click PM Tracker.exe.
5. Click PM HAS BEEN PERFORMED.
The PM Tracker window opens.
Fig. 11: PM Tracker Window
6. In the Initials field, type your initials, and in the Password field type PMTracker.
7. Click OK.
The PM Message dialog box opens.
8. Click OK.
The current date is displayed in the Date of Last PM field and the current test count
resets to 0.
9. Click Exit to close the PM Tracker and return to the desktop.
PM Run one set of customer controls on one assay to verify performance.
PM Reset the PM Tracker test count information.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 31 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
32 3 Changes to Previous Version
3.1 Version 08
Section Change Description Change Num‐
ber
( Sample Car‐ Added steps to replace the foam CHG0035595
ousel / Page 17) roller. and
LFT0014886
( Replacing the Added check point to “check LFT0015216
Vacuum Pump wash sump drain for leaks”.
Trap / Page 20)
( Final Revised step 2 and associated LFT0013579
Check / Page 31) check point to “Run one set of
customer controls on one assay
to verify performance”.
IMMULITE 2000/2000 XPi DCIM-B02.831.01.08.02 Page 32 of 34 © Siemens Healthineers, 2020
12.20 Restricted
List of Hazard IDs 4 33
There are no Hazard IDs in this document.
© Siemens Healthineers, 2020 DCIM-B02.831.01.08.02 Page 33 of 34 IMMULITE 2000/2000 XPi
Restricted 12.20
- Restricted - All documents may
only be used by authorized person‐
nel for rendering services on Sie‐
mens Healthcare Products. Any
document in electronic form may
be printed once. Copy and distribu‐
tion of electronic documents and
hardcopies is prohibited. Offenders
will be liable for damages. All other
rights are reserved.
siemens-healthineers.com
Siemens Healthineers Headquarters
Siemens Healthcare GmbH
Henkestr. 127
91052 Erlangen
Germany
Telephone: +49 9131 84-0
siemens-healthineers.com
Print No.: DCIM-B02.831.01.08.02 | Replaces: DCIM-B02.831.01.07.02
Doc. Gen. Date: 12.20 | Language: English
© Siemens Healthineers, 2020
You might also like
- Mispa Count Service ManualDocument76 pagesMispa Count Service Manuallemanhcuong1503No ratings yet
- Kirschen2000 - The Royal London Space Planning - Part 1Document8 pagesKirschen2000 - The Royal London Space Planning - Part 1drgeorgejose7818100% (2)
- Manual Roller 20 PNDocument33 pagesManual Roller 20 PNSilvio Luiz SalotiNo ratings yet
- RP500 Interfaz LISDocument14 pagesRP500 Interfaz LISJavier Andres leon HigueraNo ratings yet
- VUM108 Abacus Vet5 User's Manual v1.01 PDFDocument135 pagesVUM108 Abacus Vet5 User's Manual v1.01 PDFdmantsioNo ratings yet
- KT-6200 Recommended Spare Parts List V1.2-20190718Document1 pageKT-6200 Recommended Spare Parts List V1.2-20190718Christian PintoNo ratings yet
- Opti Cca ts2 Interface Specifications PDFDocument104 pagesOpti Cca ts2 Interface Specifications PDFnejivan100% (1)
- XS-Series PM Standard Procedure (E)Document10 pagesXS-Series PM Standard Procedure (E)Armando VargasNo ratings yet
- Use of ForceDocument7 pagesUse of ForceNewsChannel 9100% (1)
- XS Series E 03 ElectronicsDocument15 pagesXS Series E 03 Electronicsjocund55No ratings yet
- XS Series E 02 HydraulicsDocument101 pagesXS Series E 02 Hydraulicsjocund55No ratings yet
- XS Series E 04 AdjustmentDocument41 pagesXS Series E 04 Adjustmentjuan miguelNo ratings yet
- XN-L E Chap04 AdjustmentDocument65 pagesXN-L E Chap04 Adjustmentsuraj adhikariNo ratings yet
- Section 1 Specifications: Edition: 175Document40 pagesSection 1 Specifications: Edition: 175Delzi Guindra AdriNo ratings yet
- Service Manual M18 - M18vet-Rev3Document144 pagesService Manual M18 - M18vet-Rev3Ivan ParedesNo ratings yet
- K-Litte5 Service ManualDocument25 pagesK-Litte5 Service ManualJackson Njaramba100% (1)
- Service Manual Update Record MODEL: XS-seriesDocument2 pagesService Manual Update Record MODEL: XS-seriesHuseyn aliyevNo ratings yet
- XS Series E 06 Error MessagesDocument72 pagesXS Series E 06 Error Messagesjocund55No ratings yet
- SUIT Protocol Description v8.0 PDFDocument59 pagesSUIT Protocol Description v8.0 PDFEzzudien Khames HamedNo ratings yet
- XN-L - Series - (MANTENIMIENTO PREVENTIVO)Document8 pagesXN-L - Series - (MANTENIMIENTO PREVENTIVO)Omar GómezNo ratings yet
- Appendix B Installation: XS Series S/M December 13, 2007Document51 pagesAppendix B Installation: XS Series S/M December 13, 2007Huseyn aliyevNo ratings yet
- KT-6200 V2.0 Spare Parts List V5.0eDocument8 pagesKT-6200 V2.0 Spare Parts List V5.0eChristian PintoNo ratings yet
- mek7222血球仪维修手册 PDFDocument185 pagesmek7222血球仪维修手册 PDFSurendra SharmaNo ratings yet
- Mispa Count Plus Service ManualDocument331 pagesMispa Count Plus Service Manuallemanhcuong1503No ratings yet
- Urit 5380 Protocolo BidirecionalDocument5 pagesUrit 5380 Protocolo BidirecionalManuais Interfaceamento 2No ratings yet
- Scil Vet ABC Plus Hematology Analyzer Operations Manual: Scil Animal Care CompanyDocument96 pagesScil Vet ABC Plus Hematology Analyzer Operations Manual: Scil Animal Care CompanyCharity Wesley100% (1)
- MEK-9100 Overview and Operating ProceduresDocument168 pagesMEK-9100 Overview and Operating ProceduresAn VyNo ratings yet
- HydraulicsDocument101 pagesHydraulicsHuseyn aliyevNo ratings yet
- Mek-6400 Parts ListDocument32 pagesMek-6400 Parts ListAsad IqbalNo ratings yet
- Xp100 Vs Micros Es60Document2 pagesXp100 Vs Micros Es60asgbalaji100% (1)
- Service ProgramDocument47 pagesService ProgramHuseyn aliyevNo ratings yet
- Cobas U 411 ST Analyzer: Installation and Training ManualDocument21 pagesCobas U 411 ST Analyzer: Installation and Training ManualFabiolaNo ratings yet
- Astm Protocol: Diagnostica Stago S.A.S - 9, Rue Des Frères Chausson - 92600 Asnières Sur Seine (France)Document48 pagesAstm Protocol: Diagnostica Stago S.A.S - 9, Rue Des Frères Chausson - 92600 Asnières Sur Seine (France)Carlos Alberto Mollinedo GilNo ratings yet
- Ves Matic Cube ASTM Specifications 1 - 1Document10 pagesVes Matic Cube ASTM Specifications 1 - 1Jason HullNo ratings yet
- Mispa I2 Service ManualDocument25 pagesMispa I2 Service Manuallemanhcuong1503No ratings yet
- Internal Technical Note 8.60 20130102 FV - enDocument82 pagesInternal Technical Note 8.60 20130102 FV - enVu Anh100% (1)
- CD 3200 Service Manual PDFDocument334 pagesCD 3200 Service Manual PDFPhan QuanNo ratings yet
- Mek8222 SM-DDocument249 pagesMek8222 SM-Dthanhtu987No ratings yet
- Troobelsooting WideDocument34 pagesTroobelsooting WideEduardo Ricardo SilvaNo ratings yet
- Section 3 Electronics: June 5, 2008Document12 pagesSection 3 Electronics: June 5, 2008Huseyn aliyevNo ratings yet
- Trinity Amelung KC4 - Service ManualDocument34 pagesTrinity Amelung KC4 - Service ManualDenis SinedNo ratings yet
- Mek8222 TRM BDocument50 pagesMek8222 TRM BSergioUrichNo ratings yet
- Bt3000plus Servicemanual Ing PDF Ver (1) .9Document263 pagesBt3000plus Servicemanual Ing PDF Ver (1) .9Alvaro Restrepo GarciaNo ratings yet
- Combostik R 300Document1 pageCombostik R 300Laboratorium Ansari SalehNo ratings yet
- 0614 904173J Mek7300k OmDocument424 pages0614 904173J Mek7300k OmMbonimana GasanaNo ratings yet
- Microlab300 2010 PDFDocument2 pagesMicrolab300 2010 PDFgemra lumban gaol0% (1)
- Counter XS 20 Service Manual (English)Document226 pagesCounter XS 20 Service Manual (English)Josesito SifuentesNo ratings yet
- Insight - X-Series and XN-Series Analyzers - QC Data Instructions Using SNCS - 1575-TS - Rev3Document12 pagesInsight - X-Series and XN-Series Analyzers - QC Data Instructions Using SNCS - 1575-TS - Rev3Julio AtahualpaNo ratings yet
- User Biolyzer 200Document268 pagesUser Biolyzer 200Tran Chi Tien100% (1)
- Parts List (Main)Document97 pagesParts List (Main)rodeoaNo ratings yet
- Sysmex XN 1000 BrochureDocument4 pagesSysmex XN 1000 BrochureJade Kanen100% (1)
- ST Art 4 Reference ManualDocument60 pagesST Art 4 Reference ManualHernan VinetNo ratings yet
- PDFDocument526 pagesPDFjairodiaz88No ratings yet
- Aquila Service Manual VaDocument138 pagesAquila Service Manual VaZakaria ZebbicheNo ratings yet
- Celltac MEK 6500Document3 pagesCelltac MEK 6500RiduanNo ratings yet
- Alcor Carryover Intersample TestDocument9 pagesAlcor Carryover Intersample TestAmine CHAHIDNo ratings yet
- Celldyn Emerald-Interface E22ALDocument36 pagesCelldyn Emerald-Interface E22ALgustavodlr100% (1)
- IQ200 Family Protocol 300-4941JBDocument90 pagesIQ200 Family Protocol 300-4941JBADI SUSENO100% (1)
- Abx Pentra400 ManualDocument24 pagesAbx Pentra400 ManualNurNo ratings yet
- BC5000¡ BC5150 Service Training Material1.0Document180 pagesBC5000¡ BC5150 Service Training Material1.0Jaime EspinosaNo ratings yet
- Dynajet 350 - MG - PlusDocument71 pagesDynajet 350 - MG - PlusJose Maria Castro PazosNo ratings yet
- POLYDOROS R and F Family, POLYDOROS R and F Family CSTD AXD3-500.805.01 AX65-120.842.01Document34 pagesPOLYDOROS R and F Family, POLYDOROS R and F Family CSTD AXD3-500.805.01 AX65-120.842.01Izzeldin ZakiNo ratings yet
- Siemens Advia 2400 Operator's GuideDocument200 pagesSiemens Advia 2400 Operator's Guideроман жуковNo ratings yet
- Instruction Manual For Table Top Refrigerated Centrifuge Z 513 KDocument34 pagesInstruction Manual For Table Top Refrigerated Centrifuge Z 513 Kроман жуковNo ratings yet
- Heraeus Megafuge 16 Heraeus Megafuge 16R: Instruction ManualDocument56 pagesHeraeus Megafuge 16 Heraeus Megafuge 16R: Instruction Manualроман жуковNo ratings yet
- инструкция на аллегра PDFDocument64 pagesинструкция на аллегра PDFроман жуковNo ratings yet
- Jacob Wolfe - Native American Land Use WebquestDocument3 pagesJacob Wolfe - Native American Land Use Webquestapi-550219419No ratings yet
- Organic Chemistry: WorksheetDocument50 pagesOrganic Chemistry: Worksheetshahed khayyatNo ratings yet
- 3300 Xl-8mm-Proximity-transducer-system Datasheet 141194 Cda 000 0Document35 pages3300 Xl-8mm-Proximity-transducer-system Datasheet 141194 Cda 000 0Jaime Andres Quijano CossioNo ratings yet
- Neck ExercisesDocument8 pagesNeck ExercisesAnthony Dinicolantonio100% (1)
- CoverDocument177 pagesCoverBernard OwusuNo ratings yet
- VIVA - TarrifDocument1 pageVIVA - TarrifSrinivas VadtheNo ratings yet
- Kemasan HNA NO Produk SyrupDocument2 pagesKemasan HNA NO Produk SyruprianNo ratings yet
- HIRA - COVID 19 Prevention and ControlsDocument17 pagesHIRA - COVID 19 Prevention and ControlsGyanendra Narayan NayakNo ratings yet
- Q.1 Write Short Answers of The Following Questions: Ghazali Guess Chemistry 1 10thDocument9 pagesQ.1 Write Short Answers of The Following Questions: Ghazali Guess Chemistry 1 10thAwais AliNo ratings yet
- Quality Management Principles For Excellence: by - Sacchidanand Gogawale, Zen International SystemsDocument5 pagesQuality Management Principles For Excellence: by - Sacchidanand Gogawale, Zen International SystemsSanjeevani GogawaleNo ratings yet
- Hydraulic Stacker - Model No. Mn397: Operating Instructions and Parts ListDocument9 pagesHydraulic Stacker - Model No. Mn397: Operating Instructions and Parts ListAJ MusicNo ratings yet
- The Importance of Shapes Fitting Together in Cells and OrganismsDocument4 pagesThe Importance of Shapes Fitting Together in Cells and Organisms17athermonNo ratings yet
- General Description: Plug Fan ER..C With Standard MotorDocument7 pagesGeneral Description: Plug Fan ER..C With Standard MotorwildanNo ratings yet
- Transposition: Shubhangi ShuklaDocument24 pagesTransposition: Shubhangi ShuklaShubhangi ShuklaNo ratings yet
- Sexuality Research in India An UpdateDocument5 pagesSexuality Research in India An UpdatekavalapparaNo ratings yet
- SDS Biplas PL 4 MMDocument3 pagesSDS Biplas PL 4 MMAkhilNo ratings yet
- New and Future Developments in Sustainable Agriculture Advances in Microbe Based Biostimulants Harikesh Bahadur Singh Download PDF ChapterDocument52 pagesNew and Future Developments in Sustainable Agriculture Advances in Microbe Based Biostimulants Harikesh Bahadur Singh Download PDF Chaptersamantha.lara160100% (6)
- The Role of Public Health Leadership (2) FinalDocument20 pagesThe Role of Public Health Leadership (2) FinalKing Mugitah18No ratings yet
- Algae Final InteractiveDocument8 pagesAlgae Final InteractivesuziethefatcatNo ratings yet
- Homeopathic Remedies For 'Flu - Flow ChartDocument1 pageHomeopathic Remedies For 'Flu - Flow Chartisadore97% (32)
- Swift 2018Document33 pagesSwift 2018Sam Steven Hernandez JañaNo ratings yet
- Router7031 NS-01-002 CNV AllAccessGuideDocument20 pagesRouter7031 NS-01-002 CNV AllAccessGuideAHMAD FUADNo ratings yet
- 10-12si Brush Holder ReplacementDocument1 page10-12si Brush Holder Replacementrogerc1894No ratings yet
- Quiz Chapter 2 3Document25 pagesQuiz Chapter 2 3THỊNH DƯƠNG GIANo ratings yet
- Elements, Compounds and Mixtures: Gen. ScienceDocument31 pagesElements, Compounds and Mixtures: Gen. ScienceMadhavi KapadiaNo ratings yet
- Technical Data Sheet: Hanson Construction AggregatesDocument9 pagesTechnical Data Sheet: Hanson Construction AggregatesShaiful ZamriNo ratings yet
- 1 Human Reaction On Different WordsDocument13 pages1 Human Reaction On Different Wordsshamim islam limonNo ratings yet
- Abdomen SEQDocument9 pagesAbdomen SEQcataztropher100% (1)