Professional Documents
Culture Documents
MEM Project Diploma
MEM Project Diploma
Uploaded by
Joshua Kondkar0 ratings0% found this document useful (0 votes)
87 views13 pagesThis document is a certificate from Devi Mahalaxmi Polytechnic College in Titwala for a student project on preparing a chart of the iron carbide equilibrium diagram. It provides details of two students who completed the project for their Mechanical Engineering Materials course, and certifies that a faculty member guided and approved their work. It also contains an introduction and explanation of the iron-carbon phase diagram, including the different phases such as austenite, ferrite, cementite, and ledeburite that make up the diagram.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document is a certificate from Devi Mahalaxmi Polytechnic College in Titwala for a student project on preparing a chart of the iron carbide equilibrium diagram. It provides details of two students who completed the project for their Mechanical Engineering Materials course, and certifies that a faculty member guided and approved their work. It also contains an introduction and explanation of the iron-carbon phase diagram, including the different phases such as austenite, ferrite, cementite, and ledeburite that make up the diagram.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
87 views13 pagesMEM Project Diploma
MEM Project Diploma
Uploaded by
Joshua KondkarThis document is a certificate from Devi Mahalaxmi Polytechnic College in Titwala for a student project on preparing a chart of the iron carbide equilibrium diagram. It provides details of two students who completed the project for their Mechanical Engineering Materials course, and certifies that a faculty member guided and approved their work. It also contains an introduction and explanation of the iron-carbon phase diagram, including the different phases such as austenite, ferrite, cementite, and ledeburite that make up the diagram.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 13
Devi Mahalaxmi Polytechnic College , Titwala
Departments of Mechanical Engineering
Academic Year :2020-21
Course : ME-3-I Subject & Code : MEM(22343)
Project Tittle : Prepare a Chart of Iron carbide equilibrium diagram.
Project Group :
Roll No Enrollment No Name of Student
2016800086 Hiren Rajesh Kondkar.
2016800085 Nitin Sopan wavhal.
Subject Teacher H.O.D Principal
Devi Mahalaxmi Polytechnic,Titwala
Certificate
Certified that this Report submitted by,
Mr/Ms :- Hiren Rajesh Kondkar Enroll No:- 2016800086
Mr/Ms :- Nitin Sopan Wavhal Enroll No:- 2016800085
Seat no : 291091 of Mechanical Engineering course 3rd /2nd Sem/Year
as a part of PROJECT WORK as prescribed by the Maharashtra Board of Technical
Education ,Mumbai for Subject MECHANICAL ENGINEERING MATERIALS.
And that , I have Guided him for the said work from time to time and I found him to be
satisfactorily progressive during the Academic Year 2020-2021.
And that the said work has been assessed by me and I am satisfied that the same is up to the
standard envisaged for the level of course.
Date : 09/030/2021
Name & signature of Name and signature of Principal
Internal Examiner H.O.D/ External Examiner
MECHANICAL ENGINEERING MATERIALS
Topic :- Prepare a chart of Iron Carbide Equilibrium
Diagram .
INTRODUCTION :-
The Iron-Iron Carbide Equilibrium Diagram
Iron is a substance of allotropy. Alloy components, the
most significant of which is carbon, influence the
temperature at which the allotropic modifications occur
in iron. In this paper is provided information about the
part of the iron"carbon alloy scheme that is of concern to
technicians.
The Iron-Iron Carbide Diagram
The iron-carbon composite scheme diagram portion
between plain iron and an interstitial compound, iron
carbide (Fe3C), comprising 6.67 percent by weight is
called iron-iron carbide balance diagram. It may be
observed that although it is called as a diagram of
equilibrium, it is not a real diagram of equilibrium, as
equilibrium does not imply a shift of stage with moment.
The iron carbide compound actually breaks down into
metal and coal (graphite). This decomposition takes a
long time to form graphite at room temperature, even at
1300Â ° F. The metastable stage is called the iron
carbide. Thus, although the iron-iron carbide diagram
technically reflects metastable circumstances, under
comparatively gentle heating and cooling circumstances
it can be regarded as depicting alterations in equilibrium.
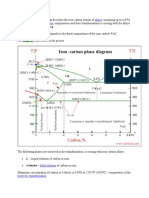
The figure above demonstrates an iron-iron carbide
balance diagram marked with Greek letters in particular
to depict the strong alternatives. However, giving unique
names to most of the constructions appearing on the
diagram is prevalent practice. The strong solution from
Î3 is called austenite. The diagram displays three
horizontal lines indicating isothermal responses.
The extended perspective of the diagram part in the
bottom left corner of the diagram is shown in the
following picture.
Due to the solid solution of the Î, this part of the diagram
is regarded as delta region. A peritectic reaction is the
horizontal line at 2720Â ° F. The peritectic response
equation can be published as
At point M, the maximum carbon solubility in b.c.c. Î ' Fe is
0.10%. Carbon presence influences the allotropic shift
between Î ' and Î3. As the carbon concentration in iron
increases, the allotropic change temperature increases at 0.1
percent carbon from 2554 to 2720Â ° F.
On cooling, the NM line represents the start of the change in
crystal structure from b.c.c. Î' Fe to f.c.c. Î3 Fe for metals
comprising less than 0.1% water. By means of a peritectic
response for alloys between 0.10 and 0.18 percent carbon,
the MP part of row MPB reflects the start of this crystal
structure shift. On drying, the start of the glass shape shift is
provided by the row NP for plastics comprising less than
0.18% oxygen. The PB part reflects the start and end of the
shift in the crystal structure through the peritectic response.
That is, the allotropic shift starts and finishes at steady
temperature for alloys between 0.18 and 0.50 percent
carbon. Any metal comprising more than 0.5% oxygen can be
seen to trim the diagram to the left of point B and solidify
straight to austenite. It will totally bypass the delta solid
solution and the allotropic shift.
The following picture indicates the equilibrium diagram of
iron-iron carbide marked with the popular names for the
constructions.
It can be seen that at 2065Â ° F there is an eutectic
response. The eutectic point E is 4.3% biomass and the
eutectic wind range CED. Whenever this row is passed
by an alloy, there must be an eutectic response. Any
liquid present at the time of reaching this line must now
solidify into the very good blend of the two stages at
either end of the horizontal line, namely austenite and
metal carbide (called cement). This eutectic mixture is
called ledeburite and can be described as a response
As shown in the figure, each alloy will consist of a
mixture of ferrite and cementite below the eutectoid
temperature line HJK.
Depending on the carbon content, dividing the iron-iron
carbide diagram into two components is prevalent
practice. Alloys that contain less than 2% of carbon are
known as steels and metals that contain more than 2%
of carbon are regarded as cast iron. The variety of metal
is further reduced by the quantity of eutectoid carbon
(0.8% C). Steels comprising less than 0.8% C are called
hypoeutectoid steels, while steels comprising between
0.8% and 2.0% C are called hypereutectoid steels.
Eutectic carbon content (4.3 percent C) also subdivides
the cast iron variety. Cast iron comprising less than
4.3% C is known as hypoeutectic cast iron and those
comprising more than 4.3% C are regarded as
hypereutectic cast iron.
Micro-constituents/Structures
Names are given to various micro-constituents
for descriptive or commemorative reason.
Information about them is given below.
Cementite or metal carbide
The formation of cementite requires a set quantity of coal
and a set quantity of metal. Fe3C is his chemical formula. It
includes by weight 6.67 percent of coal. It is a compound of
difficult and brittle interstitial poor tensile strength (about
5000 psi) but elevated compressive strength. Its
orthorhombic crystal structure. It is the easiest building on
the carbide diagram of iron-iron.
Austenite
The name provided to the strong solution Î3 is Austenite. It is
an interstitial strong carbon solution deposited in Î3 metal
with a face-centered cubic framework (f.c.c.) of crystal.
Maximum solubility at 2065Â ° F (point C) is 2% nitrogen.
Average properties of austenite are as under.
Tensile strength: 150,000 psi
Elongation: 10 % in 2 in. gage length
Hardness: Rockwell C 40
Toughness: High
Austenite at room temperature is usually volatile. Austenite
can be obtained at room temperature under certain
circumstances (as in stainless steels from austenite).
Austenite has no magnetic character.
Ledeburite
It is the eutectic mix of austenite and cement. It has a carbon
content of 4.3% and is formed at 2065Â ° F (point E). It occurs
when the fuel content exceeds 2%, which constitutes the
separating line between steel and cast iron on the balance
diagram.
Ferrite
The name provided to the α strong solution is ferrite. It is an
interstitial solid solution of a small amount of carbon
dissolved in α iron with a crystal structure centered on the
body (b.c.c.). The maximum solubility at 1333Â ° F (point H) is
0.025% oxygen, and at room temperature it dissolves only
0.008% oxygen. It is the weakest iron-iron carbide diagram
framework.
Average properties of ferrite are as under.
Tensile Strength: 40,000 psi
Elongation: 40 % in 2 in. gage length
Hardness: Less than Rockwell C 0 or less than Rockwell B 90
Toughness: Low.
The iron-carbon diagram. It should first be pointed out
that the normal equilibrium diagram really represents
the metastable equilibrium between iron and iron
carbide (cementite). Cementite is metastable, and the
true equilibrium should be between iron and graphite.
CONCLUSION :-
A study of the constitution and structure of all steels and
irons must first start with the iron-carbon equilibrium
diagram. Many of the basic features of this system
influence the behavior of even the most complex alloy
steels.
From all the above theory we should understand the chart
of iron carbide equilibrium diagram.
THANK YOU
You might also like
- Sae J1268-1995Document96 pagesSae J1268-1995Jader Pitangueira100% (6)
- What Pipeliners Need To Know About Induction Bends PDFDocument20 pagesWhat Pipeliners Need To Know About Induction Bends PDFmarcos2dami2o2de2azeNo ratings yet
- Capili Jefferson 10Document5 pagesCapili Jefferson 10Christian Al EncarnacionNo ratings yet
- Iron-Carbon Phase Diagram Explained BrieflyDocument4 pagesIron-Carbon Phase Diagram Explained BrieflyZicoNo ratings yet
- Study of Iron-Iron Carbide and Isothermal TransformationDocument14 pagesStudy of Iron-Iron Carbide and Isothermal TransformationAhmed JishanNo ratings yet
- MSM GTU Study Material E-Notes Unit-5 23112020052908AMDocument14 pagesMSM GTU Study Material E-Notes Unit-5 23112020052908AMVijayNo ratings yet
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 pagesUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongNo ratings yet
- Iron-Iron Carbide DiagramDocument10 pagesIron-Iron Carbide DiagrammusabNo ratings yet
- Lecture 1 - Smart Materials - IntroductionDocument8 pagesLecture 1 - Smart Materials - IntroductionOmar Adel MohammedNo ratings yet
- Composition of Plain Carbon Steel Carbon Steel or Plain-Carbon Steel, Is A Metal Alloy. It Is A Combination ofDocument14 pagesComposition of Plain Carbon Steel Carbon Steel or Plain-Carbon Steel, Is A Metal Alloy. It Is A Combination ofkayodeNo ratings yet
- Weldability of Metals - NPTELDocument18 pagesWeldability of Metals - NPTELKaushal Gandhi0% (1)
- Iron Carbon Phase Diagram TEDDocument6 pagesIron Carbon Phase Diagram TEDAnonymous dh6DITNo ratings yet
- Handout Chapter 5 Iron Carbon SystemDocument7 pagesHandout Chapter 5 Iron Carbon SystemBikram MuduliNo ratings yet
- Iron Carbon Note 1 2023Document23 pagesIron Carbon Note 1 2023gerrard samuelNo ratings yet
- Engineering Metallurgy: Misan University-College of EngineeringDocument27 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manNo ratings yet
- Iron Carbon DiagramDocument10 pagesIron Carbon DiagramsivakumarNo ratings yet
- Section 2: The Microstructural Nature of Carbon SteelsDocument4 pagesSection 2: The Microstructural Nature of Carbon Steelsamitkharb111195No ratings yet
- Iron Carbon Equilibrium DiagramDocument11 pagesIron Carbon Equilibrium Diagramganesh82No ratings yet
- Iron Carbon Equillibrium Diagram GandhidhamDocument22 pagesIron Carbon Equillibrium Diagram Gandhidhamcal2_uniNo ratings yet
- Steels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceDocument39 pagesSteels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceKareem YasserNo ratings yet
- Iron - Iron Carbide Iron Iron Carbide Phase Diagram: Today's TopicsDocument11 pagesIron - Iron Carbide Iron Iron Carbide Phase Diagram: Today's TopicsembargioNo ratings yet
- 06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramDocument42 pages06-Iron (Fe) - Iron Carbide (Fe3C) Phase DiagramTalaat Ahmed Mohamed El-Benawy100% (2)
- The Iron-Iron Carbide Equilibrium DiagramDocument15 pagesThe Iron-Iron Carbide Equilibrium DiagramjhangeerNo ratings yet
- Callister7E - pp290 301 (The Iron Carbon System)Document12 pagesCallister7E - pp290 301 (The Iron Carbon System)iglumacNo ratings yet
- Ch-27.3 Iron Carbon Equilibrium DiagramDocument58 pagesCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfNo ratings yet
- Iron Carbon Diagram (ChE Handbook)Document21 pagesIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- Diagram FasaDocument6 pagesDiagram Fasaolid_zoneNo ratings yet
- The IronCarbide DiagramDocument11 pagesThe IronCarbide DiagramshajjikhalidNo ratings yet
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument52 pagesCh-27.5 Iron Carbon Equilibrium DiagramManojNo ratings yet
- AceroDocument11 pagesAceroCristian FloresNo ratings yet
- Hardening From The Liquid StateDocument5 pagesHardening From The Liquid StateSinhrooNo ratings yet
- Universiti Teknologi Mara Lab 2Document9 pagesUniversiti Teknologi Mara Lab 2Ilman FaiqNo ratings yet
- Phase Diagrams and AlloysDocument4 pagesPhase Diagrams and AlloysAkhilesh KumarNo ratings yet
- Iron Carbon Phase DiagramDocument7 pagesIron Carbon Phase Diagrampratap biswasNo ratings yet
- Chapter 2 - TTA - TTT - DiagramsDocument13 pagesChapter 2 - TTA - TTT - DiagramsPrasad Mhatre100% (1)
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramnaveedNo ratings yet
- 211005IJMetcastCE CastIronFINALDocument27 pages211005IJMetcastCE CastIronFINALLucas CunhaNo ratings yet
- Dokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dDocument16 pagesDokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dAfrizal Adithya PNo ratings yet
- Fec DiagramDocument15 pagesFec DiagramShankarNo ratings yet
- Iron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingDocument7 pagesIron-Iron Carbide (Fe-Fe3C) Phase Diagram: M. Tech. (FFT) Technology of Ferrous CastingRajulapati Sunil KumarNo ratings yet
- SteelDocument4 pagesSteelgovimanoNo ratings yet
- InTech-Chemical and Physical Properties of Fluxes For Saw of Low Carbon SteelsDocument19 pagesInTech-Chemical and Physical Properties of Fluxes For Saw of Low Carbon SteelsSiap SiapNo ratings yet
- Iron Carbon Part1 PDFDocument33 pagesIron Carbon Part1 PDFErick HoganNo ratings yet
- Iron Carbon DiagramDocument9 pagesIron Carbon DiagramNagamuthu PandianNo ratings yet
- 3 Iron Carbon DiaDocument21 pages3 Iron Carbon DiaChhavi SharmaNo ratings yet
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument39 pagesFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualcacoonnymphaea6wgyct100% (17)
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument79 pagesFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualdextrermachete4amgqgNo ratings yet
- Structure of Plain SteelDocument4 pagesStructure of Plain Steelsatish_trivediNo ratings yet
- Industrial Materials: Instructed By: Dr. Sajid ZaidiDocument13 pagesIndustrial Materials: Instructed By: Dr. Sajid ZaidiarulmuruguNo ratings yet
- Fe CDocument34 pagesFe CZaza ArifinNo ratings yet
- Iron-IronCarbide Phase DiagramDocument3 pagesIron-IronCarbide Phase Diagramumangmodi32No ratings yet
- Iron Carbon Phase DiagramDocument3 pagesIron Carbon Phase DiagramrabikmNo ratings yet
- Eutectoid Hypoeutectoid Hypereutectoid Ferrite Austenite Delta IronDocument2 pagesEutectoid Hypoeutectoid Hypereutectoid Ferrite Austenite Delta IronAlex LopezNo ratings yet
- 18bem0046 Materials Da 2Document20 pages18bem0046 Materials Da 2QUANTUM SOLEOSNo ratings yet
- Metallurgy of Carbon SteelDocument5 pagesMetallurgy of Carbon SteelJayeshNo ratings yet
- Iron-Carbon Phase Diagram: 1-Peritectic Reaction: Occur at Temperature 1493Document5 pagesIron-Carbon Phase Diagram: 1-Peritectic Reaction: Occur at Temperature 1493حسين كاظم ياسينNo ratings yet
- Mse2104 Lab06Document6 pagesMse2104 Lab06মোহাম্মদ ইসমাইল হোসেনNo ratings yet
- 9A03301 Materials Science and EngineeringDocument4 pages9A03301 Materials Science and EngineeringsivabharathamurthyNo ratings yet
- Molecular Modeling of Corrosion Processes: Scientific Development and Engineering ApplicationsFrom EverandMolecular Modeling of Corrosion Processes: Scientific Development and Engineering ApplicationsNo ratings yet
- Sustainable and Green Electrochemical Science and TechnologyFrom EverandSustainable and Green Electrochemical Science and TechnologyNo ratings yet
- Endohedral Metallofullerenes: Fullerenes with Metal InsideFrom EverandEndohedral Metallofullerenes: Fullerenes with Metal InsideNo ratings yet
- EME Project Dilpoma CLGDocument10 pagesEME Project Dilpoma CLGJoshua KondkarNo ratings yet
- TEN Project Dilpoma CLGDocument13 pagesTEN Project Dilpoma CLGJoshua KondkarNo ratings yet
- SOM Project Diploma CLGDocument9 pagesSOM Project Diploma CLGJoshua KondkarNo ratings yet
- MWM Project Diploma CLGDocument12 pagesMWM Project Diploma CLGJoshua Kondkar100% (2)
- Birla Institute of Technology and Science, Pilani: Pilani Campus AUGS/ AGSR DivisionDocument6 pagesBirla Institute of Technology and Science, Pilani: Pilani Campus AUGS/ AGSR DivisionIkshwakNo ratings yet
- Cupola Furnace-Iron MakingDocument32 pagesCupola Furnace-Iron MakingSameer MdNo ratings yet
- Engineering Materials and Metallurgy 2 Mark With AnswersDocument31 pagesEngineering Materials and Metallurgy 2 Mark With AnswersbrindharajasekarNo ratings yet
- A. Metallic:: 3.1. Physical and Chemical PropertiesDocument7 pagesA. Metallic:: 3.1. Physical and Chemical PropertiesVinothKumarVinothNo ratings yet
- A Comparative Thermal and Structural Analysis of Different Disc Brake MaterialsDocument6 pagesA Comparative Thermal and Structural Analysis of Different Disc Brake MaterialsTJPRC PublicationsNo ratings yet
- 2017 Standard CatalogDocument116 pages2017 Standard CatalogpicottNo ratings yet
- Year 7 MetalsDocument8 pagesYear 7 MetalsMissparish8No ratings yet
- Tube Material GradesDocument40 pagesTube Material GradessajuxNo ratings yet
- Basics On Fatigue and Fracture MechanicsDocument1 pageBasics On Fatigue and Fracture Mechanicsdavood abbasiNo ratings yet
- Astm B333Document5 pagesAstm B333Jota JacquesNo ratings yet
- Electrode PDFDocument32 pagesElectrode PDFShrikant MojeNo ratings yet
- AnnealingDocument9 pagesAnnealingRathne AbeynayakeNo ratings yet
- Rheinfelden Silafont®, Magsimal® Und Castasil® 2012Document18 pagesRheinfelden Silafont®, Magsimal® Und Castasil® 2012Giacomo ZammattioNo ratings yet
- 3rd Sem SyllabusDocument14 pages3rd Sem SyllabusSañkü KhåñNo ratings yet
- Troubleshooting GuideDocument25 pagesTroubleshooting GuideIjabiNo ratings yet
- Astm B283-20Document16 pagesAstm B283-20Gregory Alan Francisco IINo ratings yet
- A 5.29 FCAW Consumables PDFDocument36 pagesA 5.29 FCAW Consumables PDFStephen LewellenNo ratings yet
- Alloys - Ferrous & Non-Ferrous MetalsDocument13 pagesAlloys - Ferrous & Non-Ferrous MetalsResta CafNo ratings yet
- Paper Failure Weld JointDocument6 pagesPaper Failure Weld JointWildan HamdaniNo ratings yet
- High Temperature Material Two Mark With AnswerDocument13 pagesHigh Temperature Material Two Mark With AnswerTamilselvan Selvan100% (1)
- Api 653 Exam 2018Document21 pagesApi 653 Exam 2018Akram AlhaddadNo ratings yet
- Brass and BronzeDocument7 pagesBrass and BronzeH A RanaNo ratings yet
- Corrosion AllowanceDocument35 pagesCorrosion AllowanceReni Mutiara Sari50% (2)
- Mixture Word Problems PDFDocument2 pagesMixture Word Problems PDFKevin Okech100% (1)
- Nibco Fire Protection ValvesDocument56 pagesNibco Fire Protection ValvesJuan Martinez0% (1)
- Aalco MaterialsDocument20 pagesAalco MaterialsMohamed FaragNo ratings yet
- Microstructure and Mechanical Properties of Powder Metallurgy 2024 Aluminum Alloy During Cold RollingDocument12 pagesMicrostructure and Mechanical Properties of Powder Metallurgy 2024 Aluminum Alloy During Cold Rollingjesus herazoNo ratings yet
- CZ106 Sheet Strip Foil To BS 2870Document2 pagesCZ106 Sheet Strip Foil To BS 2870zaafrNo ratings yet