Professional Documents
Culture Documents
Binx Results J2WOZRX
Binx Results J2WOZRX
Uploaded by
RyanCopyright:
Available Formats
You might also like
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMuhammad AreebNo ratings yet
- COVID PCR TestDocument1 pageCOVID PCR TestStacey ChanNo ratings yet
- Covid TestDocument2 pagesCovid TestFaizanNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesVivek VinuNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusShahzad Ali100% (1)
- BooksDocument1 pageBooksCollin LongNo ratings yet
- Burgos, Juan Bautista M 06/24/1954: "Serving The Health Care Community Since 1967"Document1 pageBurgos, Juan Bautista M 06/24/1954: "Serving The Health Care Community Since 1967"Selena BurgosNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusAli NawazNo ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRUmar BadshahNo ratings yet
- Covid-19 RT-PCR: Test Results PanelDocument1 pageCovid-19 RT-PCR: Test Results PanelPatricia Cottle-SalyerNo ratings yet
- Untitled 12Document2 pagesUntitled 12Natasa PrelevicNo ratings yet
- Aragaw 206714-1 364272Document1 pageAragaw 206714-1 364272zeine omerNo ratings yet
- CLLPatientReport05!28!2021 21-47-54Document1 pageCLLPatientReport05!28!2021 21-47-54adeel jamilNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) Virusshoaib chNo ratings yet
- MR Lokesh Wadhey - 9300401789Document2 pagesMR Lokesh Wadhey - 9300401789Aks WadheNo ratings yet
- Covid Compare Moral ImmoralDocument3 pagesCovid Compare Moral ImmoralCarlos Eduardo GuerraNo ratings yet
- COVID 19 PCR Sample ReportDocument1 pageCOVID 19 PCR Sample ReportRamanamma PosaNo ratings yet
- Divya Bangera MBBS, MD Microbiology MME Team LeadDocument2 pagesDivya Bangera MBBS, MD Microbiology MME Team LeadRajavardhanNo ratings yet
- Sars-Cov-2 RT PCR Testing: Test Description Method ResultDocument1 pageSars-Cov-2 RT PCR Testing: Test Description Method ResultabcNo ratings yet
- Methods and Limitations: Test Result InterpretationDocument1 pageMethods and Limitations: Test Result InterpretationJuan Carlos MillaresNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMuhammad HayatNo ratings yet
- Mr. Pratik P Shetty 14 Dec 2020-UnlockedDocument2 pagesMr. Pratik P Shetty 14 Dec 2020-UnlockedPratik ShettyNo ratings yet
- Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRDocument3 pagesSars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRAmar PatilNo ratings yet
- Covid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedDocument2 pagesCovid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedRonni PriceNo ratings yet
- CovidcomparemoralimmoralDocument3 pagesCovidcomparemoralimmoralapi-515083990No ratings yet
- RapidCare - RT PCR - September 5th 3Document1 pageRapidCare - RT PCR - September 5th 3দীপা পালNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMian IqbalNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMuhammad Ali KazmiNo ratings yet
- Report ViewerDocument1 pageReport Viewervoldemort killerNo ratings yet
- Fusion Diagnostics Lingad, Joselito Bohots: Sars-Cov-2 PCRDocument2 pagesFusion Diagnostics Lingad, Joselito Bohots: Sars-Cov-2 PCRjb lingadNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMaaz SiddiquiNo ratings yet
- Monique Positive Covid Test Results.Document1 pageMonique Positive Covid Test Results.Vicky KeNo ratings yet
- FEVRE13081999Document23 pagesFEVRE13081999Charly ValerioNo ratings yet
- ReportViewer 3Document1 pageReportViewer 3CH MUBASHER MAQSOOD ALAMNo ratings yet
- SR4750118 1Document1 pageSR4750118 1ac9467593No ratings yet
- RTPCRDocument1 pageRTPCRAmit TyagiNo ratings yet
- Global Calcium Diagnostic Services: Covid-19 Test ReportDocument1 pageGlobal Calcium Diagnostic Services: Covid-19 Test Reportkanmanan selvamNo ratings yet
- Marilao Medical & Diagnostic Clinic, Inc.: (Joni Villanueva Molecular Diagnostic Laboratory) Igulot RD., Brgy. IgulotDocument2 pagesMarilao Medical & Diagnostic Clinic, Inc.: (Joni Villanueva Molecular Diagnostic Laboratory) Igulot RD., Brgy. IgulotJermar LazagaNo ratings yet
- El Arte de DelegarDocument2 pagesEl Arte de DelegarGreen DusterNo ratings yet
- MR Shaikh Aabid 25 07 2021 03 41 01 PMDocument1 pageMR Shaikh Aabid 25 07 2021 03 41 01 PMKNOWLEDGE REQUIREDNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusAli NawazNo ratings yet
- Jean Durant 1940 Biarritz DR Apt 8 Miami Beach, FL 33141: Test ReportDocument1 pageJean Durant 1940 Biarritz DR Apt 8 Miami Beach, FL 33141: Test ReportStacy KestwickNo ratings yet
- Report ViewerDocument1 pageReport ViewerZeeshan JunejoNo ratings yet
- 2021 12 30 21268565v1 OmicronDocument19 pages2021 12 30 21268565v1 OmicronNancy Grisell RamirezNo ratings yet
- ResultDocument1 pageResultNandini Pritesh PatelNo ratings yet
- Sars-Cov-2 RT PCR Testing: Test Description Method ResultDocument1 pageSars-Cov-2 RT PCR Testing: Test Description Method ResultMAYUR PATELNo ratings yet
- Sars-Cov2 (Covid-19) Real Time RT PCR Test: Positive and Negative Controls For All The Three Genes Were SatisfactoryDocument1 pageSars-Cov2 (Covid-19) Real Time RT PCR Test: Positive and Negative Controls For All The Three Genes Were Satisfactorygowtham thakutNo ratings yet
- EMAIL ReportDocument1 pageEMAIL ReportGenesis CernaNo ratings yet
- AbbottDocument10 pagesAbbottSisca PrimaNo ratings yet
- Prophasedx Laboratory Phone: (855) 982-1100Document2 pagesProphasedx Laboratory Phone: (855) 982-1100ommanon15 aNo ratings yet
- Sars-Cov-2 Rna, QL, RT PCR (Covid-19) - DetailsDocument1 pageSars-Cov-2 Rna, QL, RT PCR (Covid-19) - DetailsimlimitededitionNo ratings yet
- Covidl 9 Test - Google Drive: St. Luke'sDocument2 pagesCovidl 9 Test - Google Drive: St. Luke'sAya BeeNo ratings yet
- NokolDocument1 pageNokolTanvirNo ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRirfan shabbbirNo ratings yet
- Abdul GhafoorDocument1 pageAbdul Ghafooratta ur rehmanNo ratings yet
- COVID19 Screen (ER - in Patient) 2020-10-23 00 - 53 - 02 PDFDocument1 pageCOVID19 Screen (ER - in Patient) 2020-10-23 00 - 53 - 02 PDFIsos CellNo ratings yet
- Brown, Joielle 08/24/1983 Patient Report: Ordered Items: Sars-Cov-2, NaaDocument1 pageBrown, Joielle 08/24/1983 Patient Report: Ordered Items: Sars-Cov-2, NaajoiNo ratings yet
- MithunDocument1 pageMithunMithun MukherjeeNo ratings yet
- Order Information Iwama, Moises: CommentsDocument1 pageOrder Information Iwama, Moises: CommentsLuis IwamaNo ratings yet
- SARS-CoV-2 Viral Outbreak Investigation: Laboratory Perspective: Clinical Updates in COVID-19From EverandSARS-CoV-2 Viral Outbreak Investigation: Laboratory Perspective: Clinical Updates in COVID-19Rating: 3 out of 5 stars3/5 (1)
- Applied Surface Science AdvancesDocument19 pagesApplied Surface Science Advancesneelkant sharmaNo ratings yet
- The Ancient DNA Oligopoly and The Stories People Tell About David Reich - Gene ExpressionDocument21 pagesThe Ancient DNA Oligopoly and The Stories People Tell About David Reich - Gene ExpressionPaul ChapmanNo ratings yet
- MSC Dissertation Literature ReviewDocument6 pagesMSC Dissertation Literature Reviewc5nqw54q100% (1)
- Lecture 2 - The Cell - The Basic Unit of LifeDocument10 pagesLecture 2 - The Cell - The Basic Unit of LifeKaye EvangelistaNo ratings yet
- Ohms Law in A Quantum WorldDocument4 pagesOhms Law in A Quantum WorldRawlinson IbiapinaNo ratings yet
- s12859 018 2189 Z - Samsa2 PDFDocument11 pagess12859 018 2189 Z - Samsa2 PDFSuran Ravindran NambisanNo ratings yet
- Prokaryotic CellDocument36 pagesProkaryotic CellPrecious Marie AgabeNo ratings yet
- Learning Activity 2.3: Proteins: BiomoleculesDocument5 pagesLearning Activity 2.3: Proteins: BiomoleculesaikeeNo ratings yet
- Nature Protocols 2020Document29 pagesNature Protocols 2020gorkhmazabbaszadeNo ratings yet
- Bald CatDocument2 pagesBald CatAyush sharmaNo ratings yet
- Nature or NurtureDocument11 pagesNature or NurtureChicki ChandwaniNo ratings yet
- LT Adm 15.2Document11 pagesLT Adm 15.2KristineNo ratings yet
- CHAPTER 4 Lesson 1 VirusDocument29 pagesCHAPTER 4 Lesson 1 VirusKreizel FajaNo ratings yet
- Sarmiento 2022 - Muddying The Muddle in The Middle Even MoreDocument3 pagesSarmiento 2022 - Muddying The Muddle in The Middle Even MorehioniamNo ratings yet
- Unit - 10 - General Characters and Classification of Fungi by Dr. Kirtika PadaliaDocument38 pagesUnit - 10 - General Characters and Classification of Fungi by Dr. Kirtika PadalianucleophilicmishraNo ratings yet
- Antivirals - HIV, Hepatitis, Influenza, Herpes Treatment (Note) AtfDocument16 pagesAntivirals - HIV, Hepatitis, Influenza, Herpes Treatment (Note) AtfEmaan NoorNo ratings yet
- Eukaryotic Cell Lecture PDFDocument49 pagesEukaryotic Cell Lecture PDFDaezel Deux100% (1)
- Biochemistry SAQsDocument37 pagesBiochemistry SAQscarolinegatwiri08No ratings yet
- Pearson Syndrome - A Multisystem Mitochondrial Disease With Bone Marrow Failure - PMCDocument23 pagesPearson Syndrome - A Multisystem Mitochondrial Disease With Bone Marrow Failure - PMCLeslie CordovaNo ratings yet
- Proof That The C-19 Injections Are A Chemical WeaponDocument15 pagesProof That The C-19 Injections Are A Chemical WeaponSilvo SoaresNo ratings yet
- Pretest Second QuarterDocument2 pagesPretest Second QuarterJobelle Tabinas100% (1)
- A Level Misa Meet PPT 2021Document113 pagesA Level Misa Meet PPT 2021Pooja GodfreyNo ratings yet
- Flashcards - Topic 6.16-6.20 Forensic TechniquesDocument47 pagesFlashcards - Topic 6.16-6.20 Forensic TechniquesMoataz NassefNo ratings yet
- Ex3 PostLabDocument4 pagesEx3 PostLabDaniel Seth AndalNo ratings yet
- Molecular Biology: Negative Negative NegativeDocument1 pageMolecular Biology: Negative Negative Negativeravi kumarNo ratings yet
- MSC BiotechnologyDocument82 pagesMSC BiotechnologyJanikaa Singaravel MuruganNo ratings yet
- 3rdgrad NOTES GeneticsDocument3 pages3rdgrad NOTES GeneticsKarl SiaganNo ratings yet
- Resume UpdatedDocument1 pageResume Updatedapi-398024140No ratings yet
- Biology STAAR Review Stations Day 8Document9 pagesBiology STAAR Review Stations Day 8excaliber4No ratings yet
- Gee 301 (Midterm) PDFDocument3 pagesGee 301 (Midterm) PDFPatrisha Isabelle D. DumaranNo ratings yet
Binx Results J2WOZRX
Binx Results J2WOZRX
Uploaded by
RyanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Binx Results J2WOZRX
Binx Results J2WOZRX
Uploaded by
RyanCopyright:
Available Formats
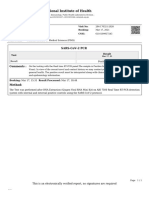
OraRisk® COVID-19 RT-PCR
FINAL REPORT
Chen, Yung-Yi Ordering Provider Sample Information
Date Of Birth: 09/21/1999 (22 yrs) Jayne Jordan MD Specimen#: 7RS30MQH Collected: 12/14/2021 15:14
Gender: Male 245 First St Riverview II Accession#: 202112-51958 Received: 12/15/2021 10:49
Patient Id: PYQ5zAHtlwfxCh32Nv5J Cambridge, MA 02142 Specimen: Nasal Swab, Dry Reported: 12/15/2021 16:15
Patient Location: binx health 8446924691
Reason for Testing: Not Provided
Related info: Not Provided
MOLECULAR DETECTION OF SARS-COV-2
Test Results
SARS-CoV-2 Not Detected
Interpretation:
The submitted sample is negative (absence of the RNA) for SARS-CoV-2, the virus that causes the disease called
COVID-19. See comments.
Guidance from the Centers for Disease Control and Prevention:
- General information about SARS-CoV-2:
https://www.cdc.gov/coronavirus/2019-ncov/index.html
- Information on testing for SARS-CoV-2:
https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html
https://www.cdc.gov/coronavirus/2019-ncov/testing/diagnostic-testing.html
- Guidance for Healthcare Providers:
https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html
https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html
Fact Sheets:
- Healthcare Provider:
https://www.fda.gov/media/140291/download
- Patient:
https://www.fda.gov/media/140292/download
Methodology: Based on 1 mL of primary sample, 300 uL is used to extract viral RNA and cellular DNA using the Chemagic Viral 300 360 HP 96 (ABI Perkin Elmer).
Following this, reverse transcription is used to create cDNA of the SARS-CoV-2 viral sequence followed by polymerase chain reaction-based amplification of regions of the
SAR-Cov-2 genome (ORF1a, RdRp, E and N genes). This rt-PCR test uses the components of the Logix Smart 2019 Novel Coronavirus kit (Co-Diagnostics, Salt Lake
City, UT). The results of this test are based on the detection of amplified viral gene sequences along with an internal control gene marker. The results are reported as
Detected, Not Detected or Inconclusive. The performance characteristics of this assay was determined by Access Genetics, LLC, d.b.a. OralDNA Labs and is assigned as
a laboratory developed test. This test has not been FDA cleared or approved. This test has been authorized by FDA under an EUA for use by Access Genetics, LLC, d.b.a.
OralDNA Labs and has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. This test is only authorized for the
duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests detection and/or diagnosis of COVID-19 under
Section 564(b)(1) of the Act 21, U.S.C. 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Ronald McGlennen MD, FCAP, FACMG, ABMG
Medical Director
©2021 OralDNA Labs, A Service of Access Genetics, LLC All Rights Reserved
7400 Flying Cloud Drive Suite 150, Eden Prairie, MN 55344 | Phone: 855-ORALDNA | Fax: 952-767-0446 | www.oraldna.com
CLIA#: 24D1033809 CAP#: 7190878
1 of 1
Web enabled system provided by:
You might also like
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMuhammad AreebNo ratings yet
- COVID PCR TestDocument1 pageCOVID PCR TestStacey ChanNo ratings yet
- Covid TestDocument2 pagesCovid TestFaizanNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesVivek VinuNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusShahzad Ali100% (1)
- BooksDocument1 pageBooksCollin LongNo ratings yet
- Burgos, Juan Bautista M 06/24/1954: "Serving The Health Care Community Since 1967"Document1 pageBurgos, Juan Bautista M 06/24/1954: "Serving The Health Care Community Since 1967"Selena BurgosNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusAli NawazNo ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRUmar BadshahNo ratings yet
- Covid-19 RT-PCR: Test Results PanelDocument1 pageCovid-19 RT-PCR: Test Results PanelPatricia Cottle-SalyerNo ratings yet
- Untitled 12Document2 pagesUntitled 12Natasa PrelevicNo ratings yet
- Aragaw 206714-1 364272Document1 pageAragaw 206714-1 364272zeine omerNo ratings yet
- CLLPatientReport05!28!2021 21-47-54Document1 pageCLLPatientReport05!28!2021 21-47-54adeel jamilNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) Virusshoaib chNo ratings yet
- MR Lokesh Wadhey - 9300401789Document2 pagesMR Lokesh Wadhey - 9300401789Aks WadheNo ratings yet
- Covid Compare Moral ImmoralDocument3 pagesCovid Compare Moral ImmoralCarlos Eduardo GuerraNo ratings yet
- COVID 19 PCR Sample ReportDocument1 pageCOVID 19 PCR Sample ReportRamanamma PosaNo ratings yet
- Divya Bangera MBBS, MD Microbiology MME Team LeadDocument2 pagesDivya Bangera MBBS, MD Microbiology MME Team LeadRajavardhanNo ratings yet
- Sars-Cov-2 RT PCR Testing: Test Description Method ResultDocument1 pageSars-Cov-2 RT PCR Testing: Test Description Method ResultabcNo ratings yet
- Methods and Limitations: Test Result InterpretationDocument1 pageMethods and Limitations: Test Result InterpretationJuan Carlos MillaresNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMuhammad HayatNo ratings yet
- Mr. Pratik P Shetty 14 Dec 2020-UnlockedDocument2 pagesMr. Pratik P Shetty 14 Dec 2020-UnlockedPratik ShettyNo ratings yet
- Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRDocument3 pagesSars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRAmar PatilNo ratings yet
- Covid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedDocument2 pagesCovid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedRonni PriceNo ratings yet
- CovidcomparemoralimmoralDocument3 pagesCovidcomparemoralimmoralapi-515083990No ratings yet
- RapidCare - RT PCR - September 5th 3Document1 pageRapidCare - RT PCR - September 5th 3দীপা পালNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMian IqbalNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMuhammad Ali KazmiNo ratings yet
- Report ViewerDocument1 pageReport Viewervoldemort killerNo ratings yet
- Fusion Diagnostics Lingad, Joselito Bohots: Sars-Cov-2 PCRDocument2 pagesFusion Diagnostics Lingad, Joselito Bohots: Sars-Cov-2 PCRjb lingadNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMaaz SiddiquiNo ratings yet
- Monique Positive Covid Test Results.Document1 pageMonique Positive Covid Test Results.Vicky KeNo ratings yet
- FEVRE13081999Document23 pagesFEVRE13081999Charly ValerioNo ratings yet
- ReportViewer 3Document1 pageReportViewer 3CH MUBASHER MAQSOOD ALAMNo ratings yet
- SR4750118 1Document1 pageSR4750118 1ac9467593No ratings yet
- RTPCRDocument1 pageRTPCRAmit TyagiNo ratings yet
- Global Calcium Diagnostic Services: Covid-19 Test ReportDocument1 pageGlobal Calcium Diagnostic Services: Covid-19 Test Reportkanmanan selvamNo ratings yet
- Marilao Medical & Diagnostic Clinic, Inc.: (Joni Villanueva Molecular Diagnostic Laboratory) Igulot RD., Brgy. IgulotDocument2 pagesMarilao Medical & Diagnostic Clinic, Inc.: (Joni Villanueva Molecular Diagnostic Laboratory) Igulot RD., Brgy. IgulotJermar LazagaNo ratings yet
- El Arte de DelegarDocument2 pagesEl Arte de DelegarGreen DusterNo ratings yet
- MR Shaikh Aabid 25 07 2021 03 41 01 PMDocument1 pageMR Shaikh Aabid 25 07 2021 03 41 01 PMKNOWLEDGE REQUIREDNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusAli NawazNo ratings yet
- Jean Durant 1940 Biarritz DR Apt 8 Miami Beach, FL 33141: Test ReportDocument1 pageJean Durant 1940 Biarritz DR Apt 8 Miami Beach, FL 33141: Test ReportStacy KestwickNo ratings yet
- Report ViewerDocument1 pageReport ViewerZeeshan JunejoNo ratings yet
- 2021 12 30 21268565v1 OmicronDocument19 pages2021 12 30 21268565v1 OmicronNancy Grisell RamirezNo ratings yet
- ResultDocument1 pageResultNandini Pritesh PatelNo ratings yet
- Sars-Cov-2 RT PCR Testing: Test Description Method ResultDocument1 pageSars-Cov-2 RT PCR Testing: Test Description Method ResultMAYUR PATELNo ratings yet
- Sars-Cov2 (Covid-19) Real Time RT PCR Test: Positive and Negative Controls For All The Three Genes Were SatisfactoryDocument1 pageSars-Cov2 (Covid-19) Real Time RT PCR Test: Positive and Negative Controls For All The Three Genes Were Satisfactorygowtham thakutNo ratings yet
- EMAIL ReportDocument1 pageEMAIL ReportGenesis CernaNo ratings yet
- AbbottDocument10 pagesAbbottSisca PrimaNo ratings yet
- Prophasedx Laboratory Phone: (855) 982-1100Document2 pagesProphasedx Laboratory Phone: (855) 982-1100ommanon15 aNo ratings yet
- Sars-Cov-2 Rna, QL, RT PCR (Covid-19) - DetailsDocument1 pageSars-Cov-2 Rna, QL, RT PCR (Covid-19) - DetailsimlimitededitionNo ratings yet
- Covidl 9 Test - Google Drive: St. Luke'sDocument2 pagesCovidl 9 Test - Google Drive: St. Luke'sAya BeeNo ratings yet
- NokolDocument1 pageNokolTanvirNo ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRirfan shabbbirNo ratings yet
- Abdul GhafoorDocument1 pageAbdul Ghafooratta ur rehmanNo ratings yet
- COVID19 Screen (ER - in Patient) 2020-10-23 00 - 53 - 02 PDFDocument1 pageCOVID19 Screen (ER - in Patient) 2020-10-23 00 - 53 - 02 PDFIsos CellNo ratings yet
- Brown, Joielle 08/24/1983 Patient Report: Ordered Items: Sars-Cov-2, NaaDocument1 pageBrown, Joielle 08/24/1983 Patient Report: Ordered Items: Sars-Cov-2, NaajoiNo ratings yet
- MithunDocument1 pageMithunMithun MukherjeeNo ratings yet
- Order Information Iwama, Moises: CommentsDocument1 pageOrder Information Iwama, Moises: CommentsLuis IwamaNo ratings yet
- SARS-CoV-2 Viral Outbreak Investigation: Laboratory Perspective: Clinical Updates in COVID-19From EverandSARS-CoV-2 Viral Outbreak Investigation: Laboratory Perspective: Clinical Updates in COVID-19Rating: 3 out of 5 stars3/5 (1)
- Applied Surface Science AdvancesDocument19 pagesApplied Surface Science Advancesneelkant sharmaNo ratings yet
- The Ancient DNA Oligopoly and The Stories People Tell About David Reich - Gene ExpressionDocument21 pagesThe Ancient DNA Oligopoly and The Stories People Tell About David Reich - Gene ExpressionPaul ChapmanNo ratings yet
- MSC Dissertation Literature ReviewDocument6 pagesMSC Dissertation Literature Reviewc5nqw54q100% (1)
- Lecture 2 - The Cell - The Basic Unit of LifeDocument10 pagesLecture 2 - The Cell - The Basic Unit of LifeKaye EvangelistaNo ratings yet
- Ohms Law in A Quantum WorldDocument4 pagesOhms Law in A Quantum WorldRawlinson IbiapinaNo ratings yet
- s12859 018 2189 Z - Samsa2 PDFDocument11 pagess12859 018 2189 Z - Samsa2 PDFSuran Ravindran NambisanNo ratings yet
- Prokaryotic CellDocument36 pagesProkaryotic CellPrecious Marie AgabeNo ratings yet
- Learning Activity 2.3: Proteins: BiomoleculesDocument5 pagesLearning Activity 2.3: Proteins: BiomoleculesaikeeNo ratings yet
- Nature Protocols 2020Document29 pagesNature Protocols 2020gorkhmazabbaszadeNo ratings yet
- Bald CatDocument2 pagesBald CatAyush sharmaNo ratings yet
- Nature or NurtureDocument11 pagesNature or NurtureChicki ChandwaniNo ratings yet
- LT Adm 15.2Document11 pagesLT Adm 15.2KristineNo ratings yet
- CHAPTER 4 Lesson 1 VirusDocument29 pagesCHAPTER 4 Lesson 1 VirusKreizel FajaNo ratings yet
- Sarmiento 2022 - Muddying The Muddle in The Middle Even MoreDocument3 pagesSarmiento 2022 - Muddying The Muddle in The Middle Even MorehioniamNo ratings yet
- Unit - 10 - General Characters and Classification of Fungi by Dr. Kirtika PadaliaDocument38 pagesUnit - 10 - General Characters and Classification of Fungi by Dr. Kirtika PadalianucleophilicmishraNo ratings yet
- Antivirals - HIV, Hepatitis, Influenza, Herpes Treatment (Note) AtfDocument16 pagesAntivirals - HIV, Hepatitis, Influenza, Herpes Treatment (Note) AtfEmaan NoorNo ratings yet
- Eukaryotic Cell Lecture PDFDocument49 pagesEukaryotic Cell Lecture PDFDaezel Deux100% (1)
- Biochemistry SAQsDocument37 pagesBiochemistry SAQscarolinegatwiri08No ratings yet
- Pearson Syndrome - A Multisystem Mitochondrial Disease With Bone Marrow Failure - PMCDocument23 pagesPearson Syndrome - A Multisystem Mitochondrial Disease With Bone Marrow Failure - PMCLeslie CordovaNo ratings yet
- Proof That The C-19 Injections Are A Chemical WeaponDocument15 pagesProof That The C-19 Injections Are A Chemical WeaponSilvo SoaresNo ratings yet
- Pretest Second QuarterDocument2 pagesPretest Second QuarterJobelle Tabinas100% (1)
- A Level Misa Meet PPT 2021Document113 pagesA Level Misa Meet PPT 2021Pooja GodfreyNo ratings yet
- Flashcards - Topic 6.16-6.20 Forensic TechniquesDocument47 pagesFlashcards - Topic 6.16-6.20 Forensic TechniquesMoataz NassefNo ratings yet
- Ex3 PostLabDocument4 pagesEx3 PostLabDaniel Seth AndalNo ratings yet
- Molecular Biology: Negative Negative NegativeDocument1 pageMolecular Biology: Negative Negative Negativeravi kumarNo ratings yet
- MSC BiotechnologyDocument82 pagesMSC BiotechnologyJanikaa Singaravel MuruganNo ratings yet
- 3rdgrad NOTES GeneticsDocument3 pages3rdgrad NOTES GeneticsKarl SiaganNo ratings yet
- Resume UpdatedDocument1 pageResume Updatedapi-398024140No ratings yet
- Biology STAAR Review Stations Day 8Document9 pagesBiology STAAR Review Stations Day 8excaliber4No ratings yet
- Gee 301 (Midterm) PDFDocument3 pagesGee 301 (Midterm) PDFPatrisha Isabelle D. DumaranNo ratings yet