Professional Documents
Culture Documents
Slater Rule
Slater Rule
Uploaded by
Ashutosh KumarOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Slater Rule
Slater Rule
Uploaded by
Ashutosh KumarCopyright:
Available Formats
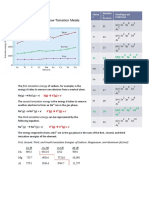
Calculated Effective Nuclear Charge (Z*) Values
letters Element Electron Z*(3d) Z*(4s) av
Configuration
Slater’s Rules and Electron Configurations 3.00 3.00
Sc [Ar 3d14s2 3.00
To the Editor: [Ar 3d24s1 2.65 2.50 2.60
[Ar 3d3 2.30 2.30
The article “The Use of Effective Nuclear Charge (Z)
Calculations to Illustrate the Relative Energies of ns and Ti [Ar]3d24s2 3.65 3.15 3.40
3.30 2.65 3.14
(n l)d Orbitals” by Christina Poth Brink (I) describes the
- [Ar]3d34s1
use of Slater’s rules to account for the fact that while the [Ar]3d4 2.95 2.95
4s orbital fills before the 3d orbitals for the 4th row ele-
V 4.30 3.30 3.90
ments, the 4s electrons are the first to be removed on ion- [Ar]3d34s2
3.95 2.80 3.72
ization. Slater’s rules had been used previously to justify [Ar]3d44s1
[Ar]3d5 3.60 3.60
that the ground state electron configuration for potassium
is [ArMs1 rather than [ArjSd1 (2, 3). Also, I have used a Cr [Ar]3d44s2 4.95 3.45 4.45
similar approach to that described by Brink (I) to illustr- [Ar]3d54s1 4.60 2.95 4.33
ate that the 3d orbitals become progressively more stable [Ar]3d6 4.25 4.25
than the 4s orbital on going through the first transition
Mn [Ar]3d54s2 5.60 3.60 5.03
series. At this point, students often ask why, for example, 5.25 3.10 4.94
[Ar]3d64s1
the ground state electron configuration for Ti is [Ar]3d24s2 [Ar] 3d7 4.90 4.90
rather than [Ar]3d4. However, this problem can be solved
again with the use of Slater’s rules, which lead to an effec- Fe [Ar]3d64s2 6.25 3.75 5.63
tive nuclear charge of 3.15 for 4s electrons and 3.65 for 3d [Ar]3d74s1 5.90 3.25 5.57
electrons if the electron configuration of Ti is [Ar]3d24s2 [Ar]3d8 5.55 5.55
(1). On the other hand, if the electron configuration of Ti Co 6.90 3.90 6.23
[Ar]3d74s2
had been [Ar]3d4, the effective nuclear charge that the 3d [Ar]3d84s1 6.55 3.40 6.20
electrons would have experienced is: [Ar]3d9 6.20 6.20
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
{ls2)(2s22p6)(3s23p6)(3d4) Ni [Ar]3d84s2 7.55 4.05 6.85
S = (3 x (0.35)) + (18 x (1.00)) 19.05 6.84
Downloaded via 115.248.191.25 on August 16, 2019 at 09:33:49 (UTC).
=
[Ar]3d94s1 7.20 3.55
Z* = 22.00 19.05 = 2.95
-
[Ar]3d10 6.85 6.85
Consequently, despite the fact that the 3d orbitals are Cu [Ar]3d94s2 8.20 4.20 7.47
lower in energy than the 4s orbital, both 3d and 4s elec- [Ar]3d104s1 7.85 3.70 7.47
trons of Ti [Ar]3d24s2 feel a higher effective nuclear charge
and are therefore more stable than the 3d electrons of Ti number for polyelectronic atoms. This misleading diagram
[Ar]3d4. Accordingly, the ground state electron configura- showing that the orbital energy for the 4s orbital becomes
tion of Ti will be [Ar]3d24s2 rather than [Ar]3d4. Similarly, lower than for 3d orbitals for certain values of atomic num-
calculations can be performed for the effective nuclear ber is still contained in some inorganic chemistry text-
charge experienced by the 3d and 4s electrons for the pos- books published during the last decade (8-12).
sible [Ar]3dn4s2, [Ar]3dn+14s1 and [Ar]3d"+24s° electron In conclusion, despite the apparent usefulness of Slater’s
configurations of the first transition metal row elements. rules to account for the ground state electron configuration
The results are summarized in the table, where Z av is the of the 4th row elements, they lead to the erroneous idea
average of the effective nuclear charge calculated for the that for potassium the 4s orbital is lower in energy than
3d and 4s electrons. It can be seen from the table that the 3d orbital. Rather, the energy due to the [Ar]4s1 config-
while the difference between the effective nuclear charge uration is lower than that due to the [Ar]3dT configuration
experienced by the 3d and 4s electrons increases along the because of the electron repulsion term, in spite of the fact
first transition series, the average effective nuclear charge that the 4s orbital energy is higher than the 3d orbital en-
for the different possible electron configurations becomes ergy (6). Nevertheless, the simplicity of the Slater’s rules
more similar from Sc to Cu, so that exceptions to the elec- makes them useful, for teaching purposes, to discern
tron configuration [Ar]3d"4s2 are not surprising. trends along the 4th row elements.
It is important to note that the calculations reported above
and in reference (1) are useful to discern trends along the 4th Literature Cited
Brink, C. J. Chem. Educ. 1991, 68, 376-377.
row elements, but the actual numerical values must be inter-
1. P.
2. Sharpe, A. G. Inorganic Chemistry, 2nd ed.; Longman: London, 1986; Chapter 3, p 67.
preted with considerable caution. More specifically, Slater’s 3. Butler, I. S.; Harrod. J. F. Inorganic Chemistry, Benjamin-Cummings: Redwood City,
rules are based on simplifying assumptions that lead to poor CA, 1989; Chapter 2, p 55. The possible electron configurations for potassium are
misprinted as [Ar]3s23pfi3dI and [Ar|3s“3pb4s'.
agreement between the calculated and true effective nuclear 4. Huheey, J. E. Inorganic Chemistry, 3rd ed.; Harper and Row: New York, 1983; Chap-
charges (3), and, therefore, the electronic energies estimated ter 2, p 37.
5. Porterfield, W. W. Inorganic Chemistry; Addison-Wesley: Reading, MA, 1984; Chap-
by Slater’s rules are often not veiy accurate (4). In particular, ter 2, p 38.
Slater’s rules underestimate the effective nuclear charge val- 6. Pilar, F. L. J. Chem. Educ. 1978, 55, 2-6.
ues for 3d electrons (5). Furthermore, the very concept of effec- 7. Scerri, E. R. J. Chem. Educ. 1989, 66, 481-483,
8. Moeller, T. Inorganic Chemistry, A Modern Introduction', Wiley: New York, 1982;
tive nuclear charge is a crude and incomplete way of taking Chapter 3, pp 57,58.
electron-electron repulsions into account (6). Consequently, 9. Cotton, F. A.; Wilkinson, G.; Gaus, P. L. Basic Inorganic Chemistry, Wiley: New York,
1987; Chapter 2, p 46.
the use of Slater’s rides leads to the result that for potassium 10. Cotton, F. A,; Wilkinson, G. Advanced Inorganic Chemistry, 5th ed.; Wiley: New
the 4s orbital is lower in energy than the 3d orbital (i), while York, 1988; Chapter 17, p 628.
it is known that the 4s orbital energy is always above the 3d 11. Reference (3), p 34.
12. Shriver, D. F.; Atkins, P. W.; Langford, C. H. Inorganic Chemistry, Oxford University
orbital energy (6). As pointed by Pilar (6) and Scerri (7), the Press: Oxford, 1990; Chapter 1, p 23.
erroneous notion that the 4s orbital ever has a lower energy David Tudeia
than that of 3d is extended by textbooks containing a diagram Universidad Autonoma de Madrid
that represents the energy of atomic orbitals versus atomic 28049-Madrid (SPAIN)
956 Journal of Chemical Education
You might also like
- Full Download Test Bank For Oral Radiology Principles and Interpretation 6th Edition White Isbn 10 0323049834 Isbn 13 9780323049832 PDF Full ChapterDocument36 pagesFull Download Test Bank For Oral Radiology Principles and Interpretation 6th Edition White Isbn 10 0323049834 Isbn 13 9780323049832 PDF Full Chapterdumose.animose.h8wp100% (21)
- Ebook Chemical Peels Procedures in Cosmetic Dermatology Series PDF Full Chapter PDFDocument58 pagesEbook Chemical Peels Procedures in Cosmetic Dermatology Series PDF Full Chapter PDFsandy.wicker653100% (34)
- Radiographic Imaging and Exposure 4th Edition Fauber Test BankDocument25 pagesRadiographic Imaging and Exposure 4th Edition Fauber Test BankAngelaPhillipsbeomf100% (47)
- B.Sc. Chemistry Syllabus, National P.G. College, LucknowDocument11 pagesB.Sc. Chemistry Syllabus, National P.G. College, Lucknow621 605ManishNo ratings yet
- StriplineDocument18 pagesStriplineJamilNo ratings yet
- Unit 5 - FEA - HalfDocument14 pagesUnit 5 - FEA - Halfhahaha hahaNo ratings yet
- IE RevisionDocument2 pagesIE RevisionSains Pismp 17No ratings yet
- IE RevisionDocument2 pagesIE RevisionSains Pismp 17No ratings yet
- XIIPhys. HW 2Document1 pageXIIPhys. HW 2Sahil LakhyaniNo ratings yet
- 3.1 Aerodynamic Resistance CalculationDocument9 pages3.1 Aerodynamic Resistance CalculationparkourtracerNo ratings yet
- Assessment of Seismic Behavior of 3D Asymmetric Steel Buildings Retrofitted With TADAS Device Based On Incremental Dynamic Analysis (IDA)Document10 pagesAssessment of Seismic Behavior of 3D Asymmetric Steel Buildings Retrofitted With TADAS Device Based On Incremental Dynamic Analysis (IDA)Elyar ZafarkhahNo ratings yet
- ES 2232 Lab 02 Planetary AtmospheresTamlaMenditaDocument7 pagesES 2232 Lab 02 Planetary AtmospheresTamlaMenditaCynthia AndatiNo ratings yet
- 3-2: A.C Conductivity. 3-2.0: Introduction.: T Do DDocument43 pages3-2: A.C Conductivity. 3-2.0: Introduction.: T Do DM.M. El HawaryNo ratings yet
- 2608 PCM Paper With Sol MorningDocument25 pages2608 PCM Paper With Sol Morningpalshivanshu23No ratings yet
- Physics: 25 July 2021 (SHIFT - 1) Question With AnswerDocument9 pagesPhysics: 25 July 2021 (SHIFT - 1) Question With Answereliezer hembromNo ratings yet
- N3 Engineering Science August 2021 MemorandumDocument8 pagesN3 Engineering Science August 2021 MemorandumNkazimulo MbonaniNo ratings yet
- Inbound 2526419856430552992Document3 pagesInbound 2526419856430552992Javes L. JunioNo ratings yet
- Design Aid For Unstiffened Triangular Steel Brackets Based On Elastic StabilityDocument12 pagesDesign Aid For Unstiffened Triangular Steel Brackets Based On Elastic StabilitySid ShendgeNo ratings yet
- Final Jee-Main Examination - August, 2021: Physics Test Paper With SolutionDocument25 pagesFinal Jee-Main Examination - August, 2021: Physics Test Paper With Solutionfunny videoNo ratings yet
- Ijce V3i12p102Document10 pagesIjce V3i12p102William PolNo ratings yet
- 2 - DeterminanDocument23 pages2 - DeterminanHILDA BERNIKSNo ratings yet
- Cohesive and Thermal Properties of Sodium Cyanide-Halide Mixed CrystalsDocument6 pagesCohesive and Thermal Properties of Sodium Cyanide-Halide Mixed CrystalsAJAST JournalNo ratings yet
- IE RevisionDocument2 pagesIE RevisionRadamael MaembongNo ratings yet
- 30 01 2023 Physics - Paper+With+Ans - MorningDocument9 pages30 01 2023 Physics - Paper+With+Ans - Morningrushirajparmar1818No ratings yet
- Adobe Scan Aug 19, 2022Document5 pagesAdobe Scan Aug 19, 2022Kunal GoutamNo ratings yet
- Physics For Global Scientists and Engineers Volume 2 2Nd Edition Raymond A Serway All ChapterDocument67 pagesPhysics For Global Scientists and Engineers Volume 2 2Nd Edition Raymond A Serway All Chapterjoyce.bongiorno295100% (9)
- Curriculum: Vehicle Design: HCMC University of Technology and EducationDocument12 pagesCurriculum: Vehicle Design: HCMC University of Technology and EducationHải ĐăngNo ratings yet
- Homework #4Document2 pagesHomework #4ranjidsarhanNo ratings yet
- Engineering M12 Solutions Chapter 03 MSEDocument5 pagesEngineering M12 Solutions Chapter 03 MSEmelbaz1No ratings yet
- jp3054857 Si 001Document10 pagesjp3054857 Si 001odunmoolorun dorcasNo ratings yet
- JEE Main Online Exam 2019: Questions & Solutions (Memory Based)Document5 pagesJEE Main Online Exam 2019: Questions & Solutions (Memory Based)V Veeramani ManiNo ratings yet
- Me Mains Paper 2 Final 96Document45 pagesMe Mains Paper 2 Final 96Ritesh DevrajNo ratings yet
- Chapter 3 TorsionDocument7 pagesChapter 3 Torsionmeseretab1289No ratings yet
- Arc Length and Curvature.Document15 pagesArc Length and Curvature.aayangreatgreatNo ratings yet
- To Investigation The Structure and Morphology of Iron Metallic by Difference TechniquesDocument5 pagesTo Investigation The Structure and Morphology of Iron Metallic by Difference TechniquesDIPANKAR POKHRELNo ratings yet
- Buckling ExampleDocument3 pagesBuckling ExampleSrdjan KosoricNo ratings yet
- Enhancement of Thermoelectric Performance of Transition Metal Doped Bi Te by Retaining Topological Insulating PhaseDocument36 pagesEnhancement of Thermoelectric Performance of Transition Metal Doped Bi Te by Retaining Topological Insulating PhaseProf. Yogeshchandra SharmaNo ratings yet
- Sri Chaitanya IIT Academy., India.: A Right Choice For The Real Aspirant ICON Central Office - Madhapur - HyderabadDocument16 pagesSri Chaitanya IIT Academy., India.: A Right Choice For The Real Aspirant ICON Central Office - Madhapur - HyderabadvisheshNo ratings yet
- 3001 - Physics - Paper With Sol - EveningDocument7 pages3001 - Physics - Paper With Sol - EveningAnsh RaoNo ratings yet
- 2608 Physics Paper With Sol MorningDocument9 pages2608 Physics Paper With Sol MorningTheManASHNo ratings yet
- May Jun 2023-4Document3 pagesMay Jun 2023-4Manas DeshmukhNo ratings yet
- 153639-30-01-2023 Physics Paper+With+Ans EveningDocument7 pages153639-30-01-2023 Physics Paper+With+Ans Eveningrushirajparmar1818No ratings yet
- T630 - Engineering Science N2 APRIL 21 MG Sign OffDocument6 pagesT630 - Engineering Science N2 APRIL 21 MG Sign Offseete.sybilNo ratings yet
- Be Mechanical Engineering Semester 6 2023 May Computer Aided Engineering Cae Pattern 2019Document5 pagesBe Mechanical Engineering Semester 6 2023 May Computer Aided Engineering Cae Pattern 2019abhijit patilNo ratings yet
- X PM Office LensDocument5 pagesX PM Office LensRuchir NagarNo ratings yet
- Influence of Internal Parametric and Kinematic Excitation On Nonlinear Gearbox VibrationDocument2 pagesInfluence of Internal Parametric and Kinematic Excitation On Nonlinear Gearbox VibrationarctanxNo ratings yet
- Effects of The Hydrostatic Pressure and Temperature On The Properties of The (Gaas) Single Quantum Dot in A Magnetic FieldDocument73 pagesEffects of The Hydrostatic Pressure and Temperature On The Properties of The (Gaas) Single Quantum Dot in A Magnetic FieldolehkuzykNo ratings yet
- Neet - 2 Test Series Rotational Motion and Gravitation 1 FinalDocument4 pagesNeet - 2 Test Series Rotational Motion and Gravitation 1 Finalumved singh yadavNo ratings yet
- Oct 2022 Bxe Que PaperDocument2 pagesOct 2022 Bxe Que Papersanskrutisonawane0305No ratings yet
- Applications of Trigonometry: BY: Charvi Saini 10164Document15 pagesApplications of Trigonometry: BY: Charvi Saini 10164charvisainiNo ratings yet
- CHAPTER 3 - CALCULUS OF TENSORS - 1994 - Continuum MechanicsDocument46 pagesCHAPTER 3 - CALCULUS OF TENSORS - 1994 - Continuum MechanicsAnonymous PO7VwbBnNo ratings yet
- Physics Paper 2 SLDocument20 pagesPhysics Paper 2 SLSaniya DautovaNo ratings yet
- Full Download Book Physics For Global Scientists and Engineers Volume 2 PDFDocument41 pagesFull Download Book Physics For Global Scientists and Engineers Volume 2 PDFjeanine.chapin893100% (24)
- Bragg VS LaueDocument7 pagesBragg VS LaueSHIVAM GUPTANo ratings yet
- Strip LineDocument18 pagesStrip LineJamilNo ratings yet
- Chapter 03Document5 pagesChapter 03Ahmad Reza Tabbakhi MamaghaniNo ratings yet
- ReportDocument16 pagesReportMaxx PowerrNo ratings yet
- 2016 Aakash Anthe Junior Sample Paper Class10Document16 pages2016 Aakash Anthe Junior Sample Paper Class10ats edu100% (1)
- Pages From ASME B31.3-2014-2Document1 pagePages From ASME B31.3-2014-2Godwin A.udo-akanNo ratings yet
- Introduction To GraphsDocument6 pagesIntroduction To GraphsRajshree SinghNo ratings yet
- Characteristic Modes: Theory and Applications in Antenna EngineeringFrom EverandCharacteristic Modes: Theory and Applications in Antenna EngineeringNo ratings yet
- Endohedral Metallofullerenes: Fullerenes with Metal InsideFrom EverandEndohedral Metallofullerenes: Fullerenes with Metal InsideNo ratings yet
- Reverberation Chambers: Theory and Applications to EMC and Antenna MeasurementsFrom EverandReverberation Chambers: Theory and Applications to EMC and Antenna MeasurementsNo ratings yet
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyFrom EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNo ratings yet
- Time Monday Tuesday Wednesday Thursday FridayDocument1 pageTime Monday Tuesday Wednesday Thursday FridayAshutosh KumarNo ratings yet
- Private Car Package Policy - Zone B Motor Insurance Certificate Cum Policy ScheduleDocument2 pagesPrivate Car Package Policy - Zone B Motor Insurance Certificate Cum Policy ScheduleAshutosh KumarNo ratings yet
- National Institute of Technology, DelhiDocument5 pagesNational Institute of Technology, DelhiAshutosh KumarNo ratings yet
- Submitted By: Ashutosh Kumar Class-IX-B Roll No.6 Submitted To: Kiran Mam Biology TeacherDocument12 pagesSubmitted By: Ashutosh Kumar Class-IX-B Roll No.6 Submitted To: Kiran Mam Biology TeacherAshutosh KumarNo ratings yet
- Chemistry CBSE 11th 2023 Sample PaperDocument6 pagesChemistry CBSE 11th 2023 Sample PaperAlpha StarNo ratings yet
- Fourier-Transform Infrared Spectroscopy (FTIR)Document14 pagesFourier-Transform Infrared Spectroscopy (FTIR)Anushri VaidyaNo ratings yet
- Electrophilic Aromatic Substitution: Generic ReactionDocument33 pagesElectrophilic Aromatic Substitution: Generic ReactionEr Bipin VermaNo ratings yet
- Big History Threshold 3aDocument39 pagesBig History Threshold 3aLaiza BautistaNo ratings yet
- S-Block ElementsDocument17 pagesS-Block ElementsPiggu SurfersNo ratings yet
- XII PhysicsDocument283 pagesXII PhysicsNripendraNo ratings yet
- NEET 2024 Answer Key Aakash R3Document47 pagesNEET 2024 Answer Key Aakash R3kaleemofficial21No ratings yet
- Jablonski Diagram e PathshalaDocument9 pagesJablonski Diagram e PathshalaSriNo ratings yet
- 01 - GR # Modern Physics-1 - With SolutionDocument17 pages01 - GR # Modern Physics-1 - With Solutionydprince111No ratings yet
- S Block (Micro)Document17 pagesS Block (Micro)Anant JainNo ratings yet
- KLEEE Old Question Paper PDFDocument27 pagesKLEEE Old Question Paper PDFkattukoluNo ratings yet
- Ionized Gases - Von EngelDocument169 pagesIonized Gases - Von EngelGissela MartinezNo ratings yet
- Detailed Lesson Plan in Modern PhysicsDocument17 pagesDetailed Lesson Plan in Modern PhysicsDolores FilipinoNo ratings yet
- Hsslive XI CH 3 Chemistry Notes by AkDocument8 pagesHsslive XI CH 3 Chemistry Notes by AkkundrapupNo ratings yet
- Chemistry 6th Edition Mcmurry Solutions ManualDocument35 pagesChemistry 6th Edition Mcmurry Solutions Manualthrenodyvoxlkio100% (28)
- Highly Nonlinear Dipolar Exciton-Polaritons in Bilayer MoS2Document7 pagesHighly Nonlinear Dipolar Exciton-Polaritons in Bilayer MoS2Roni RahmatNo ratings yet
- PHY212-Exam-2012-13-ms-JUNE-SEPT2013-Modern PhysicsDocument7 pagesPHY212-Exam-2012-13-ms-JUNE-SEPT2013-Modern PhysicsMARK MICHAELNo ratings yet
- Introduction - Matter and Measurement 1.1 Multiple-Choice Questions AnswerDocument104 pagesIntroduction - Matter and Measurement 1.1 Multiple-Choice Questions AnswerNgọc PhụngNo ratings yet
- A Quick History of ChemistryDocument48 pagesA Quick History of ChemistryFaisal BachaNo ratings yet
- Microsoft PowerPoint - Chapter 6Document16 pagesMicrosoft PowerPoint - Chapter 6endgnahkNo ratings yet
- Chemistry 1 11 Q2 M1Document14 pagesChemistry 1 11 Q2 M1Gaelle Mariposa100% (1)
- Chemistry Study Guide Exam2Document11 pagesChemistry Study Guide Exam2Aaron WoodNo ratings yet
- Grade 9 Chemistry Annual Lesson Plan For 2015 Year: Unit One: Structure of The AtomDocument3 pagesGrade 9 Chemistry Annual Lesson Plan For 2015 Year: Unit One: Structure of The Atombasha0% (1)
- Average Atomic Mass Problems (Mazz 9-2018) - 1Document3 pagesAverage Atomic Mass Problems (Mazz 9-2018) - 1mclark25No ratings yet
- 2 3 Worksheet WarmupDocument2 pages2 3 Worksheet WarmupRudyline HiposNo ratings yet
- Chem Notes6Document54 pagesChem Notes6Mohamed KimoNo ratings yet