Professional Documents
Culture Documents
Green Park Educational Institutions, Namakkal: Long Term - Chemistry (Worksheet)
Green Park Educational Institutions, Namakkal: Long Term - Chemistry (Worksheet)
Uploaded by
Monalisa Premkumar0 ratings0% found this document useful (0 votes)
240 views4 pages1. This document contains a chemistry worksheet on haloalkanes and haloarenes with 22 multiple choice questions.
2. The questions cover topics like reactivity of alkyl halides, SN1 and SN2 reaction mechanisms, synthesis of alkyl halides from alcohols, and properties of haloarenes.
3. Key reactions mentioned include the Williamson ether synthesis, Sandmeyer reaction, Finkelstein reaction, and Lucas test.
Original Description:
Original Title
Haloalkanes and haloarenes
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1. This document contains a chemistry worksheet on haloalkanes and haloarenes with 22 multiple choice questions.
2. The questions cover topics like reactivity of alkyl halides, SN1 and SN2 reaction mechanisms, synthesis of alkyl halides from alcohols, and properties of haloarenes.
3. Key reactions mentioned include the Williamson ether synthesis, Sandmeyer reaction, Finkelstein reaction, and Lucas test.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
240 views4 pagesGreen Park Educational Institutions, Namakkal: Long Term - Chemistry (Worksheet)
Green Park Educational Institutions, Namakkal: Long Term - Chemistry (Worksheet)
Uploaded by
Monalisa Premkumar1. This document contains a chemistry worksheet on haloalkanes and haloarenes with 22 multiple choice questions.
2. The questions cover topics like reactivity of alkyl halides, SN1 and SN2 reaction mechanisms, synthesis of alkyl halides from alcohols, and properties of haloarenes.
3. Key reactions mentioned include the Williamson ether synthesis, Sandmeyer reaction, Finkelstein reaction, and Lucas test.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 4
GREEN PARK EDUCATIONAL INSTITUTIONS, NAMAKKAL
LONG TERM - CHEMISTRY (WORKSHEET)
24. Haloalkanes and Haloarenes
Choose the correct answer :
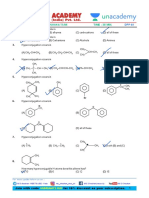
1. Order of melting points for the following 3) CH 3 − CH − CH 2 − I
compounds. |
I
4) CH3-CH=CH-I
6. Best method of preparing alkyl chloride is
Pyridine
1) ROH + SOCl2 →
2) ROH + PCl5
→
(A) (B) (C)
3) ROH + PCl3
→
1) A > B > C 2) C > B > A
anhy.ZnCl2
3) B > C > A 4) C > A > B 4) ROH + HCl →
2. Match the following 7. In the reactions given below.
List – I List – II i) KCN
R-Cl → Product A
ii) LiAlH 4
Compound Its use i) AgCN
R-Cl → Product B
A) CCl2F2 I) Insecticide ii) LiAlH 4
B) CHI3 II) Antiseptic The compounds A and B are
C) D.D.T III) Fire extinguisher 1) Chain Isomers
D) CCl4 IV) Refrigerent 2) Position isomers
The correct match is 3) Functional isomers
A B C D 4) Geometrical isomers
1) I II III IV HBr
8. → ? (Major)
2) II III IV I
3) IV II I III
4) III IV I II 1) 2)
3. Number of possible mono chlorination
products obtained from isopentane (Exculding
stereo isomerism) 3) 4)
1) 2 2) 6
9. x C2H5OH + PCl3 → x C2H5Cl
3) 4 4) 5
y C2H5OH + PCl5 → y C2H5Cl
4. Which of the following reagents may not be
used to convert alkyl chlorides and alkyl Here x : y is
bromides into alkyl fluorides? 1) 1 : 3 2) 3 : 1
1) Hg2F2 2) SbF5 3) 2 : 1 4) 1 : 2
3) AgF 4) CaF2 10. Dipole moment is maximum for
alc.KOH 1) CH3F 2) CH3Cl
5. In the reaction CH 3CHCH 3 →A
| 3) CH3Br 4) CH3I
Br CH 3
HBr
→ NaI
B → C, C is |
HCl
Peroxide Acetone 11. CH 3 − C − CH 2 OH
ZnCl2
→ Product
1) CH3CH2CH2I |
CH 3
2) CH 3 − CH − CH 3
| The main product is
I
2
CH 3 1) C6H5CH2CH2CH2Br 2) C6 H5CH - CH 2 Br
| |
1) CH 3 − C − CH 2 − OH CH 3
| CH 3
CH 3 |
CH 3 3) C6 H 5 - C - Br 4) C6 H5CH 2CH - CH 3
| | |
CH 3 Br
2) CH 3 − C − CH 2 − CH 3
| 16. Which of the following is fastly undergoes
Cl
dehydrohalogenation?
3) CH 3 − C = CH − CH 3
|
CH 3 1) 2)
4) CH 3 − CH − CH = CH 2
| Br
CH 3 3) 4)
12.
17. The order of reactivities of the following alkyl
halides for a SN2 reaction is
(A) predominantly is 1) RF > RCl > RBr > RI
1) 2) 2) RF > RBr > RCl > RI
3) RCl > RBr > RF > RI
4) RI > RBr > RCl > RF
3) 4)
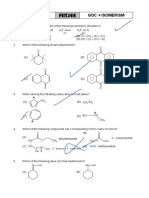
18. Which of the following is a secondary alkyl
halide?
13. Most reactive towards SN1 reaction is 1) Isobutyl chloride
2) Isopentyl chloride
3) Neopentyl chloride
1) 2) 4) Isopropyl chloride
19. C2H5OH + SOCl2 → X + Y + Z. What are X,
Y, Z
1) C2H4Cl2, SO2, HCl
2) C2H5Cl, SO3, HCl
3) C2H5Cl, SO2, HCl
3) 4)
4) C2H4, SO2, Cl2
20. The synthesis of alkyl fluorides is best
accomplished by
14. Correct order of reactivity for SN1 reaction for 1) free radical fluorination
the following 2) Sandmeyer’s reaction
3) Finkelstein reaction
4) Swarts reaction
21. The compound which reacts fastest with Lucas
reagent at room temperature is
1) butan-l-ol
(P) (Q) (R) (S) 2) butanol-2
1) S > R > Q > P 2) R > S > Q > P 3) 2-methyl propan-l-ol
3) Q > R > S > P 4) P > Q > R > S 4) 2-methyl propan-2-ol
15. Which of the following alkyl halides would be 22. When the concentration of alkyl halide is
the most reactive in an SN1 reaction? triple and concentration of OH- is reduced to
half, the rate of SN2 reaction increased by
3
1) 3 times 2) 1.5 times
3)
3) 2 times 4) 6 times
23. Which of the following statements regarding
the SN1 reaction shown by alkyl halide is not 4)
correct?
1) The added nucleophile plays no kinetic role 29. Which of the following is least reactive
in SN1 reaction towards nucleophilic substitution?
2) The SN1 reaction involves the inversion of
configuration of the optically active
substrate 1) 2)
3) The SN1 reaction on the chiral starting
material ends up with racemization of the
product
4) The more stable carbocation intermediate
the faster the SN1 reaction 3) 4)
24. The correct order of reactivity of the halides,
30. Which of the following will produce
ethyl chloride (I) isopropyl chloride (II) and
benzyl chloride (III) in SN1 reactions is chlorobenzene as a major product?
NaNO2 ∆
1) III > II > I 2) I > II > III 1) C6H5NH2
HCl;0o C
→ →
3) II > I > III 4) I > III > II NaNO2 CuCl
2) C6H5NH2
HCl;0o C
→ →
25. Which of the following is more reactive
AlCl3
towards SN1 reaction 3) C6H6 + C6H5COCl →
hν
4) C6H6 + Cl2 →
31. Which of the following undergo hydrolysis
1) 2) most easily?
1) 2)
3) 4)
26. Which of the chloride is less reactive towards
hydrolysis? 3) 4)
1) Vinyl chloride 2) Allyl chloride
3) Ethyl chloride 4) t-butyl chloride
27. Which of the following compounds undergo 32. When Friedel-Crafts alkylation of benzene is
E2 reactions with maximum rate? carried out with n-propyl bromide, the major
product is
1) 2) CH3(CH2)2CH2Cl 1) n-propyl benzene
2) isopropyl benzene
3) 2-ethyl benzene
3) CH3(CH2)2CH2I 4) 4) none of these
acetone
33. R-X + NaI → R – I + NaX. This is
28. Which of the following may be classified as
known as _______ reaction.
an aryl halide?
1) Finkelstein 2) Swart’s
1) 3) Wurtz-Fittig 4) Friedel-Craft’s
34. Which of the following is called Sandmeyer
reaction?
2) NaOH
1) 2HCHO → CH3OH + HCOONa
CuCl
2) C6H5N2Cl → C6H5Cl
4
Br2 + Red P
AlCl3 1) C2H5OH →
3) + CH3Cl → CH3 SOCl2
2) C2H5OH →
COOH KBr + Conc H 2SO4
3) C2H5OH →
4) All
CO2
4) OH
NaOH
→ OH 42. End product of the given reaction is
35. A reaction between methyl magnesium
bromide and ethyl alcohol gives
1) Butane 2) Ethane
3) Propane 4) Methane 1) 2)
36. C2H5Cl + AgF → C2H5F + AgCl
The above reaction is called 3) 4)
1) Hunsdiecker 2) Swarts
43. What products are formed when the following
3) Strecker 4) Wurtz
2 compounds is treated with Br2 in the presence
37. Rate of SN reaction is maximum when the
of FeBr3 ?
solvent is
CH3
1) Methyl alcohol 2) Water
3) Dimethyl sulphoxide 4) Benzene
38. Which of the following is most suitable for
CH3
SN 2 mechanism
1) 2) 1)
3) 4)
2)
39. The IUPAC name of
CH 3 I
| |
CH 3 − C − CH − CH − CH 2 − CH 3 is
| | 3)
CH 3CH 3
1) 3-iodo-4,5,5-trimethyl hexane 4) All of these
2) 4-iodo-1,1,3-trimethyl hexane
3) 4-iodo-2,2-dimethyl heptane 44. Centeral nervous system can be depressed by
4) 4-iodo-2,2,3-trimethyl hexane the use of which of the following
40. The IUPAC name of the given structure 1) Chloroform 2) Freon
3) Idodofrom 4) DDT
45. Solvent which is used in the synthesis of
chlorofluoro carbons
1) 5-Bromo-6-chloro cyclohex-1-en-3-yne 1) Iodoform
2) 6-Bromo-5-chloro cyclohex-1-en-3-yne 2) chloroform
3) 6-Bromo-5-chloro cyclohex-3-en-1-yne 3) carbontetra chloride
4) 4-Bromo-3-chloro cyclohex-1-en-5-yne 4) methylene chloride
41. Which of the following leads to the formation
of an alkyl halide
You might also like
- Volume Test - Ii: Long Term ChemistryDocument11 pagesVolume Test - Ii: Long Term Chemistrysachin sakuNo ratings yet
- HydrocarbonDocument15 pagesHydrocarbonzohaibsalamNo ratings yet
- LT - Batch A - Unit Test - 6 - CHE & BOT - 23.03.2023 - A Type PDFDocument16 pagesLT - Batch A - Unit Test - 6 - CHE & BOT - 23.03.2023 - A Type PDFVENUGOPALARAONo ratings yet
- Substitution - EliminationDocument36 pagesSubstitution - EliminationSachin SinghalNo ratings yet
- Electrophilic Aromatic Substitution DPPDocument35 pagesElectrophilic Aromatic Substitution DPPAsif Hoda0% (1)
- Organic Chemistry: Daily Practice ProblemsDocument35 pagesOrganic Chemistry: Daily Practice ProblemsRaju SinghNo ratings yet
- Ummeed: Isomerism NEET Organic Chemistry Crash CourseDocument7 pagesUmmeed: Isomerism NEET Organic Chemistry Crash CourseAman PandeyNo ratings yet
- Micro - OC - DPP Without Answer (110-116)Document24 pagesMicro - OC - DPP Without Answer (110-116)Ayush JaiswalNo ratings yet
- R IS IR: Iupac & NomenclatureDocument11 pagesR IS IR: Iupac & NomenclatureDhruv KuchhalNo ratings yet
- ADV. I 57 - 64 (Exercise 3)Document8 pagesADV. I 57 - 64 (Exercise 3)Aditya ShahNo ratings yet
- Concentration Terms and Eudiometry: (Physical Chemistry) Exercise (O-I) Introduction of Concentration Terms 1Document59 pagesConcentration Terms and Eudiometry: (Physical Chemistry) Exercise (O-I) Introduction of Concentration Terms 1Jayarj singh100% (1)
- ElectrochemistryDocument11 pagesElectrochemistrysaranya ganesanNo ratings yet
- Isomerism ExerciseDocument23 pagesIsomerism ExerciseAryhaNo ratings yet
- Index: Hydrocarbons (Alkanes, Alkenes & Alkynes)Document31 pagesIndex: Hydrocarbons (Alkanes, Alkenes & Alkynes)Harsh VardhanNo ratings yet
- P-Block (Group 13 To 14) NM Solution (-1) Chem PDFDocument31 pagesP-Block (Group 13 To 14) NM Solution (-1) Chem PDFChauhan RonakNo ratings yet
- Coordination Compound - Ex. Module-3-2Document18 pagesCoordination Compound - Ex. Module-3-2Raju SinghNo ratings yet
- Mole Concept Solution Practice Set Objective by S.K.sinha See Chemistry Animations atDocument1 pageMole Concept Solution Practice Set Objective by S.K.sinha See Chemistry Animations atmyiitchemistry50% (2)
- Quantitative and QualitativeDocument15 pagesQuantitative and QualitativesquadralsupremeNo ratings yet
- Reaction Mechanism Jeemain - GuruDocument12 pagesReaction Mechanism Jeemain - Guruprexa indiaNo ratings yet
- CLS Aipmt-19-20 XIII Che Study-Package-3 Level-1 Chapter-15 PDFDocument40 pagesCLS Aipmt-19-20 XIII Che Study-Package-3 Level-1 Chapter-15 PDFThavasimariselvam N100% (1)
- Redox Reactions NEET PYQ SOLUTIONDocument6 pagesRedox Reactions NEET PYQ SOLUTIONsomeone nooneNo ratings yet
- DPP 2Document2 pagesDPP 2ashutosh99878No ratings yet
- 5 DPP - 56to81 - FinalDocument44 pages5 DPP - 56to81 - FinalArnab KumarNo ratings yet
- Practice TestDocument14 pagesPractice TestHimanshu JindalNo ratings yet
- 13 DPP 03g Goc Excel Acid+BaseDocument5 pages13 DPP 03g Goc Excel Acid+BasekljNo ratings yet
- ThepblockelementsDocument56 pagesThepblockelementsAshutosh Ganesan92% (13)
- Hydrocarbons Jumbo Sheet by MKA SirDocument44 pagesHydrocarbons Jumbo Sheet by MKA SirRahul SinghNo ratings yet
- Complete Course in Organic Chemistry by Vineet Khatri Sir: Class: Xi Time: 35 Min. DPP. NO.17Document4 pagesComplete Course in Organic Chemistry by Vineet Khatri Sir: Class: Xi Time: 35 Min. DPP. NO.17Arnab KumarNo ratings yet
- Aldol Reaction - ChemistryDocument7 pagesAldol Reaction - ChemistryGamer HelperNo ratings yet
- Aromaticity Assingment PDFDocument10 pagesAromaticity Assingment PDFGaurav YadavNo ratings yet
- 3.AcidBases FinalDocument35 pages3.AcidBases FinalSoham RaneNo ratings yet
- Hydrocarbons DPPsDocument37 pagesHydrocarbons DPPsAsif HodaNo ratings yet
- Jitendra Hirwani: Daily Practice Problem OF Physical Chemistry For NeetDocument8 pagesJitendra Hirwani: Daily Practice Problem OF Physical Chemistry For NeetabhishekNo ratings yet
- Alkyl Halides and Aryl Halides - QBDocument23 pagesAlkyl Halides and Aryl Halides - QBNETHAKANI SUJATHA100% (1)
- Goc & Eas Test-IiDocument7 pagesGoc & Eas Test-IiAniket GuptaNo ratings yet
- Benzoin CondensationDocument3 pagesBenzoin Condensationprivatesanket710No ratings yet
- A - 1 (Isomerism, Reaction Mechanism) - Question PaperDocument11 pagesA - 1 (Isomerism, Reaction Mechanism) - Question PaperSachin DedhiaNo ratings yet
- Raj7 PDFDocument22 pagesRaj7 PDFAvdhoot GautamNo ratings yet
- Bond Angle Practice SheetDocument3 pagesBond Angle Practice Sheetgaurav100% (1)
- Physics DPPDocument6 pagesPhysics DPPHarshika KatariyaNo ratings yet
- Alcohol, Ether & Phenol - QuestionDocument3 pagesAlcohol, Ether & Phenol - Questionbest badmintonNo ratings yet
- Ms Chouhan & Team: Organic ChemistryDocument16 pagesMs Chouhan & Team: Organic ChemistryPrithviraj GhoshNo ratings yet
- Cls Jeead-18-19 Xii Che Target-7 Set-2 Chapter-12Document47 pagesCls Jeead-18-19 Xii Che Target-7 Set-2 Chapter-12DxNo ratings yet
- MCQ Chapter 9 Haloalkanes and HaloarenesDocument2 pagesMCQ Chapter 9 Haloalkanes and HaloarenesNinaNo ratings yet
- Aldehydes, Ketones & Carboxylic AcidsDocument35 pagesAldehydes, Ketones & Carboxylic AcidsMD MoonNo ratings yet
- Goc + IsomerismDocument5 pagesGoc + IsomerismRohail HussainNo ratings yet
- Chemistry Advanced Level Problem Solving (ALPS-5) - SolutionDocument12 pagesChemistry Advanced Level Problem Solving (ALPS-5) - SolutionAnanmay Chauhan100% (1)
- Aromaticity DPP 4Document4 pagesAromaticity DPP 4SubhadeepNo ratings yet
- Unacademy - IOCXII MegaDPP 23withoutDocument2 pagesUnacademy - IOCXII MegaDPP 23withoutAaryan KeshanNo ratings yet
- GRiGNARD REAGENT!!Document22 pagesGRiGNARD REAGENT!!GazalNo ratings yet
- Chem Academy: ThermodynamicsDocument5 pagesChem Academy: ThermodynamicsHamit RanaNo ratings yet
- Bakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveDocument3 pagesBakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveRitesh SonawaneNo ratings yet
- Previous HSE Questions From The Chapter "Alcohols, Phenols and Ethers"Document3 pagesPrevious HSE Questions From The Chapter "Alcohols, Phenols and Ethers"alan ChackoNo ratings yet
- Goc Ques Bank IIT JEEDocument39 pagesGoc Ques Bank IIT JEEJyöt SîlvērNo ratings yet
- ALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestDocument6 pagesALDEHYDES, KETONES, ACIDS-01-170419: Neet-Crash-2017 Chemistry TestPoorvaBakshiNo ratings yet
- BOC Complete 1 To 5 DPPDocument5 pagesBOC Complete 1 To 5 DPPBhawna SharmaNo ratings yet
- D and F Block DPPDocument4 pagesD and F Block DPPKalyan ReddtNo ratings yet
- Anic Chemistry Carbonyl CompoundsDocument6 pagesAnic Chemistry Carbonyl Compoundseamcetmaterials100% (1)
- M-Caps-34: Chemistry: NEET & AIIMS 2018-19Document5 pagesM-Caps-34: Chemistry: NEET & AIIMS 2018-19Vishal SinghNo ratings yet
- Xicbse Che Haloalkanes Haloarenes 2 QPDocument3 pagesXicbse Che Haloalkanes Haloarenes 2 QPtanishkakannan3253No ratings yet
- Csir Model QuestionsDocument15 pagesCsir Model QuestionsbaluNo ratings yet
- Name ReactionsDocument32 pagesName ReactionsM.NandabalanNo ratings yet
- Act 7 Reducing Properties of Sugars 1Document3 pagesAct 7 Reducing Properties of Sugars 1Annamae Moleño PinedaNo ratings yet
- Name ReactionDocument15 pagesName Reactionnirbhay shukla100% (1)
- Kinetics of The Selective Hydrogenation of Phenol To Cyclohexanone Over A Pd-Alumina CatalystDocument8 pagesKinetics of The Selective Hydrogenation of Phenol To Cyclohexanone Over A Pd-Alumina CatalystTaylor PennaNo ratings yet
- Teknik Reaksi Kimia 1: Prepared By: Is Sulistyati PR, PHDDocument9 pagesTeknik Reaksi Kimia 1: Prepared By: Is Sulistyati PR, PHDMusya RofahNo ratings yet
- AspenPolymers+Vol1V7 3-UsrDocument554 pagesAspenPolymers+Vol1V7 3-UsrFirman Suryalaga Dikusuma100% (1)
- Modern Strategies For Heterocycle SynthesisDocument374 pagesModern Strategies For Heterocycle SynthesisKAREN DAYANNA BARBOSA CEPEDANo ratings yet
- Haloalkanes and Haloarenes. Set 1Document7 pagesHaloalkanes and Haloarenes. Set 1Achyuta GajurelNo ratings yet
- Chapter 4Document162 pagesChapter 4kaslana kianaNo ratings yet
- Solucionario PDFDocument34 pagesSolucionario PDFSergio Rugerio0% (1)
- TBOC FMOC Protocols in Peptide SynthesisDocument30 pagesTBOC FMOC Protocols in Peptide Synthesissantosh kumar sahoo100% (1)
- Rhodium Catalyzed Hydroformylation - CH 07Document14 pagesRhodium Catalyzed Hydroformylation - CH 07maildesantiagoNo ratings yet
- Chemical Kinetics ExercisesDocument2 pagesChemical Kinetics ExercisesBanana CrazyNo ratings yet
- III Sem - POC II - Question Bank-NewDocument3 pagesIII Sem - POC II - Question Bank-NewNirmal TomyNo ratings yet
- Combustion Chemistry PDFDocument2 pagesCombustion Chemistry PDFCynthiaNo ratings yet
- Chapter 4 - Isothermal Reactor Design PDFDocument38 pagesChapter 4 - Isothermal Reactor Design PDFKai Faha LukumNo ratings yet
- BH10Document87 pagesBH10Neel PatelNo ratings yet
- 3 PDFDocument6 pages3 PDFTysir SarhanNo ratings yet
- Preparation and Reactions of EthersDocument54 pagesPreparation and Reactions of EthersEj AgsaldaNo ratings yet
- CH CooDocument2 pagesCH CooPawan SharmaNo ratings yet
- Problem Set # 3Document4 pagesProblem Set # 3Lightning GeeNo ratings yet
- Chapter 1, Chemical KineticsDocument68 pagesChapter 1, Chemical KineticsTesfamariam Setargew MesfinNo ratings yet
- Sandwich StoichiometryDocument2 pagesSandwich StoichiometryMaysaa El HarakehNo ratings yet
- Modeling of A Radialflow Movingbed Reactor For Dehydrogenation of IsobutaneDocument7 pagesModeling of A Radialflow Movingbed Reactor For Dehydrogenation of IsobutaneForcus onNo ratings yet
- Factors Affecting Enzymes ActivityDocument11 pagesFactors Affecting Enzymes ActivityZubair AslamNo ratings yet
- 4571 Chap14 Catalysis IntroDocument14 pages4571 Chap14 Catalysis IntroSankar SasmalNo ratings yet
- Lecture2 Chapter1 Molebalancepart1Document42 pagesLecture2 Chapter1 Molebalancepart1Nur SafiahNo ratings yet
- The Rate Law For The Hydrolysis o Ethyl Acetate by Aqueous Sodium Hydroxide at 298 KDocument2 pagesThe Rate Law For The Hydrolysis o Ethyl Acetate by Aqueous Sodium Hydroxide at 298 KJosé Kaisor Angara100% (1)
- CRE - Catalyst FundamentalDocument17 pagesCRE - Catalyst Fundamentalandono kusuma jatiNo ratings yet