Professional Documents
Culture Documents
Relative Atomic Mass - Toni Vidal
Relative Atomic Mass - Toni Vidal
Uploaded by
Tuni VidalCopyright:
Available Formats
You might also like
- Chemistry 12th Edition Chang Solutions ManualDocument24 pagesChemistry 12th Edition Chang Solutions ManualChristianDavisijsn100% (52)
- Empirical Study DataDocument2 pagesEmpirical Study DataHadi Moheb-AlizadehNo ratings yet
- CHM ART Activity 4 Atoms and Isotopes PDFDocument4 pagesCHM ART Activity 4 Atoms and Isotopes PDFMark Vincent DoriaNo ratings yet
- App 1 Surayala FuelDocument15 pagesApp 1 Surayala FuelAldiarsoNo ratings yet
- Choice of Coal Vs Design Asb13 - 1-4Document51 pagesChoice of Coal Vs Design Asb13 - 1-4Jerry MateoNo ratings yet
- Tutor 2Document4 pagesTutor 2Nguyễn Minh KhuêNo ratings yet
- Data Kolom DistilasiDocument28 pagesData Kolom DistilasiM Ragil ZandisyahNo ratings yet
- Chapter 4Document19 pagesChapter 4Diệu ThiệnNo ratings yet
- ED 20220805-Tabel Nilai Kontrol HematologiDocument1 pageED 20220805-Tabel Nilai Kontrol HematologiAhmad Rahmadi AdheNo ratings yet
- Lead Alloy Calculators 082311Document6 pagesLead Alloy Calculators 082311Rafael Blancas ChaucaNo ratings yet
- AS Chemistry Atomic Structure WS 2Document4 pagesAS Chemistry Atomic Structure WS 2Malik Ammad AnjumNo ratings yet
- Cálculos Bbas - 13Document30 pagesCálculos Bbas - 13Cristian Riquelme ContrerasNo ratings yet
- Stoichiometry Atomic and Molecular Mass Worksheet - AnswerDocument3 pagesStoichiometry Atomic and Molecular Mass Worksheet - AnswerFoxy world 152No ratings yet
- Part 3Document152 pagesPart 3Nithin KumarNo ratings yet
- Chemistry Chapter 1 QPDocument10 pagesChemistry Chapter 1 QPAsif AyazNo ratings yet
- Lampiran A Contoh PerhitunganDocument10 pagesLampiran A Contoh PerhitunganIka Safitri RachmawatiNo ratings yet
- Activity 2Document1 pageActivity 2josemanuelambito16No ratings yet
- RESULT and DISCUSSIONDocument5 pagesRESULT and DISCUSSIONnisasoberiNo ratings yet
- Return Saham Bulan A B C rA-rA RB-RBDocument8 pagesReturn Saham Bulan A B C rA-rA RB-RBCitra ParwatiNo ratings yet
- CHEM 1411 Chapter 5 Homework AnswersDocument8 pagesCHEM 1411 Chapter 5 Homework AnswersGrothendieck Langlands ShtukasNo ratings yet
- Tabel Nilai Control Hematologi Beckman Coulter DXH 500: Abnormal LowDocument1 pageTabel Nilai Control Hematologi Beckman Coulter DXH 500: Abnormal LowAhmad Rahmadi AdheNo ratings yet
- Convert WT% To At% (And Reserve) in An Alloy-Ver.2Document13 pagesConvert WT% To At% (And Reserve) in An Alloy-Ver.2HanLe DuyNo ratings yet
- Alloy 12Document4 pagesAlloy 12lojatatticosNo ratings yet
- Reaksi Kimia LimestoneDocument6 pagesReaksi Kimia LimestoneRifka FadillahNo ratings yet
- Batch DistillationDocument12 pagesBatch DistillationBrennie GohNo ratings yet
- GasificaciónDocument27 pagesGasificaciónStiven SofanNo ratings yet
- Ball Mill SimulatorDocument4 pagesBall Mill SimulatorIrshad HussainNo ratings yet
- Experiment 2 SKF3013Document9 pagesExperiment 2 SKF3013Nurfariha SafarNo ratings yet
- Year X 1 5.6 2 2.7 Mode 3 7.3 Median 4 3.5 Mean 5 0.01 Variance Standard Deviation Skewness KurtosisDocument36 pagesYear X 1 5.6 2 2.7 Mode 3 7.3 Median 4 3.5 Mean 5 0.01 Variance Standard Deviation Skewness KurtosisauroralpearlNo ratings yet
- APPENDIX 2: Experimental Data: 2A Thermodynamic Data at 25°CDocument7 pagesAPPENDIX 2: Experimental Data: 2A Thermodynamic Data at 25°CJenny ZevallosNo ratings yet
- Coal NCV and GCVDocument5 pagesCoal NCV and GCVirfanNo ratings yet
- Syn CompressorDocument23 pagesSyn CompressorManish GautamNo ratings yet
- P R O D U C T O: Cabeza, TMS Cabeza, TMSDocument16 pagesP R O D U C T O: Cabeza, TMS Cabeza, TMSPaul Perez MatosNo ratings yet
- Atkins Appendix 2A Thermodynamic DataDocument7 pagesAtkins Appendix 2A Thermodynamic DataEric MñzNo ratings yet
- UntitledDocument2 pagesUntitledRahmat Nurul KhatamiNo ratings yet
- AF Ash IncorporationDocument7 pagesAF Ash Incorporationirfan100% (1)
- Pages From CBTL Final ReportDocument1 pagePages From CBTL Final ReportNrsPersonNo ratings yet
- Analisis MinsurDocument5 pagesAnalisis MinsurArce NiloNo ratings yet
- Sponge Iron Heat & Mass BalanceDocument33 pagesSponge Iron Heat & Mass BalanceNILESH JAIN100% (1)
- Isotope Practice QuestionsDocument5 pagesIsotope Practice QuestionsocNo ratings yet
- Aim: How To Calculate The Average Atomic Mass?: Do Now: (10 Min) Complete Worksheet FromDocument10 pagesAim: How To Calculate The Average Atomic Mass?: Do Now: (10 Min) Complete Worksheet FromMAYA SMITHNo ratings yet
- Grafcbn 12Document1 pageGrafcbn 12khoirul abidinNo ratings yet
- 2 How Atoms Differ 2022Document3 pages2 How Atoms Differ 2022alexandraNo ratings yet
- Black Hill Coking CoalDocument1 pageBlack Hill Coking Coalnaresh adusumilliNo ratings yet
- Atomic Structure WorksheetDocument2 pagesAtomic Structure Worksheetrangerblue94% (17)
- Bam m384b eDocument6 pagesBam m384b eterecuaNo ratings yet
- Nec B310-9Document2 pagesNec B310-9Mohamed MahrousNo ratings yet
- Geochemistry & Earth ProcessesDocument27 pagesGeochemistry & Earth ProcessesYoussef OuahziziNo ratings yet
- Random VariablesDocument12 pagesRandom VariablesChong Ray JieNo ratings yet
- Laporan Oos p4 Mei Jabar New FormatDocument520 pagesLaporan Oos p4 Mei Jabar New FormatRia Maria DjumhanaNo ratings yet
- OK AristoRod 12.50 SWR 10014-En US-FactSheet Main-01Document4 pagesOK AristoRod 12.50 SWR 10014-En US-FactSheet Main-01Poltak SianiparNo ratings yet
- 1 Balance MetalurgicoDocument15 pages1 Balance MetalurgicoMAYERNo ratings yet
- Libro 1Document4 pagesLibro 1Michael ParraviciniNo ratings yet
- Bimetallic ZN and HF On Silica Catalysts For The Conversion of Ethanol To 1,3-ButadieneDocument10 pagesBimetallic ZN and HF On Silica Catalysts For The Conversion of Ethanol To 1,3-ButadieneTalitha AdhyaksantiNo ratings yet
- Source of Mineral Premix: No Ingredient Source Price DM Ash Ca P Na CL K FeDocument8 pagesSource of Mineral Premix: No Ingredient Source Price DM Ash Ca P Na CL K FeAndi Ummul KhairNo ratings yet
- Tabel Dasar Teori Praktikum Utilitas PDFDocument3 pagesTabel Dasar Teori Praktikum Utilitas PDFBima FernandoNo ratings yet
- Interpretation of MS-MS Mass Spectra of Drugs and PesticidesFrom EverandInterpretation of MS-MS Mass Spectra of Drugs and PesticidesNo ratings yet
- Radioactive Isotopes UsesDocument10 pagesRadioactive Isotopes UsesTuni VidalNo ratings yet
- Ionic and Covalent Bonding WorksheetDocument5 pagesIonic and Covalent Bonding WorksheetTuni VidalNo ratings yet
- Chemistry FormulasDocument1 pageChemistry FormulasTuni VidalNo ratings yet
- Tonvid ExcretionDocument2 pagesTonvid ExcretionTuni VidalNo ratings yet
- Endocrine SystemDocument2 pagesEndocrine SystemTuni VidalNo ratings yet
- Solubility LabDocument4 pagesSolubility LabTuni VidalNo ratings yet
Relative Atomic Mass - Toni Vidal
Relative Atomic Mass - Toni Vidal
Uploaded by
Tuni VidalOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Relative Atomic Mass - Toni Vidal
Relative Atomic Mass - Toni Vidal
Uploaded by
Tuni VidalCopyright:
Available Formats
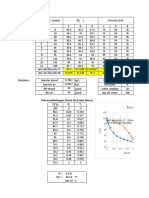
Name: _____________________________
RELATIVE ATOMIC MASS
The relative atomic mass (Ar) of atoms is the average mass of all the different isotopes of an element (taking into
12
account the amount of each isotope) on a scale where C atoms have a mass of exactly 12.
Imagine you have 90 balls with mass 200 g, and 10 balls with mass 300 g. The average mass of the balls is
given by:
Average mass of balls = total mass of all the balls = [ (90 x 200) + (10 x 300) ] = 21000 = 210 g
total number of balls 90 + 10 100
The relative atomic mass of atoms is worked out in a similar way:
Relative atomic mass (Ar) = total mass of all atoms
total number of atoms
Element Isotopes Abundance Relative atomic mass (Ar) (to 3sf)
Chlorine 35 75.8%
17Cl
37 75.8 + 24.2 100
17Cl 24.2%
Lithium 6
3Li 7.6%
7
3Li 92.4%
Bromine 79 50.7%
35Br
81 49.3%
35Br
Copper 63
29Cu 69.2%
65 30.8%
29Cu
Fluorine 19
9F 100.0%
Magnesium 24
12Mg 79.0%
25 10.0%
12Mg
26 11.0%
12Mg
Iron 54 5.8%
26Fe
56 91.8%
26Fe
57 2.1%
26Fe
58 0.3%
26Fe
Krypton 78
36Kr 0.4%
80
36Kr 2.3%
82
36Kr 11.6%
83
36Kr 11.5%
84
36Kr 57.0%
85 17.3%
36Kr
You might also like
- Chemistry 12th Edition Chang Solutions ManualDocument24 pagesChemistry 12th Edition Chang Solutions ManualChristianDavisijsn100% (52)
- Empirical Study DataDocument2 pagesEmpirical Study DataHadi Moheb-AlizadehNo ratings yet
- CHM ART Activity 4 Atoms and Isotopes PDFDocument4 pagesCHM ART Activity 4 Atoms and Isotopes PDFMark Vincent DoriaNo ratings yet
- App 1 Surayala FuelDocument15 pagesApp 1 Surayala FuelAldiarsoNo ratings yet
- Choice of Coal Vs Design Asb13 - 1-4Document51 pagesChoice of Coal Vs Design Asb13 - 1-4Jerry MateoNo ratings yet
- Tutor 2Document4 pagesTutor 2Nguyễn Minh KhuêNo ratings yet
- Data Kolom DistilasiDocument28 pagesData Kolom DistilasiM Ragil ZandisyahNo ratings yet
- Chapter 4Document19 pagesChapter 4Diệu ThiệnNo ratings yet
- ED 20220805-Tabel Nilai Kontrol HematologiDocument1 pageED 20220805-Tabel Nilai Kontrol HematologiAhmad Rahmadi AdheNo ratings yet
- Lead Alloy Calculators 082311Document6 pagesLead Alloy Calculators 082311Rafael Blancas ChaucaNo ratings yet
- AS Chemistry Atomic Structure WS 2Document4 pagesAS Chemistry Atomic Structure WS 2Malik Ammad AnjumNo ratings yet
- Cálculos Bbas - 13Document30 pagesCálculos Bbas - 13Cristian Riquelme ContrerasNo ratings yet
- Stoichiometry Atomic and Molecular Mass Worksheet - AnswerDocument3 pagesStoichiometry Atomic and Molecular Mass Worksheet - AnswerFoxy world 152No ratings yet
- Part 3Document152 pagesPart 3Nithin KumarNo ratings yet
- Chemistry Chapter 1 QPDocument10 pagesChemistry Chapter 1 QPAsif AyazNo ratings yet
- Lampiran A Contoh PerhitunganDocument10 pagesLampiran A Contoh PerhitunganIka Safitri RachmawatiNo ratings yet
- Activity 2Document1 pageActivity 2josemanuelambito16No ratings yet
- RESULT and DISCUSSIONDocument5 pagesRESULT and DISCUSSIONnisasoberiNo ratings yet
- Return Saham Bulan A B C rA-rA RB-RBDocument8 pagesReturn Saham Bulan A B C rA-rA RB-RBCitra ParwatiNo ratings yet
- CHEM 1411 Chapter 5 Homework AnswersDocument8 pagesCHEM 1411 Chapter 5 Homework AnswersGrothendieck Langlands ShtukasNo ratings yet
- Tabel Nilai Control Hematologi Beckman Coulter DXH 500: Abnormal LowDocument1 pageTabel Nilai Control Hematologi Beckman Coulter DXH 500: Abnormal LowAhmad Rahmadi AdheNo ratings yet
- Convert WT% To At% (And Reserve) in An Alloy-Ver.2Document13 pagesConvert WT% To At% (And Reserve) in An Alloy-Ver.2HanLe DuyNo ratings yet
- Alloy 12Document4 pagesAlloy 12lojatatticosNo ratings yet
- Reaksi Kimia LimestoneDocument6 pagesReaksi Kimia LimestoneRifka FadillahNo ratings yet
- Batch DistillationDocument12 pagesBatch DistillationBrennie GohNo ratings yet
- GasificaciónDocument27 pagesGasificaciónStiven SofanNo ratings yet
- Ball Mill SimulatorDocument4 pagesBall Mill SimulatorIrshad HussainNo ratings yet
- Experiment 2 SKF3013Document9 pagesExperiment 2 SKF3013Nurfariha SafarNo ratings yet
- Year X 1 5.6 2 2.7 Mode 3 7.3 Median 4 3.5 Mean 5 0.01 Variance Standard Deviation Skewness KurtosisDocument36 pagesYear X 1 5.6 2 2.7 Mode 3 7.3 Median 4 3.5 Mean 5 0.01 Variance Standard Deviation Skewness KurtosisauroralpearlNo ratings yet
- APPENDIX 2: Experimental Data: 2A Thermodynamic Data at 25°CDocument7 pagesAPPENDIX 2: Experimental Data: 2A Thermodynamic Data at 25°CJenny ZevallosNo ratings yet
- Coal NCV and GCVDocument5 pagesCoal NCV and GCVirfanNo ratings yet
- Syn CompressorDocument23 pagesSyn CompressorManish GautamNo ratings yet
- P R O D U C T O: Cabeza, TMS Cabeza, TMSDocument16 pagesP R O D U C T O: Cabeza, TMS Cabeza, TMSPaul Perez MatosNo ratings yet
- Atkins Appendix 2A Thermodynamic DataDocument7 pagesAtkins Appendix 2A Thermodynamic DataEric MñzNo ratings yet
- UntitledDocument2 pagesUntitledRahmat Nurul KhatamiNo ratings yet
- AF Ash IncorporationDocument7 pagesAF Ash Incorporationirfan100% (1)
- Pages From CBTL Final ReportDocument1 pagePages From CBTL Final ReportNrsPersonNo ratings yet
- Analisis MinsurDocument5 pagesAnalisis MinsurArce NiloNo ratings yet
- Sponge Iron Heat & Mass BalanceDocument33 pagesSponge Iron Heat & Mass BalanceNILESH JAIN100% (1)
- Isotope Practice QuestionsDocument5 pagesIsotope Practice QuestionsocNo ratings yet
- Aim: How To Calculate The Average Atomic Mass?: Do Now: (10 Min) Complete Worksheet FromDocument10 pagesAim: How To Calculate The Average Atomic Mass?: Do Now: (10 Min) Complete Worksheet FromMAYA SMITHNo ratings yet
- Grafcbn 12Document1 pageGrafcbn 12khoirul abidinNo ratings yet
- 2 How Atoms Differ 2022Document3 pages2 How Atoms Differ 2022alexandraNo ratings yet
- Black Hill Coking CoalDocument1 pageBlack Hill Coking Coalnaresh adusumilliNo ratings yet
- Atomic Structure WorksheetDocument2 pagesAtomic Structure Worksheetrangerblue94% (17)
- Bam m384b eDocument6 pagesBam m384b eterecuaNo ratings yet
- Nec B310-9Document2 pagesNec B310-9Mohamed MahrousNo ratings yet
- Geochemistry & Earth ProcessesDocument27 pagesGeochemistry & Earth ProcessesYoussef OuahziziNo ratings yet
- Random VariablesDocument12 pagesRandom VariablesChong Ray JieNo ratings yet
- Laporan Oos p4 Mei Jabar New FormatDocument520 pagesLaporan Oos p4 Mei Jabar New FormatRia Maria DjumhanaNo ratings yet
- OK AristoRod 12.50 SWR 10014-En US-FactSheet Main-01Document4 pagesOK AristoRod 12.50 SWR 10014-En US-FactSheet Main-01Poltak SianiparNo ratings yet
- 1 Balance MetalurgicoDocument15 pages1 Balance MetalurgicoMAYERNo ratings yet
- Libro 1Document4 pagesLibro 1Michael ParraviciniNo ratings yet
- Bimetallic ZN and HF On Silica Catalysts For The Conversion of Ethanol To 1,3-ButadieneDocument10 pagesBimetallic ZN and HF On Silica Catalysts For The Conversion of Ethanol To 1,3-ButadieneTalitha AdhyaksantiNo ratings yet
- Source of Mineral Premix: No Ingredient Source Price DM Ash Ca P Na CL K FeDocument8 pagesSource of Mineral Premix: No Ingredient Source Price DM Ash Ca P Na CL K FeAndi Ummul KhairNo ratings yet
- Tabel Dasar Teori Praktikum Utilitas PDFDocument3 pagesTabel Dasar Teori Praktikum Utilitas PDFBima FernandoNo ratings yet
- Interpretation of MS-MS Mass Spectra of Drugs and PesticidesFrom EverandInterpretation of MS-MS Mass Spectra of Drugs and PesticidesNo ratings yet
- Radioactive Isotopes UsesDocument10 pagesRadioactive Isotopes UsesTuni VidalNo ratings yet
- Ionic and Covalent Bonding WorksheetDocument5 pagesIonic and Covalent Bonding WorksheetTuni VidalNo ratings yet
- Chemistry FormulasDocument1 pageChemistry FormulasTuni VidalNo ratings yet
- Tonvid ExcretionDocument2 pagesTonvid ExcretionTuni VidalNo ratings yet
- Endocrine SystemDocument2 pagesEndocrine SystemTuni VidalNo ratings yet
- Solubility LabDocument4 pagesSolubility LabTuni VidalNo ratings yet