Professional Documents
Culture Documents
A Review of Lumbar Radiculopathy, Diagnosis, and Treatment
A Review of Lumbar Radiculopathy, Diagnosis, and Treatment
Uploaded by
Matthew PhillipsOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
A Review of Lumbar Radiculopathy, Diagnosis, and Treatment
A Review of Lumbar Radiculopathy, Diagnosis, and Treatment
Uploaded by
Matthew PhillipsCopyright:
Available Formats
Open Access Review
Article DOI: 10.7759/cureus.5934
A Review of Lumbar Radiculopathy,
Diagnosis, and Treatment
James A. Berry 1 , Christopher Elia 1 , Harneel S. Saini 2 , Dan E. Miulli 1
1. Neurosurgery, Riverside University Health System Medical Center, Moreno Valley, USA 2. Neurology,
Alleghany Health Network, Pittsburgh, USA
Corresponding author: James A. Berry, jamesberrydo@gmail.com
Disclosures can be found in Additional Information at the end of the article
Abstract
We review the epidemiology, etiology, symptomatology, clinical presentation, anatomy,
pathophysiology, workup, diagnosis, non-surgical and surgical management, postoperative
care, outcomes, long-term management, and morbidity of lumbar radiculopathy. We review
when outpatient conservative management is appropriate and "red flag" warning symptoms
that would necessitate an emergency evaluation. Diagnostic modalities, including magnetic
resonance imaging (MRI), computerized tomography (CT), contrast myelogram,
electromyogram (EMG), and nerve conduction velocity (NCV), are involved in the diagnosis and
decision-making are discussed. Treatment of lumbar radiculopathy requires a multimodal and
multispecialty team. We review indications for the involvement of other professionals,
including physical therapy (PT), occupational therapy (OT), physical and rehabilitation
medicine (PMR), and pain management.
Categories: Neurosurgery, Orthopedics, Pain Management
Keywords: lumbar radiculopathy, spine neurosurgery, lumbar spine
Introduction And Background
Lumbar radiculopathy is one of the most common complaints evaluated by a spine surgeon. Its
prevalence has been estimated to be 3%-5% of the population, affecting both men and
women. Age is a primary risk factor, as it occurs secondary to the degenerative process within
the spinal column. Symptoms typically begin in midlife, with men often affected in the
40s while women are affected in the 50s and 60s [1-2]. Females have a higher risk in certain
populations, with physically demanding careers such as service in the military. In the general
population, there is a male preponderance [3]. Degenerative spondyloarthropathies are the
primary cause of lumbar radiculopathy [1]. Patients commonly present with back pain that is
associated with their radiculopathy. By definition, radiculopathy describes pain that radiates
Received 04/29/2019
down the legs and is often described by patients as electric, burning, or sharp. The most
Review began 09/11/2019
Review ended 09/20/2019 common underlying cause of radiculopathy is irritation of a particular nerve, which can occur
Published 10/17/2019 at any point along the nerve itself and is most often a result of a compressive force. In the case
of lumbar radiculopathy, this compressive force may occur within the thecal sac, as the nerve
© Copyright 2019
Berry et al. This is an open access root exits the thecal sac within the lateral recess, as the nerve root traverses the neural
article distributed under the terms of foramina or even after the nerve root as exited the foramina. It may be related to disc bulging or
the Creative Commons Attribution herniation, facet or ligamentous hypertrophy, spondylolisthesis, or even neoplastic and
License CC-BY 3.0., which permits
infectious processes. The diagnosis of the causative agent and subsequent treatment starts with
unrestricted use, distribution, and
reproduction in any medium, provided
a thorough physical exam.
the original author and source are
credited.
Review
How to cite this article
Berry J A, Elia C, Saini H S, et al. (October 17, 2019) A Review of Lumbar Radiculopathy, Diagnosis, and
Treatment. Cureus 11(10): e5934. DOI 10.7759/cureus.5934
Diagnosis
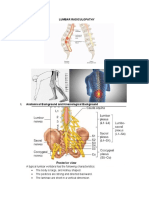
The initial exam should include a complete history and physical exam, including manual muscle
testing, sensory testing, deep tendon reflexes, and Lasegue’s sign [4]. Lasegue’s sign is assessed
with the patient lying in the supine position, the knee extended, the ankle dorsiflexed, and the
cervical spine flexed. The examiner lifts the patient’s lower extremity off the table towards 90
degrees, which will elicit radicular pain as the nerve root is stretched. Classically, when
radiculopathy is caused by nerve root compression pain, sensory loss occurs in a dermatomal
pattern (Figure 1). Motor loss may occur in a myotomal pattern (Table 1). The distribution of
pain and motor findings on physical exam should guide the neurosurgeon to the region of the
spine to focus on, with further modalities such as MRI and electrodiagnostic testing.
FIGURE 1: Dermatomes
Anatomical map of the sensory dermatomes of the Lumbosacraloccygeal region
Image provided by the National University of Córdoba with permission for use.
2019 Berry et al. Cureus 11(10): e5934. DOI 10.7759/cureus.5934 2 of 7
Spinal Nerve Myotome
L2 Hip Flexion Iliopsoas
L3 Knee Extension
L4 Ankle Dorsiflexion Tibialis Anterior
L5 Ankle Eversion (peronous longus and brevis) Great Toe Extension Extensor Hallucis Longus
S1 Plantar Flexion Gastrocnemius, Soleus
TABLE 1: Lumbosacral myotomes
Anatomical distribution of lumbosacral myotomes
After a thorough physical exam, diagnostic imaging should be reviewed. The optimal imaging
modality for the evaluation of radiculopathy is MRI of the lumbar spine without contrast, which
can show compression of the nerve root (see Figure 2). Contrast-enhanced MRI may be useful or
indicated in cases where a tumor, infection, or prior surgery has occurred. In cases where MRI is
not available or possible, a CT myelogram is a reasonable alternative.
FIGURE 2: A. T2 sagittal MRI of the lumbar spine w/o contrast
B. Axial MRI of the lumbar spine w/o contrast
A. Sagittal T2 w/o contrast MRI lumbar spine shows a large 9 mm L5/S1 paracentral disc protrusion
with mass effect on the thecal sac.
B. Axial T2 w/o contrast MRI lumbar spine; the same patient shows compression of the right exiting
S1 nerve root, which has caused this patient to experience right S1 radiculopathy.
Clinical Pearl
Ensure that you evaluate the MRI with the patient’s clinical exam in mind. Often, a far lateral
2019 Berry et al. Cureus 11(10): e5934. DOI 10.7759/cureus.5934 3 of 7
disc herniation can be missed when not specifically looking for it. MRI is a triplanar modality
that necessitates utilizing the axial, sagittal, and coronal sequences. The sagittal sequences can
demonstrate far lateral disc herniations with the foramina. The coronal sequence shows nerve
roots and foraminal and extraforaminal regions where a far lateral disc herniation occurs.
When there is a lack of correlation between the exam findings and imaging studies,
electrodiagnostic testing may be employed. Electromyography (EMG) and nerve conduction
velocities (NCV), as well as somatosensory evoked potentials (SSEP), can help differentiate
between radiculopathy and more diffuse disorders of the peripheral nervous system. It is
important to note that while EMG and NCV studies may be a useful diagnostic tool when
combined with a thorough history, clinical examination, and other diagnostic studies, they do
have limitations and potential pitfalls. EMG and NCV studies are affected by the patients’ level
of cooperation, which may be limited by pain, temperature of room, electrolyte and fluid
balance, pre-existing medical comorbidities, such as diabetes mellitus, thyroid disease, or renal
failure that can produce peripheral neuropathy, medications such as statins, which can produce
myopathy, movement disorders that produce tremors, prior surgeries, such as laminectomy,
which may give paraspinous muscle false-positives, body habitus with extreme obesity
preventing the full insertion of needles into muscle, congenital anatomical variations, for
example, Martin-Gruber nerve anastomosis, and subjective interpretation of the data by the
individual clinician [5]. Diagnostic nerve root blocks may also help to localize the symptomatic
level [6].
Treatment
Non-Surgical
The need for surgical intervention, the timing of surgery, and surgical approaches has been
extensively studied, yet, controversy still exists. Guidelines for approaching lumbar
radiculopathy favor an initial trial of conservative management, including patient education,
staying active/exercise, manual therapy (such as McKenzie exercises), and non-steroidal anti-
inflammatory drugs (NSAIDs) as first-line treatments [7-9]. The use of McKenzie exercises has
been demonstrated to provide some acute symptomatic relief in patients undergoing
conservative management for lumbar radiculopathy [10]. Oral corticosteroids prescribed as a
taper may benefit patients in the acute phase [11]. Often, the next step in treatment is pain
injections, which may include epidural steroid injections, facet injection, or transforaminal
injections, which have been shown to provide long-term relief of symptoms [12]. These
injections typically consist of a combination of an anti-inflammatory agent, such as a
glucocorticoid, and a long-lasting anesthetic such as Marcaine. In situations where the pain
generator is indeterminate, spinal injections can be both diagnostic and therapeutic. For
example, a patient with significantly low back pain and some numbness in her left foot who has
extensive arthritic changes throughout her spinal column receives an injection in her facets
with profound symptomatic relief. The injection into her lumbar spinal facet joints provided her
with significant symptomatic relief, demonstrating that arthritic changes in her facet joints,
not lumbar radiculopathy from a compressed nerve root, were her pain generator. However, a
patient with significant lower extremity pain in an L5 dermatomal pattern experiences
significant relief after an epidural injection, indicating the pain generator is likely the
compressed left L5 nerve root, not arthritic changes in the facet joints.
Surgical Decision-Making
When conservative treatments fail to provide symptom relief, surgical intervention is
considered. The timing between surgery and when conservative measures can be designated as
failed therapy typically ranges between four and eight weeks [13]. However, the question as to
who would benefit from surgery and who should continue conservative therapy is debatable.
2019 Berry et al. Cureus 11(10): e5934. DOI 10.7759/cureus.5934 4 of 7
The Spine Patient Outcomes Research Trial (SPORT) trail attempted to answer this question
[14]. It evaluated 501 patients with herniated lumbar discs and compared surgical vs. non-
surgical treatments. The primary outcome being SF-36, which is a benefit-cost ratio of lumbar
fusion in comparison to other surgical Interventions and Oswestry Disability Index (ODI) scores
at specific intervals. In the end, it found that both the surgery and the non-operative treatment
groups improved substantially over a two-year period, with improvements consistently in favor
of surgery for all periods but that were small and not statistically significant [14].
A significant contribution from this study was that most patients improve given time, either
with or without surgery. At eight years after symptom onset, those patients who benefited most
from a surgical intervention were patients with sequestered disc fragments, symptom duration
of greater than six months, those with higher levels of low back pain, or who were neither
working nor disabled at baseline [4].
The ultimate timing of surgery is often based on the severity of the patient’s symptoms and
clinical experience. Overall, surgery has been shown to be of benefit to patients with more
severe symptoms [15].
Surgical Techniques
The gold standard surgical procedure for simple lumbar disc herniation remains a discectomy.
In 1939, Semmes presented a subtotal laminectomy and retraction of the dural sac to remove
the herniated disc [16]. Since then, many iterations of this procedure focussing on less invasive
techniques have been developed. In 1977 and 1978, Caspar and Williams reported refinements
in the approach with the use of a microsurgical technique [17]. In 1997, Foley introduced the
microendoscopic discectomy (MED) procedure [18-19].
Surgical options include open laminectomy with discectomy, the so-called “mini-open”
hemilaminectomy with a microdiscectomy, minimally invasive hemilaminectomy with
microdiscectomy via tubular retractors, and MED. Studies have shown MED to be superior to
open surgical techniques in producing less irritation of the nerve by intraoperative EMG studies
[20], less requirement of postoperative analgesia during the hospital stay, less mean operative
blood loss, and a lower mean number of rest days [21-22]. Less invasive methods may also
produce less joint destabilization due to less destructive techniques as well as decreased
surgical and hospital costs [22]. Minimally invasive techniques are not without limitations such
as a restricted cone field of vision for the surgeon and inability to approach pathology from
other angles. Minimally invasive techniques may be appropriate under the correct conditions
and should be evaluated on a case-by-case basis.
For traditional open discectomy, localization of the level is first obtained with fluoroscopy and
a midline incision is made at the level of the disc. The incision is then continued down in a
subperiosteal fashion to expose the lamina of the upper level and ligamentum flavum over the
interspace laterally. A retractor is then placed. The microscope is then brought in and a
hemilaminectomy and partial medial facetectomy is performed with a high-speed drill and
Kerrison Roungeurs. The ligamentum is then detached from the lamina and removed, exposing
the nerve root crossing over the disc. The root and thecal sac are retracted medially and the
annulus exposed. A box incision in the disc annulus is made and disc material removed. A
nerve hook can be used to sweep anterior to the thecal sac to retrieve any herniated fragments.
Loose fragments within the disc space can be flushed out from the disc space with
irrigation. The advantages of this approach are increased visualization, the ability to use a
wider variety of instruments, better visualization, and the ability to approach pathology from
multiple trajectories not limited to a specific trajectory such as minimally invasive surgery
(MIS) approaches.
2019 Berry et al. Cureus 11(10): e5934. DOI 10.7759/cureus.5934 5 of 7
For a minimally invasive (MIS) approach, a paramedian incision 2 centimeters off the midline is
made. A small stab incision is made in the lumbodorsal fascia and a K-wire or initial tubular
retractor is docked on the facet joint at the level of the disc space. A muscle splitting approach
is utilized by the introduction of a series of sequential dilators. The microscopic is then brought
in or in the case of MED, a rigid endoscope is inserted. The laminar facet junction is visualized
and laminotomy, medial facetectomy, and microdiscectomy are performed in a similar fashion
to the open technique.
Clinical Pearl
When using the MIS approach, it is essential to direct the trajectory of the tube perpendicular
to the disc of interest. An altered trajectory will limit the ability to fully visualize and surgically
decompress this nerve root. Confirm the trajectory with fluoroscopy.
Conclusions
Lumbar radiculopathy is one of the most common neurological complaints to be evaluated by a
neurosurgeon practicing in a rural environment. While the pathology has not changed, newer,
less invasive techniques are being developed to surgically treat these patients in the evolving
field of spine surgery. Intimate knowledge of the signs, symptoms, red-flag warning signs,
radiographic imaging, diagnostic tools, and conservative and surgical interventions is a
necessity. The red-flag warning signs that would prompt an emergent evaluation include saddle
anesthesia, incontinence to bowel or bladder, and sudden paresis in an extremity.
Additional Information
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors
declare the following: Payment/services info: All authors have declared that no financial
support was received from any organization for the submitted work. Financial relationships:
All authors have declared that they have no financial relationships at present or within the
previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or
activities that could appear to have influenced the submitted work.
References
1. Tarulli A, Raynor EM: Lumbosacral radiculopathy. Neurol Clin. 2007, 25:387-405.
10.1016/j.ncl.2007.01.008
2. Schoenfeld A; Laughlin M; Bader J, Bono C: Characterization of the incidence and risk factors
for the development of lumbar radiculopathy. J Spinal Disord Tech. 2012, 25:163-167.
10.1097/BSD.0b013e3182146e55
3. Jordon J, Konstantinou K, O'Dowd J: Herniated lumbar disc. BMJ Clin Evid. 2009, 26:1118.
4. Kreiner DS, Hwang SW, Easa EJ, et al.: An evidence-based clinical guideline for the diagnosis
and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014, 14:180-191.
10.1016/j.spinee.2013.08.003
5. Gutman L: Pearls and pitfalls in the use of electromyography and nerve conduction studies .
Semin Neurol. 2003, 23:77-82. 10.1055/s-2003-40754
6. Mondelli M, Aretini A, Arrigucci U, Ginanneschi F, Greco G, Sicurelli F: Clinical findings and
electrodiagnostic testing in 108 consecutive cases of lumbosacral radiculopathy due to
herniated disc [Article in French]. Neurophysiol Clin. 2013, 43:205-215.
10.1016/j.neucli.2013.05.004
7. Stochkendahl, Per Kajer, Hartivsen J, et al.: National Clinical Guidelines for non-surgical
treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J.
2017, 27:12-42. 10.1007/s00586-017-5099-2
2019 Berry et al. Cureus 11(10): e5934. DOI 10.7759/cureus.5934 6 of 7
8. Wong JJ, Côté P, Sutton DA, et al.: Clinical practice guidelines for the noninvasive
management of low back pain: a systematic review by the Ontario Protocol for Traffic Injury
Management (OPTIMa) Collaboration. Eur J Pain. Pain, 21:201-216. 10.1002/ejp.931
9. Machado LA, Maher C, Herbert E, Clare H, McAuley J: The effectiveness of the McKenzie
method in addition to first-line care for acute low back pain: a randomized controlled trial.
BMC Med. 2010, 8:8-10. 10.1186/1741-7015-8-10
10. Halliday MH, Pappas E, Hancock MJ, Clare HA, Pinto RZ, Robertson G, Ferreira PH: A
randomized controlled trial comparing the McKenzie method to motor control exercises in
people with chronic low back pain and a directional preference. J Orthop Sports Phys Ther.
2016, 46:514-522. 10.2519/jospt.2016.6379
11. Ko S, Sungguk K, Jaejung K, Oh T: The effectiveness of oral corticosteroids for management of
lumbar radiating pain: randomized, controlled trial study. Clin Orthop Surg. 2016, 8:262-267.
10.4055/cios.2016.8.3.262
12. Abdi S, Datta S, Trescot AM, et al.: Epidural steroids in the management of chronic spinal
pain: a systematic review. Pain Physician. 2007, 10:185-212.
13. Alentado VJ, Lubelski D, Steinmetz M, Benzel EC, Mroz TE: Optimal duration of conservative
management prior to surgery for cervical and lumbar radiculopathy: a literature review.
Global Spine J. 2014, 4:279-286. 10.1055/s-0034-1387807
14. Weinstein JN, Tosteson T, Lurie J, et al.: Surgical vs nonoperative treatment for lumbar disk
herniation. The Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA.
2006, 296:2441-2450.
15. Williams DK, Davcis M, Richard V, et al.: Low back pain disorders intervertebral disc .
Campbell’s Operative Orthopaedics, 11th Edition. Davis (ed): Mosby/Elsevier, Philadelphia,
PA; 2008. 2210-2212.
16. Palmer S: Use of a tubular retractor system in microscopic lumbar discectomy: 1 year
prospective results in 135 patients. Neurosurg Focus. 2002, 13:2-5. 10.3171/foc.2002.13.2.6
17. Kulkarni G, Bassi A, Dhruv A: Microendoscopic lumbar discectomy: technique and results of
188 cases. Indian J Orthop. 2014, 48:81-87. 10.4103/0019-5413.125511
18. Smith MW, Zhuang S, Mao Z, Chen H: Microendoscopic discectomy for lumbar disc herniation
surgical technique and outcome in 873 consecutive cases. Spine. 2006, 31:289-294.
19. Lawrence MM, Hayek SM: Minimally invasive lumbar decompression: a treatment for lumbar
spinal stenosis. Curr Opin Anaesthesiol. 2013, 5:573-579.
10.1097/01.aco.0000432520.24210.54
20. Brayda-Bruno M, Cinnella P: Posterior endoscopic discectomy (and other procedures) . Eur
Spine J. 2000, 9:24-29. 10.1007/pl00010018
21. Joaquim A, Botelho R, Mudo M, Silvinato A: Lumbar herniated disc - endoscopic discectomy
treatment. Rev Assoc Med Bras. 2018, 64:397-407. 10.1590/1806-9282.64.05.397
22. Asgarzadie F, Khoo T: Spinal stenosis: overview of early and long-term outcomes . Orthop Clin
North Am. 2007, 38:387-399. 10.1016/j.ocl.2007.02.006
2019 Berry et al. Cureus 11(10): e5934. DOI 10.7759/cureus.5934 7 of 7
You might also like
- Best of Five MCQs For The Rheumatology SCEDocument225 pagesBest of Five MCQs For The Rheumatology SCElittle lulu100% (2)
- MTRH I.Med ProtocolsDocument42 pagesMTRH I.Med ProtocolsRoberto Maina100% (3)
- Justice in Oral Health CareDocument371 pagesJustice in Oral Health CareSara Maria Menck SangiorgioNo ratings yet
- ISTDP Coughlin KatzmanDocument4 pagesISTDP Coughlin Katzmanmilane84No ratings yet
- A Review of Lumbar Radiculopathy, Diagnosis, and TreatmentDocument7 pagesA Review of Lumbar Radiculopathy, Diagnosis, and TreatmentZuraidaNo ratings yet
- Original Paper: Lumbar Disk Herniations - Clinical Status, Diagnosis, Imaging, Surgical Treatment and Global OutcomeDocument6 pagesOriginal Paper: Lumbar Disk Herniations - Clinical Status, Diagnosis, Imaging, Surgical Treatment and Global Outcomeasep budiyantoNo ratings yet
- Nursing Care of The Patient Undergoing Lumbar Spinal Fusion: ReviewsDocument10 pagesNursing Care of The Patient Undergoing Lumbar Spinal Fusion: ReviewsMuammar100% (1)
- Case Report: Coexistence of Ankylosing Spondylitis and Neurofibromatosis Type 1Document4 pagesCase Report: Coexistence of Ankylosing Spondylitis and Neurofibromatosis Type 1Lupita Mora LobacoNo ratings yet
- On The Brain PDFDocument6 pagesOn The Brain PDFNezrin BaxisliNo ratings yet
- 2011 Article 9218Document8 pages2011 Article 9218RenaldiPrimaSaputraNo ratings yet
- 2011 Article 9218 PDFDocument8 pages2011 Article 9218 PDFAgnesya GunawanNo ratings yet
- Vertebral Fractures: Clinical PracticeDocument9 pagesVertebral Fractures: Clinical PracticeIndraYudhiNo ratings yet
- Case StudyDocument15 pagesCase StudyWincy Faith SalazarNo ratings yet
- PR Ujian Neuro AuliaDocument10 pagesPR Ujian Neuro AuliaAULIA ANGRAININo ratings yet
- Backache Sciatica: The Practical Treatment of - andDocument114 pagesBackache Sciatica: The Practical Treatment of - andsujit kumarNo ratings yet
- MRI Evaluation of Lumbar Disc Degenerative Disease: Abst TDocument6 pagesMRI Evaluation of Lumbar Disc Degenerative Disease: Abst TDevi NugrahaNo ratings yet
- Imaging in RadiculopathyDocument17 pagesImaging in RadiculopathyFerdy DamaraNo ratings yet
- EpicondilteDocument7 pagesEpicondilteRicardo fariaNo ratings yet
- Facet Joint SyndromeDocument17 pagesFacet Joint Syndromepaulvaso27100% (1)
- Non-Specific Neck Pain and Evidence-Based Practice: Giannoula Tsakitzidis, PT Roy Remmen, MD, PHDDocument19 pagesNon-Specific Neck Pain and Evidence-Based Practice: Giannoula Tsakitzidis, PT Roy Remmen, MD, PHDLuis AndradeNo ratings yet
- Pathologic Basis of Lumbar Radicular Pain: BackgroundDocument8 pagesPathologic Basis of Lumbar Radicular Pain: BackgroundVizaNo ratings yet
- Management of Degenerative Lumbar Spinal Stenosis in The ElderlyDocument7 pagesManagement of Degenerative Lumbar Spinal Stenosis in The ElderlySandroNo ratings yet
- Lower Back PainDocument9 pagesLower Back PainKrishelle Kate PannigNo ratings yet
- 1 s2.0 S2255497115302111 MainDocument6 pages1 s2.0 S2255497115302111 MainkkkjaegiNo ratings yet
- Lumbar Radiculopathy Medback Castillo Mendez EDITEDDocument12 pagesLumbar Radiculopathy Medback Castillo Mendez EDITEDSteve ColbertNo ratings yet
- 2011 Article 9218Document8 pages2011 Article 9218E=MC2No ratings yet
- Lateral EpicondilitisDocument6 pagesLateral EpicondilitisJacks CanalsNo ratings yet
- IndianJClinAnaesth 9 3 374 378Document5 pagesIndianJClinAnaesth 9 3 374 378bhaskaracharya dontabhaktuniNo ratings yet
- Lumbosacral Radiculopathy - StatPearls - NCBI BookshelfDocument5 pagesLumbosacral Radiculopathy - StatPearls - NCBI BookshelfmusdalifahNo ratings yet
- Hip Vs SpineDocument12 pagesHip Vs Spinevaibhav gowdaNo ratings yet
- Seminar: Mitchell R Lunn, Ching H WangDocument14 pagesSeminar: Mitchell R Lunn, Ching H WangCatalina AlzogarayNo ratings yet
- The Treatment of Neck and Upper Back Pain With AcuDocument6 pagesThe Treatment of Neck and Upper Back Pain With AcuMohmmad Hossein NazariNo ratings yet
- Evaluationandtreatmentof Cervicalradiculopathy: Cayce A. Onks,, Gregory BillyDocument12 pagesEvaluationandtreatmentof Cervicalradiculopathy: Cayce A. Onks,, Gregory BillyAndrés MardonesNo ratings yet
- Spinal Tuberculosis: Imaging Features On MRIDocument6 pagesSpinal Tuberculosis: Imaging Features On MRIEster JuanitaNo ratings yet
- Probyn L. 2023. High-Resolution US in Evaluation Adult Hip.Document16 pagesProbyn L. 2023. High-Resolution US in Evaluation Adult Hip.Javier MartinNo ratings yet
- Uso de Ultrasonografia para Medial EpycondilitisDocument5 pagesUso de Ultrasonografia para Medial EpycondilitisSantiago BianchiNo ratings yet
- Mano 4Document3 pagesMano 4juanNo ratings yet
- Clinical Differentiation of Upper Extremity Pain EtiologiesDocument9 pagesClinical Differentiation of Upper Extremity Pain EtiologiesmarcosgabNo ratings yet
- Bamboo Spine - X-Ray Findings of Ankylosing Spondylitis RevisitedDocument3 pagesBamboo Spine - X-Ray Findings of Ankylosing Spondylitis RevisitedpanjiNo ratings yet
- Management of Spasticity After Spinal Cord Injury: Current Techniques and Future DirectionsDocument11 pagesManagement of Spasticity After Spinal Cord Injury: Current Techniques and Future DirectionslabsoneducationNo ratings yet
- Treatment of Gluteal Tendinopathy A Systematic Review and Stage-Adjusted Treatment RecommendationDocument12 pagesTreatment of Gluteal Tendinopathy A Systematic Review and Stage-Adjusted Treatment RecommendationDeivisonNo ratings yet
- Shen 2016Document38 pagesShen 2016Joice BragaNo ratings yet
- Telaah 1Document3 pagesTelaah 1anri albarruNo ratings yet
- Anatomical Variation of Sciatic Nerve Course in Saudi Population A Magnetic Resonance Imaging StudyDocument8 pagesAnatomical Variation of Sciatic Nerve Course in Saudi Population A Magnetic Resonance Imaging StudyInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- RG 2021200079Document16 pagesRG 2021200079Rahul KashyapNo ratings yet
- Radiol 13121947Document7 pagesRadiol 13121947Eduardo Agustín Vargas CamposNo ratings yet
- Ki 1.1 Rotator Cuff Any 2 ASDocument29 pagesKi 1.1 Rotator Cuff Any 2 ASAgus TianyNo ratings yet
- Neurosurgery Cases and Reviews NCR 4 056Document11 pagesNeurosurgery Cases and Reviews NCR 4 056Surya PangeranNo ratings yet
- Back Pain Printable VersionDocument43 pagesBack Pain Printable VersionSabin ParajuliNo ratings yet
- Lumbarspinalstenosisin Olderadults: Anna M. Lafian,, Karina D. TorralbaDocument12 pagesLumbarspinalstenosisin Olderadults: Anna M. Lafian,, Karina D. TorralbaIhsan KNo ratings yet
- Sindrome Do Nervo SupraescapularDocument9 pagesSindrome Do Nervo SupraescapularAna CarolineNo ratings yet
- Acute Hip Pain. Mimics of A Femoral Neck Fracture Ingles.Document9 pagesAcute Hip Pain. Mimics of A Femoral Neck Fracture Ingles.jesus marinNo ratings yet
- Surgical Management of Sacral Chordomas - Illustrative Cases and Current Management ParadigmsDocument7 pagesSurgical Management of Sacral Chordomas - Illustrative Cases and Current Management ParadigmsHugo JBNo ratings yet
- Jcis 8 6Document4 pagesJcis 8 6boy bionardoNo ratings yet
- Pediatric Supracondylar Fractures of The Distal Humerus: Provided by Springer - Publisher ConnectorDocument7 pagesPediatric Supracondylar Fractures of The Distal Humerus: Provided by Springer - Publisher ConnectorBison_sonNo ratings yet
- J Jhsa 2017 10 014Document5 pagesJ Jhsa 2017 10 014Nicolas AdrianoNo ratings yet
- Bydon 2019Document6 pagesBydon 2019julyanetorquatoNo ratings yet
- Tens 1Document8 pagesTens 1marcelogascon.oNo ratings yet
- Efficacy of Contra-Lateral Neurodynamics On Median Nerve Extensibility in Cervical Radiculopathy PatientsDocument6 pagesEfficacy of Contra-Lateral Neurodynamics On Median Nerve Extensibility in Cervical Radiculopathy PatientsSylvia Grace100% (1)
- Accuracy of Physical Examination in Subacromial Impingement SyndromeDocument5 pagesAccuracy of Physical Examination in Subacromial Impingement SyndromeClaudia BuitragoNo ratings yet
- Spinal Epidural Cavernous Hemangiomas in The 2024 International Journal of SDocument3 pagesSpinal Epidural Cavernous Hemangiomas in The 2024 International Journal of SRonald QuezadaNo ratings yet
- Compression of The Posterior Interosseous Nerve Sec 2024 International JournDocument4 pagesCompression of The Posterior Interosseous Nerve Sec 2024 International JournRonald QuezadaNo ratings yet
- MRI of Degenerative Disease of the Spine: A Case-Based AtlasFrom EverandMRI of Degenerative Disease of the Spine: A Case-Based AtlasNo ratings yet
- Interventional Radiology of the Spine: Image-Guided Pain TherapyFrom EverandInterventional Radiology of the Spine: Image-Guided Pain TherapyJ. Kevin McGrawNo ratings yet
- Articulo Febrero 25Document13 pagesArticulo Febrero 25Matthew PhillipsNo ratings yet
- Muscle & Nerve Volume 31 Issue 5 2005 (Doi 10 - CopiarDocument5 pagesMuscle & Nerve Volume 31 Issue 5 2005 (Doi 10 - CopiarMatthew PhillipsNo ratings yet
- Biomechanical Design Considerations For Transradial Prosthetic Interface: A ReviewDocument12 pagesBiomechanical Design Considerations For Transradial Prosthetic Interface: A ReviewMatthew PhillipsNo ratings yet
- Amputee Mobility Predictor-Bilateral: A Performance-Based Measure of Mobility For People With Bilateral Lower-Limb LossDocument8 pagesAmputee Mobility Predictor-Bilateral: A Performance-Based Measure of Mobility For People With Bilateral Lower-Limb LossMatthew PhillipsNo ratings yet
- Osseointegration Amputation Prostheses On The Upper Limbs: Methods, Prosthetics and RehabilitationDocument11 pagesOsseointegration Amputation Prostheses On The Upper Limbs: Methods, Prosthetics and RehabilitationMatthew PhillipsNo ratings yet
- Articulo 2Document10 pagesArticulo 2Matthew PhillipsNo ratings yet
- A Narrative Review: Current Upper Limb Prosthetic Options and DesignDocument11 pagesA Narrative Review: Current Upper Limb Prosthetic Options and DesignMatthew PhillipsNo ratings yet
- Medical Engineering and Physics: Nguiadem Clautilde, Raison Maxime, Achiche SofianeDocument10 pagesMedical Engineering and Physics: Nguiadem Clautilde, Raison Maxime, Achiche SofianeMatthew PhillipsNo ratings yet
- Muscle & Nerve Volume 24 Issue 9 2001 (Doi 10 - CopiarDocument8 pagesMuscle & Nerve Volume 24 Issue 9 2001 (Doi 10 - CopiarMatthew PhillipsNo ratings yet
- Palermo Et Al-2017-Annals of The American Thoracic SocietyDocument8 pagesPalermo Et Al-2017-Annals of The American Thoracic SocietyMatthew PhillipsNo ratings yet
- Convery 1998Document7 pagesConvery 1998Matthew PhillipsNo ratings yet
- Medical Engineering & Physics: The Patellar Tendon Bar! Is It A Necessary Feature?Document6 pagesMedical Engineering & Physics: The Patellar Tendon Bar! Is It A Necessary Feature?Matthew PhillipsNo ratings yet
- Lower Extremity Socket Design and Suspension: Kevin Carroll, MS, CP, FAAOPDocument18 pagesLower Extremity Socket Design and Suspension: Kevin Carroll, MS, CP, FAAOPMatthew PhillipsNo ratings yet
- Prevention and Management of Foot Problems in Diabetes: A Summary Guidance For Daily Practice 2015, Based On The IWGDF Guidance DocumentsDocument9 pagesPrevention and Management of Foot Problems in Diabetes: A Summary Guidance For Daily Practice 2015, Based On The IWGDF Guidance DocumentsMatthew PhillipsNo ratings yet
- Visual Evoked Potentials in Clinical Neurology: MethodsDocument2 pagesVisual Evoked Potentials in Clinical Neurology: MethodsMatthew PhillipsNo ratings yet
- Journal Pre-Proof: The American Journal of MedicineDocument29 pagesJournal Pre-Proof: The American Journal of MedicineMatthew PhillipsNo ratings yet
- Marcha ArtDocument13 pagesMarcha ArtMatthew PhillipsNo ratings yet
- Long-Term Outcome of Weekly Bisphosphonates: René Rizzoli, MDDocument5 pagesLong-Term Outcome of Weekly Bisphosphonates: René Rizzoli, MDMatthew PhillipsNo ratings yet
- Acido Zolendronico PDFDocument14 pagesAcido Zolendronico PDFMatthew PhillipsNo ratings yet
- Clariant SDS Master Batch ED 95 BR Brazil EnglishDocument12 pagesClariant SDS Master Batch ED 95 BR Brazil Englishjesus bartolo100% (1)
- 01smartez Brochure Epiciv0003may18Document2 pages01smartez Brochure Epiciv0003may18Rucha PavagadhiNo ratings yet
- The Sleeping Habits of Selected Grade 10 Students in Dalandanan National High School and Its Effect in Their Academic PerformanceDocument18 pagesThe Sleeping Habits of Selected Grade 10 Students in Dalandanan National High School and Its Effect in Their Academic PerformanceJeffrey Dela CruzNo ratings yet
- Injury Prevention in Youth Sports.Document5 pagesInjury Prevention in Youth Sports.drmanupvNo ratings yet
- AlgorithmBLS Ped CA Single RescuerCOVID 200406Document1 pageAlgorithmBLS Ped CA Single RescuerCOVID 200406Karla HernandezNo ratings yet
- سمنار الاشعة ct scanDocument13 pagesسمنار الاشعة ct scanDiana DedoNo ratings yet
- Tacrolimus Ointment Is Used To Treat The Symptoms of EczemaDocument2 pagesTacrolimus Ointment Is Used To Treat The Symptoms of EczemaSharan SahotaNo ratings yet
- Bataan National High School Senior High School Bataan National High School Senior High SchoolDocument57 pagesBataan National High School Senior High School Bataan National High School Senior High SchoolAnonymous 7WTn9kzZfSNo ratings yet
- NP1 Family Nursing 2Document2 pagesNP1 Family Nursing 2Jonas Marvin AnaqueNo ratings yet
- Reimbursment Form AXA Insurance 29082017Document1 pageReimbursment Form AXA Insurance 29082017Melford LapnawanNo ratings yet
- Urticaria, Angioedema, and Anaphylaxis AGO 2020Document12 pagesUrticaria, Angioedema, and Anaphylaxis AGO 2020Elizabeth HendersonNo ratings yet
- TOPIC: Financial Stability of Grade 11 Students: Firsts Question 1.what Is The Topic/problem About?Document2 pagesTOPIC: Financial Stability of Grade 11 Students: Firsts Question 1.what Is The Topic/problem About?Heinrich VergaraNo ratings yet
- Nursing Care Plan For Patient With PNEUMONIA (Geriatrics)Document4 pagesNursing Care Plan For Patient With PNEUMONIA (Geriatrics)CHRISTIE MONTANO0% (1)
- Forensic NursingDocument25 pagesForensic NursingAnonymous jQwBrGlNGNo ratings yet
- Understanding InnovationDocument48 pagesUnderstanding InnovationMichael J. KeeganNo ratings yet
- Nursing Health History 3rd YrDocument3 pagesNursing Health History 3rd Yrshin_2173100% (1)
- Most Common Hospital Departments: Breast CancerDocument7 pagesMost Common Hospital Departments: Breast Cancerdeddy ramadhanNo ratings yet
- Ped001 - Lesson 1Document12 pagesPed001 - Lesson 1JERE-ANN MANAMBAYNo ratings yet
- (Download pdf) Principles And Practice Of Clinical Research 4Th Edition John I Gallin full chapter pdf docxDocument69 pages(Download pdf) Principles And Practice Of Clinical Research 4Th Edition John I Gallin full chapter pdf docxelnmrraeez100% (4)
- Connective Tissue DiseaseDocument3 pagesConnective Tissue DiseaseAmberNo ratings yet
- Strategies in Liver TraumaDocument9 pagesStrategies in Liver TraumaLuis Miguel Díaz VegaNo ratings yet
- Dilated CardiomyopathyDocument125 pagesDilated CardiomyopathyAbnet WondimuNo ratings yet
- Improving The Physical Health of People With Mental Health ProblemsDocument61 pagesImproving The Physical Health of People With Mental Health ProblemsThierry UhawenimanaNo ratings yet
- Acute Zonal Occult Outer Retinopathy A Classification Based On Multimodal ImagingDocument10 pagesAcute Zonal Occult Outer Retinopathy A Classification Based On Multimodal ImagingStefanieNo ratings yet
- 25. Đề thi thử TN THPT 2021 - Môn Tiếng anh - THPT Chuyên Nguyễn Trãi - Hải Dương - Lần 1 - File word có lời giảiDocument28 pages25. Đề thi thử TN THPT 2021 - Môn Tiếng anh - THPT Chuyên Nguyễn Trãi - Hải Dương - Lần 1 - File word có lời giảiThinh LeNo ratings yet
- Personal Health 1Document21 pagesPersonal Health 1Lgbtqia BuhiNo ratings yet