Professional Documents
Culture Documents
Nmat 2384
Nmat 2384
Uploaded by
Luis M. MolinaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Nmat 2384
Nmat 2384
Uploaded by
Luis M. MolinaCopyright:
Available Formats
LETTERS
PUBLISHED ONLINE: 8 FEBRUARY 2009 DOI: 10.1038/NMAT2384
Subnanometre platinum clusters as highly
active and selective catalysts for the oxidative
dehydrogenation of propane
Stefan Vajda1,2,3 *, Michael J. Pellin4 , Jeffrey P. Greeley2 , Christopher L. Marshall1 ,
Larry A. Curtiss1,2,4 *, Gregory A. Ballentine1† , Jeffrey W. Elam5 , Stephanie Catillon-Mucherie1 ,
Paul C. Redfern1 , Faisal Mehmood4 and Peter Zapol1,2,4
Small clusters are known to possess reactivity not observed a b c
in their bulk analogues, which can make them attractive
for catalysis1–6 . Their distinct catalytic properties are often
hypothesized to result from the large fraction of under-
coordinated surface atoms7–9 . Here, we show that size-
preselected Pt8−10 clusters stabilized on high-surface-area Al2O3 Pt Al2O3 Pt SnO
supports are 40–100 times more active for the oxidative
dehydrogenation of propane than previously studied platinum
and vanadia catalysts, while at the same time maintaining Figure 1 | Depiction of the catalytic system. a,b, Illustration of Pt clusters
high selectivity towards formation of propylene over by- deposited in AAO membrane with ALD coating of Al2 O3 (a), and with
products. Quantum chemical calculations indicate that under- added SnO (b). c, Structure of Pt8 cluster on an Al2 O3 surface from density
coordination of the Pt atoms in the clusters is responsible for functional calculations. In this structure there are four Pt–O distances
the surprisingly high reactivity compared with extended sur- between 2.2 and 2.4 Å and the closest Pt–Al distance is 2.55 Å. See
faces. We anticipate that these results will form the basis for Supplementary Information for more details.
development of a new class of catalysts by providing a route to
bond-specific chemistry, ranging from energy-efficient and en- Methods for producing size-selected clusters and soft-landing
vironmentally friendly synthesis strategies to the replacement them on catalytic supports are now well established13–16 . However,
of petrochemical feedstocks by abundant small alkanes10,11 . testing these catalytic clusters under realistic reaction conditions
The oxidative dehydrogenation (ODH) of alkanes is a reaction requires supports on which the clusters resist sintering. Both
that is exothermic overall and is, thus, an attractive alternative alumina and tin oxide surfaces are known to stabilize platinum
to dehydrogenation of alkanes, which is an endothermic process clusters15,17 . We used atomic layer deposition (ALD) to coat porous
requiring significant energy input. However, current ODH catalysts anodized aluminium oxide (AAO, Anopore) membranes with
have limited activity and/or poor selectivity resulting from an alumina (Al2 O3 /AAO) before Pt-cluster deposition as illustrated
inability to prevent complete oxidation12 . The reaction scheme in Fig. 1. The ALD process ensures a uniform surface chemistry18
for propane ODH is shown in formula (1) and includes other for the attachment of the clusters. Membranes were used in this
competing pathways that lead to COx species. investigation because they provide a high surface area so that high
dispersions of size-selected clusters can be achieved, and catalytic
A B tests under well-defined conditions can be carried out. The Pt8−10
C3H 8 C 3H 6 C 3H 4
clusters were mass-selected, and a total of 900 (±135) ng Pt was
soft-landed on the large pore side (∼200 nm diameter) of the
D
(1) membrane. A pair of membranes with identical Pt8−10 and alumina
C E loadings were synthesized; one was left as-is and one was treated
with an equivalent of two-monolayer-thick tin oxide ALD, resulting
COx
in an overcoat of the alumina support around the platinum clusters
with tin oxide (SnO/Al2 O3 /AAO, Fig. 1).
In formula (1), channel A corresponds to the pathway for Synchrotron grazing-incidence small-angle X-ray scattering
propylene production and channels B, C, D and E result in studies of alumina-supported size-selected Pt clusters carried out
less desirable products. in our laboratory15 have provided evidence for the stability and
1 Chemical Sciences and Engineering Division, Argonne National Laboratory, 9700 South Cass Avenue, Argonne, Illinois 60439, USA, 2 Center for
Nanoscale Materials, Argonne National Laboratory, 9700 South Cass Avenue, Argonne, Illinois 60439, USA, 3 Department of Chemical Engineering,
School of Engineering & Applied Science, Yale University, 9 Hillhouse Avenue, New Haven, Connecticut 06520, USA, 4 Materials Science Division,
Argonne National Laboratory, 9700 South Cass Avenue, Argonne, Illinois 60439, USA, 5 Energy Systems Division, Argonne National Laboratory,
9700 South Cass Avenue, Argonne, Illinois 60439, USA. † Present Address: Max-Planck-Institut für Metallforschung, Stuttgart, Germany.
*e-mail: vajda@anl.gov; curtiss@anl.gov.
NATURE MATERIALS | VOL 8 | MARCH 2009 | www.nature.com/naturematerials 213
LETTERS NATURE MATERIALS DOI: 10.1038/NMAT2384
Selectivity Activity
a CO2 d
16.3% Pt8¬10 /SnO/Al2O3 /AAO, 400 °C
CO Pt8¬10 /SnO/Al2O3 /AAO, 500 °C
Propylene
83.7% 0% Pt8¬10 /Al2O3 /AAO, 550 °C
b CO2
15.7%
VOx /Al2O3, 1.4 V nm¬1, 390 °C
Catalyst
Propylene CO VOx /Al2O3, 8.0 V nm¬1, 390 °C
64% 20.3% VOx /Al2O3, 16.6 V nm¬1, 390 °C
VOx /Al2O3, 34.2 V nm¬1, 390 °C
c CO2
32% Pt /monolith, 400 °C
Propylene Pt /monolith, 500 °C
68% CO
0% 1E-3 0.01 0.1 1 10
TOF (s¬1)
Figure 2 | Catalyst activity and selectivity. a–c, Selectivity of the Pt8−10 -based catalysts at various temperatures and support compositions: SnO/Al2 O3
at 400 ◦ C (a), SnO/Al2 O3 at 500 ◦ C (b) and Al2 O3 at 550 ◦ C (c). d, TOFs of propylene produced on the Pt8−10 catalysts (green) and reference ODH
catalysts (grey) expressed as number of propylene molecules formed per metal atom. Pt monolith and vanadia data from refs 29 and 22, respectively. See
Supplementary Information for more details.
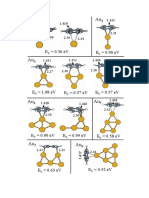
Pt4 + C3H8(g)
0.24 eV 0.42
0.54
Pt4---C3H8
0.37
0.95
Pt4-(H,C3H7)
Pt4-(2H,C3H6)
Figure 3 | Reaction path. Diagram of primary reaction steps in channel A (see formula (1)) from DFT calculations for the dehydrogenation of propane on a
Pt4 cluster leading to formation of propylene adsorbed on the cluster. Energies (in eV) of the equilibrium structures are relative to the reactants. Energy
barriers for the transition state structures are relative to the preceding equilibrium structure (‘true’ barriers). The first barrier corresponds to breaking of
the first C–H bond (on the CH2 group) and the second barrier corresponds to breaking of the second C–H bond (on a CH3 group). A number of other
reaction steps involving hydrogen migration are not included in this diagram. The dotted lines in the structures indicate partial bonds. See Supplementary
Information for more details of structures and energies and other reaction pathways.
shape of the Pt clusters. The supported clusters, similar to those below). In addition, we carried out calculations to investigate the
used in this study, showed no evidence of agglomeration over a stability of a positively charged Pt8 cluster on alumina and found
temperature range of 20–400 ◦ C, and they maintained a three- that it withdraws electrons from the surface and becomes negatively
dimensional structure. At the higher temperatures used in the charged as in the case of the supported neutral cluster (see below).
catalytic testing in this study, there was also no evidence of any In a sense, these highly uniform clusters can be considered an ideal
change in cluster size, as there was no change in selectivity or activity model of a single-site catalyst on technologically relevant supports,
after 30 h of testing. We have also carried out density functional in which all active sites closely resemble each other2,19,20 .
theory (DFT) calculations14 to investigate the structure of a Pt8 The catalyst tests were carried out under atmospheric pressure
cluster on a θ -alumina surface. The structure (Fig. 1) indicates that in a flow reactor at temperatures from 400 to 550 ◦ C by using
the cluster maintains its three-dimensional structure, as is found 10 s.c.c.m. total flow of reactants in argon carrier gas with 2.63 and
in the X-ray studies. The Pt cluster forms Pt–O bonds with the 2.73 mol% of oxygen and propane, respectively. The temperature
surface, resulting in significant charge transfer to the cluster. The range was chosen to give a direct comparison with the performance
binding of the cluster to the surface (∼3 eV) is consistent with of reported Pt-and VOx -based ODH catalysts. The measured
the stability of the subnanometre Pt8 clusters on alumina, but turnover frequencies (TOFs) are shown in Fig. 2, along with the
does not significantly affect the cluster’s chemical reactivity (see highest reported values for platinum and vanadia. The TOFs were
214 NATURE MATERIALS | VOL 8 | MARCH 2009 | www.nature.com/naturematerials
NATURE MATERIALS DOI: 10.1038/NMAT2384 LETTERS
calculated as the number of propylene molecules produced per Pt H
atom per second (see Supplementary Information for details of the H3CHCCH3
method of calculation). The activity and selectivity at 500 ◦ C was A
H
unchanged over 30 h of testing. H2CCCH3
The main finding of this study is that the activity of the
Pt8−10 catalyst is markedly higher than any reported platinum H-CH2CHCH2 B
or vanadia-based ODH catalysts. In fact, at 400 ◦ C, the observed
TOFs are 40–100 times higher than those previously reported. H-CHCHCH3
The product distribution (Fig. 2) indicates that selectivity for
H3C-CH2CH3 C
propylene over the formation of carbon oxide species is also
achieved. The total conversion approaches 25% ± 4% at high H2CHC-CH3
temperatures. The uncertainty in the reported TOF is around
D
15%, determined primarily by the uncertainty in the amount of H2C=CHCH3
Pt loading. The uncertainty in conversion rates is given by the
precision at determining the diameter of the Pt spots on the
0 0.5 1.0 1.5 2.0 2.5 3.0
membrane using grazing-incidence small-angle X-ray scattering on Barrier (eV)
poreless flat-alumina supported Pt clusters prepared under identical
Figure 4 | Energy barriers of bond breaking. ‘True’ barriers for breaking
deposition conditions.
C–C and C–H bonds in propane and propylene reactions on a Pt4 cluster.
To understand the observed high activity of small Pt clusters
Bonds that are broken are shown in red. Letters correspond to channels in
for propane ODH, we carried out DFT calculations on key steps
formula (1). All of the barriers plotted here correspond to energies relative
for channel A in the reaction diagram in formula (1). Channel
to the reactants (propane or propylene). In propane, the C–H bond
A is the predominant channel because up to 84% of the product
breaking on the centre carbon (A) is favoured over a terminal carbon. In
formed is propylene (Fig. 2). A tetrahedral Pt4 cluster was used
propylene C–H bond breaking all three sites (B) have similar barriers. See
as a model for the Pt8−10 clusters because the three-coordinated
Supplementary Information for more details of structures and energies.
Pt in this cluster is representative of the under-coordination of Pt
found in the larger Pt8−10 clusters. The use of a free cluster model to fully elucidate the effect of the substrate, these results are
(without a substrate) is justified by the fact that the observed activity strong evidence that the support has little effect on the reaction
results for alumina and alumina/tin oxide substrates are similar barrier in this case. In contrast to the low C–H bond scission
(Fig. 2) and also by some model calculations done to investigate the barrier found on Pt4 , DFT calculations for C–H bond scission in
substrate effect (see below). propane on a Pt(111) surface give an apparent activation barrier
The calculated transition states and intermediates in reaction 1 eV larger than the corresponding barrier on Pt4 . In addition, the
channel A (formula (1)), leading to formation of propylene on C–H bond cleavage barriers on a supported V2 O5 dimer are close
the Pt4 cluster, are shown in Fig. 3. The ‘true’ barrier to breaking to 1.2 eV (ref. 22) from experiment and about 2 eV from theory
of the first C–H bond is only 0.42 eV. The corresponding barrier for a V2 O5 surface23 .
referenced to gas-phase propane (the ‘apparent’ barrier) is slightly Thus, both theory and experiment lead to the conclusion that
smaller, at 0.18 eV; this barrier was found to be similarly small under-coordinated Pt sites in small Ptn clusters are much more
(0.05 eV) when recalculated on a Pt8 cluster. We note that, in active than a Pt surface for propane ODH. This can be explained
spite of the small magnitude of the energetic barrier, this step will by the attractive interaction between the under-coordinated Pt and
probably be rate-limiting because of the very large entropic loss, propane. The DFT calculations show that the initial adsorption
and consequentially lower pre-exponential factor, associated with complex between propane and the Pt4 cluster (Fig. 3) results in sig-
propane adsorption at high temperatures on the clusters. The rest nificant charge transfer from a propane C–H bonding orbital to the
of the pathway is thermodynamically downhill to formation of cluster. This weakens the C–H bond as demonstrated by its length-
propylene, which binds to Pt4 by its π bond. Interestingly, our ening and a concomitant lowering of the C–H vibrational frequency
calculations also indicate that dissociated oxygen atoms adsorbed by ∼500 cm−1 . In contrast, propane is very weakly adsorbed on a
on the cluster21 do not significantly affect the calculated C–H bond Pt(111) surface with essentially no C–H bond lengthening or charge
activities in this pathway. In the overall reaction scheme, oxygen transfer owing to higher coordination of the surface Pt atoms.
serves as a means for removal of hydrogen as water on the basis The observed product distribution, favouring propylene
of these calculations. formation over COx (84% versus 16% at 400 ◦ C—Fig. 2), suggests
The experimental barrier for propylene formation from propane that C–C or C=C cleavage on the Pt clusters is less favourable
on the subnanometre Pt clusters can be estimated to pro- than is C–H cleavage. Calculated activation barriers for various
vide comparison with theory. The barrier determined from the such reactions (Fig. 4) confirm this conclusion. For example, the
experimental data for the Pt8−10 /SnO/Al2 O3 /AAO catalyst at 400 barrier for breaking a C–C bond in propane is greater than 1 eV.
and 500 ◦ C is 0.2 eV. This is consistent with the barrier found in In addition, propylene itself is relatively unreactive; the barriers
our DFT investigation for channel A. Similarly good agreement for C–C and C=C breaking range from 2.2–2.7 eV. In addition,
is found when we include models for the Al2 O3 support in our the barrier to break a C–H bond in the methyl group of adsorbed
calculations. First, we considered a negatively (−1) charged Pt4 propylene is quite large (1.27 eV). The relatively large magnitude
cluster, which represents the charge transfer that occurs to the of this barrier is due to the considerable structural rearrangement
cluster when it is supported on alumina. The propane C–H reaction required for the methyl group in propylene to come in contact with
apparent barrier is 0.21 eV, an insignificant change from the neutral the Pt4 cluster. These results, taken together, indicate that propylene
cluster. Second, we calculated the barrier for a Pt8 cluster on a will be easily formed, but other by-products, such as allene and COx ,
θ-alumina surface. The apparent barrier is 0.19 eV, also a small will not form easily.
change from the result for the gas-phase Pt8 cluster. The lack The calculated selectivity trends can be understood from the
of a significant support effect is consistent with a larger binding electronic structures of the C–C and C–H bond-breaking transition
energy per atom (∼3 eV per atom) calculated for Pt8 and Pt4 states. The larger barriers observed for C–C versus C–H bond
clusters compared with the energy of each of the three Pt–alumina breaking are probably due to the sp3 directionality of the orbitals on
surface bonds (∼1 eV). Although more detailed studies are needed C compared with the spherical nature of the orbital on hydrogen,
NATURE MATERIALS | VOL 8 | MARCH 2009 | www.nature.com/naturematerials 215
LETTERS NATURE MATERIALS DOI: 10.1038/NMAT2384
which results in poorer overlap between the adsorbate and the 7. Lemire, C., Meyer, R., Shaikhutdinov, S. & Freund, H.-J. Do quantum size
reaction site orbitals in the transition state for breaking the C–C effects control CO adsorption on gold nanoparticles? Angew. Chem. Int. Ed. 43,
bond compared with that for the C–H bond. This argument has 118–121 (2004).
8. Wei, J. & Iglesia, E. Mechanism and site requirements for activation and
been put forward by Blomberg et al. for other surface reactions24 . chemical conversion of methane on supported Pt clusters and turnover rate
Computations indicate that CH4 (ref. 25) and H2 (ref. 26) have a comparisons among noble metals. J. Phys. Chem. B 108, 4094–4103 (2004).
small barrier and no barrier, respectively, for dissociation on a Pt4 9. Hvolbaek, B. et al. Catalytic activity of Au nanoparticles. Nano Today 2,
cluster, which supports the overlap explanation. 14–18 (2007).
10. Hutchings, G. J., Scurrell, M. S. & Woodhouse, J. R. Oxidative coupling of
To our knowledge, the work reported here is the first
methane using oxide catalysts. Chem. Soc. Rev. 18, 251–283 (1989).
investigation of size-preselected Pt clusters under realistic high- 11. Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond
temperature catalytic conditions. It has revealed a very high activity activation. Nature 417, 507–509 (2002).
of subnanometre Pt-cluster-based catalysts for the ODH of propane 12. Cavani, F., Ballarini, N. & Cericola, A. Oxidative dehydrogenation of ethane
to propylene. Combined with quantum chemical studies, this work and propane: How far from commercial implementation? Catal. Today 127,
113–131 (2007).
has shown that the high activity is due to the under-coordination 13. Benz, L. et al. Landing of size-selected Ag+ n clusters on single crystal TiO2
of the Pt in the clusters and that the clusters favour the scission of (110)-(1 × 1) surfaces at room temperature. J. Chem. Phys. 122, 081102 (2005).
C–H bonds relative to C–C or C=C bonds. Some recent work in our 14. Lee, S., Fan, C., Wu, T. & Anderson, S. L. CO oxidation on Aun /TiO2
laboratory demonstrates that small gold clusters (Au6−10 ) are highly catalysts produced by size-selected cluster deposition. J. Am. Chem. Soc. 126,
active for propylene epoxidation, thus providing further evidence 5682–5683 (2004).
15. Winans, R. E. et al. Reactivity of supported platinum nanoclusters studied by in
for the unique catalytic properties of subnanometre clusters27 . In situ GISAXS: Clusters stability under hydrogen. Top. Catal. 39, 145–149 (2006).
the future, size-selected clusters stabilized on appropriate supports 16. Yoon, B. et al. Charging effects on bonding and catalyzed oxidation of CO on
with uniform surface chemistry hold great promise for design of Au8 clusters on MgO. Science 307, 403–407 (2005).
new catalytic materials. It will be a challenging task to scale up the 17. Sadykov, V. A. et al. Oxidative dehydrogenation of propane over monoliths at
production of size-selected clusters by more conventional chemical short contact times. Catal. Today 61, 93–99 (2000).
18. Pellin, M. J. et al. Mesoporous catalytic membranes: Synthetic control of pore
methods, but there are very encouraging efforts suggesting that this size and wall composition. Catal. Lett. 102, 127–130 (2005).
will ultimately be possible2,3 . 19. Bell, A. T. The impact of nanoscience on heterogeneous catalysis. Science 299,
1688–1691 (2003).
Methods 20. Somorjai, G. A., Contreras, A. M., Montano, M. & Rioux, R. M. Cluster
Production of narrow cluster size distributions. The clusters emerging from the chemistry and dynamics special feature: Clusters, surfaces, and catalysis.
continuous beam cluster source possess identical velocity. Owing to the variation Proc. Natl Acad. Sci. 103, 10577–10583 (2006).
in their kinetic energy with size, in addition to the single mass selection on a 21. Xu, Y., Shelton, W. A. & Schneider, W. F. Thermodynamic equilibrium
quadrupole mass filter, narrow distributions of clusters with one to four sizes can be compositions, structures, and reaction energies of Ptx Oy (x = 1–3) clusters
isolated using a quadrupole deflector operated in energy filter mode. predicted from first principles. J. Phys. Chem. B 110, 16591–16599 (2006).
22. Argyle, M. D., Chen, K., Bell, A. T. & Iglesia, E. Effect of catalyst structure
Determination of the fraction of clusters in the pores acting as active catalysts. on oxidative dehydrogenation of ethane and propane on alumina-supported
As only 10.5% of the facing area of the membrane was exposed to clusters, and to vanadia. J. Catal. 208, 139–149 (2002).
the propane feed, a multiplication factor of 9.5 is applied to obtain the corrected 23. Redfern, P. C. et al. Quantum chemical study of mechanisms for oxidative
propane conversion rate on the Pt-cluster-coated fraction of the AAO membrane. dehydrogenation of propane on vanadium oxide. J. Phys. Chem. B 110,
By calculating the relative pore opening area to the total surface area of the 8363–8371 (2006).
membrane, 28% of the Pt8−10 particles, corresponding to 252 ng of Pt metal, enter 24. Blomberg, M. R. A., Siegbahn, P. E. M., Nagashima, U. & Wennerberg, J.
the pores. The rest forms metallic platinum on the face of the AAO membrane as Theoretical study of the activation of alkane C–H and C–C bonds by different
confirmed by X-ray photoemission spectroscopy. Metallic Pt is known to possess transition metals. J. Am. Chem. Soc. 113, 424–433 (1991).
orders of magnitude lower TOFs for ODH of propane28 . Thus, 252 ng of Pt was 25. Xiao, L. & Wang, L. Methane activation on Pt and Pt4 : A density functional
used for the calculation of the TOFs. theory study. J. Phys. Chem. B 111, 1657–1663 (2007).
26. Cruz, A., Bertin, V., Poulain, E., Benitez, J. I. & Castillo, S. Theoretical study of
Activation barrier calculation from the experimental data. Using Arrhenius law the H2 reaction with a Pt4 (111) cluster. J. Chem. Phys. 120, 6222–6225 (2004).
and assuming that the conversion rate is roughly proportional to the fraction 27. Lee, S. et al. Selective propene epoxidation on immobilized Au6−10 clusters: The
of activated C–H bonds, an estimate was obtained of the activation barrier effect of hydrogen and water on selectivity and activity. Angew. Chem. Int. Ed.
from two available experimental values for Pt8−10 /SnO/Al2 O3 /AAO catalyst at (in the press, 2008) <http://dx.doi.org/10.1002/anie.200804154>.
different temperatures. 28. Yu, C., Xu, H., Ge, Q. & Li, W. Properties of the metallic phase of zinc-doped
platinum catalysts for propane dehydrogenation. J. Mol. Catal. A 266,
Theoretical methods. The calculations were done using the B3LYP functional 80–87 (2007).
with the LANL2DZ basis set for Pt and 6-31G∗ for Al, C, H and O. The Pt(111) 29. Silberova, B., Fathi, M. & Holmen, A. Oxidative dehydrogenation of ethane
results were obtained with plane-wave calculations using the RPBE functional. See and propane at short contact time. Appl. Catal. A 276, 17–28 (2004).
Supplementary Information for more details.
Acknowledgements
Received 21 July 2008; accepted 12 January 2009; The work at Argonne National Laboratory was supported by the US Department
published online 8 February 2009 of Energy, BES-Chemical Sciences, BES-Materials Sciences, and BES-Scientific User
Facilities under Contract DE-AC-02-06CH11357 with UChicago Argonne, LLC,
References Operator of Argonne National Laboratory. S.V. gratefully acknowledges the support by
1. Xu, Z. et al. Size-dependent catalytic activity of supported metal clusters. the Air Force Office of Scientific Research. We acknowledge grants of computer time at
Nature 372, 346–348 (1994). the Laboratory Computing Resource Center (LCRC) at Argonne National Laboratory,
2. Gates, B. C. Supported metal clusters: Synthesis, structure, and catalysis. the National Energy Research Scientific Computing Center (NERSC) at Lawrence
Chem. Rev. 95, 511–522 (1995). Berkeley National Laboratory and the Molecular Science Computing Facility (MSCF) at
3. Argo, A. M., Odzak, J. F., Lai, F. S. & Gates, B. C. Observation of ligand effects Pacific Northwest National Laboratory. The authors are indebted to E. Iglesia and P. Stair
during alkene hydrogenation catalysed by supported metal clusters. Nature for valuable discussions, A. Holmen for providing the exact dimensions of the monolith
415, 623–623 (2002). used in their studies of Pt-based catalysts and thank J. Moore for carrying out X-ray
4. Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active Nonmetallic photoemission spectroscopy analysis of the Pt/AAO sample.
Au and Pt species on ceria-based water-gas shift catalysts. Science 301,
935–938 (2003). Additional information
5. Campbell, C. T. The active site in nanoparticle gold catalysis. Science 306, Supplementary Information accompanies this paper on www.nature.com/naturematerials.
234–235 (2004). Reprints and permissions information is available online at http://npg.nature.com/
6. Chen, M. S. & Goodman, D. W. The structure of catalytically active gold on reprintsandpermissions. Correspondence and requests for materials should be
titania. Science 306, 252–255 (2004). addressed to S.V. or L.A.C.
216 NATURE MATERIALS | VOL 8 | MARCH 2009 | www.nature.com/naturematerials
You might also like
- Lect 1.2 Principles of Food Process DesignDocument43 pagesLect 1.2 Principles of Food Process Designmahmoud hassanNo ratings yet
- Sizing and Rating BLOWDOWN - ExerciseDocument19 pagesSizing and Rating BLOWDOWN - Exercisepolaris44No ratings yet
- Jurnal Perancangan AlatDocument6 pagesJurnal Perancangan AlatFreeQueenNo ratings yet
- The Electrooxidation of Carbon Monoxide On Unsupported PT AgglomeratesDocument12 pagesThe Electrooxidation of Carbon Monoxide On Unsupported PT Agglomeratesmirybendecida91No ratings yet
- Korean Journal of Chemical Engineering, 28 (2), 383-387 (2011)Document5 pagesKorean Journal of Chemical Engineering, 28 (2), 383-387 (2011)KhangKGBNo ratings yet
- Electrochemical Behavior of Layered Solid Solution Li Mno 2limo (M 5 Ni, MN, Co) Li-Ion Cathodes With and Without Alumina CoatingsDocument7 pagesElectrochemical Behavior of Layered Solid Solution Li Mno 2limo (M 5 Ni, MN, Co) Li-Ion Cathodes With and Without Alumina CoatingsEYERUSALEM TADESSENo ratings yet
- Materials Research Bulletin: Lu Pan, Jing Tang, Fengwu WangDocument6 pagesMaterials Research Bulletin: Lu Pan, Jing Tang, Fengwu WangSHERLY KIMBERLY RAMOS JESUSNo ratings yet
- Fabrication of Ag Po /tio Composite and Its Photodegradation of Formaldehyde Under Solar RadiationDocument9 pagesFabrication of Ag Po /tio Composite and Its Photodegradation of Formaldehyde Under Solar Radiationcesafilho.idtNo ratings yet
- For Paper - 12Document13 pagesFor Paper - 12AlisaNo ratings yet
- Zou 2021Document7 pagesZou 202112334sohakamNo ratings yet
- Methanol OxidationDocument7 pagesMethanol OxidationJohndannNo ratings yet
- Journal of Colloid and Interface Science: Mohamed Mokhtar Mohamed, K.S. KhairouDocument9 pagesJournal of Colloid and Interface Science: Mohamed Mokhtar Mohamed, K.S. KhairouDMNo ratings yet
- Effect of Additives On Decomposition of Sodium Carbonate: Precombustion CO Capture Sorbent RegenerationDocument10 pagesEffect of Additives On Decomposition of Sodium Carbonate: Precombustion CO Capture Sorbent RegenerationKaededon't Deserve itNo ratings yet
- Reduced Graphene Oxide-Polypyrrole Composite As A Catalyst For Oxygen Electrode of High Rate Rechargeable Li-O CellsDocument7 pagesReduced Graphene Oxide-Polypyrrole Composite As A Catalyst For Oxygen Electrode of High Rate Rechargeable Li-O Cellswora123potNo ratings yet
- Self-Assembled Palladium Nanoparticles On Carbon NanofibersDocument6 pagesSelf-Assembled Palladium Nanoparticles On Carbon NanofiberswwNo ratings yet
- Mammen Jchemphys2015Document6 pagesMammen Jchemphys2015Luca BrunoNo ratings yet
- Referencia 74Document5 pagesReferencia 74XDEWSZAQNo ratings yet
- TS. Trương Thái Giang - Hội thảo khoa học Đại học Thành ĐôDocument10 pagesTS. Trương Thái Giang - Hội thảo khoa học Đại học Thành ĐôLưu Thu HàNo ratings yet
- Hrtem PTCDocument6 pagesHrtem PTCLuis Ramón GómezNo ratings yet
- Seventeen, Jan 2011Document4 pagesSeventeen, Jan 2011emediageNo ratings yet
- Ja8005918 BaozhenanDocument2 pagesJa8005918 BaozhenanYonggang ZhenNo ratings yet
- Arc Plasma Processing of PT and PD Catalysts SupportedDocument4 pagesArc Plasma Processing of PT and PD Catalysts SupportedFei ZhouNo ratings yet
- Removal of Lead From Crude Antimony by Using Napo As Lead Elimination ReagentDocument7 pagesRemoval of Lead From Crude Antimony by Using Napo As Lead Elimination ReagentTacachiri Chocamani JaimeNo ratings yet
- Co Nano Crystals On AluminaDocument6 pagesCo Nano Crystals On AluminaRamakanta SahuNo ratings yet
- AREA SUPERFICIALjanz2010Document7 pagesAREA SUPERFICIALjanz2010TOmmy QuantoNo ratings yet
- A Transient Response Study of The Selective Catalytic Oxidation of Ammonia To Nitrogen On Pt-CuO-Al2O3 Olofsson Et Al Chem. Eng. Sci. 2004Document11 pagesA Transient Response Study of The Selective Catalytic Oxidation of Ammonia To Nitrogen On Pt-CuO-Al2O3 Olofsson Et Al Chem. Eng. Sci. 2004juan davidNo ratings yet
- ABanuJOAMv12n5 p1189Document5 pagesABanuJOAMv12n5 p1189Alexandra BanuNo ratings yet
- Carbon Dioxide Reforming of Methane Over NiAl2O3 Treated With Glow Discharge PlasmaDocument6 pagesCarbon Dioxide Reforming of Methane Over NiAl2O3 Treated With Glow Discharge PlasmaViệtDũng TôNo ratings yet
- Carbon Dioxide Reforming of Methane Over NiAl2O3 Treated With Glow Discharge PlasmaDocument6 pagesCarbon Dioxide Reforming of Methane Over NiAl2O3 Treated With Glow Discharge PlasmaViệtDũng TôNo ratings yet
- 3 - Electrochem Acta 52 385 (2006)Document9 pages3 - Electrochem Acta 52 385 (2006)Érico Teixeira NetoNo ratings yet
- Mo2C-HYDocument11 pagesMo2C-HYioanaandra5690No ratings yet
- Wilder GetterDocument13 pagesWilder GetterFerhat Bozduman100% (1)
- Official URLDocument12 pagesOfficial URLHân TrầnNo ratings yet
- Gas Sensing Characteristics of Ultrathin Tio2-X Films Investigated With and in Situ XPS, TPD Resistance MeasurementsDocument5 pagesGas Sensing Characteristics of Ultrathin Tio2-X Films Investigated With and in Situ XPS, TPD Resistance MeasurementsRizky SåbillyNo ratings yet
- 1 s2.0 S096014819900097X MainDocument11 pages1 s2.0 S096014819900097X MaincimavNo ratings yet
- Inverse Spinel StructureDocument6 pagesInverse Spinel StructureJay Blų Flame FitzgeraldNo ratings yet
- Unusual High Oxygen Reduction Performance in All-Carbon ElectrocatalystsDocument7 pagesUnusual High Oxygen Reduction Performance in All-Carbon ElectrocatalystsVipin GNo ratings yet
- The Influence of The Silica/sodium Ratio On The Fly Ash Geopolymer BinderDocument6 pagesThe Influence of The Silica/sodium Ratio On The Fly Ash Geopolymer BinderJHON WILMAR CARDENAS PULIDONo ratings yet
- AmoolDocument12 pagesAmoolirfan k shahNo ratings yet
- SR Fe Mo O As Anodes For Solid Oxide Fuel Cells: Journal of Power SourcesDocument4 pagesSR Fe Mo O As Anodes For Solid Oxide Fuel Cells: Journal of Power SourcesJosé Luis Rosas HuertaNo ratings yet
- Electrocatalysis-of-CO-Tolerance-in-Hydrogen-Oxidation-Reaction-in-PEM-Fuel-CellsDocument11 pagesElectrocatalysis-of-CO-Tolerance-in-Hydrogen-Oxidation-Reaction-in-PEM-Fuel-CellsFaseeh KKNo ratings yet
- IR Ni LanthanaDocument6 pagesIR Ni LanthanaNelly RojasNo ratings yet
- Applied Catalysis, 31 (1987) 113-118 Elsevier Science Publishers B.V., Amsterdam - Printed in The Netherlands 113Document6 pagesApplied Catalysis, 31 (1987) 113-118 Elsevier Science Publishers B.V., Amsterdam - Printed in The Netherlands 113snurain_3No ratings yet
- Polyoxotungstate@Carbon Nanocomposites As Oxygen Reduction Reaction (ORR) ElectrocatalystsDocument12 pagesPolyoxotungstate@Carbon Nanocomposites As Oxygen Reduction Reaction (ORR) ElectrocatalystsHugo DuarteNo ratings yet
- Model NSR Catalysts Fabrication and Reactivity of Barium at Room TemperatureDocument16 pagesModel NSR Catalysts Fabrication and Reactivity of Barium at Room TemperatureSabri AeroChemNo ratings yet
- ManuscriptDocument4 pagesManuscriptapi-3728640No ratings yet
- Au-Pt/Co O Catalyst For Methane Combustion: LetterDocument4 pagesAu-Pt/Co O Catalyst For Methane Combustion: LetterfafaporkNo ratings yet
- Characterization of Anodic Spark-Converted Titanium Surfaces For Biomedical ApplicationsDocument5 pagesCharacterization of Anodic Spark-Converted Titanium Surfaces For Biomedical ApplicationstamilnaduNo ratings yet
- in Uence of TiO2 Crystal Structure On Acrylonitrile Decomposition Over Ag-TiO2Document4 pagesin Uence of TiO2 Crystal Structure On Acrylonitrile Decomposition Over Ag-TiO2Ngoc Ha NguyenNo ratings yet
- Suntivich JElectrochemSoc (2010) B1263Document7 pagesSuntivich JElectrochemSoc (2010) B1263Steph VazgalNo ratings yet
- SYNTHESIS AND APPLICATIONS OF TiO2 NANOPARTICLESDocument10 pagesSYNTHESIS AND APPLICATIONS OF TiO2 NANOPARTICLESSoheil MirtalebiNo ratings yet
- B9NR00186GDocument4 pagesB9NR00186Gcnu5322No ratings yet
- Nano-Engineering of Prussian Blue Analogues To Core-Shell ArchitecturesDocument7 pagesNano-Engineering of Prussian Blue Analogues To Core-Shell ArchitecturesprototyposNo ratings yet
- The EFTTRA-T3 Irradiation Experiment On Inert Matrix FuelsDocument11 pagesThe EFTTRA-T3 Irradiation Experiment On Inert Matrix FuelsWalid BadrNo ratings yet
- Effect of Non-Ionic Reagent Adsorption On Zeta Potential of Fine Coal Particles (#143037) - 124460Document13 pagesEffect of Non-Ionic Reagent Adsorption On Zeta Potential of Fine Coal Particles (#143037) - 124460ummuNo ratings yet
- For Paper - 16Document7 pagesFor Paper - 16AlisaNo ratings yet
- Comparision of The Flow in Co-Rotating and Counter-Rotating TwinscrewDocument11 pagesComparision of The Flow in Co-Rotating and Counter-Rotating TwinscrewChauNo ratings yet
- Cobalt Aluminate Spinel-DerivedDocument39 pagesCobalt Aluminate Spinel-DerivedMahdy HajienayatiNo ratings yet
- Electroflotation of Wastewater 41011686Document6 pagesElectroflotation of Wastewater 41011686Mardaru AnamariaNo ratings yet
- Platinum-Gold Nanoparticles: A Highly Active Bifunctional Electrocatalyst For Rechargeable Lithium-Air BatteriesDocument4 pagesPlatinum-Gold Nanoparticles: A Highly Active Bifunctional Electrocatalyst For Rechargeable Lithium-Air BatteriesAnthony RussellNo ratings yet
- 02.8 Differential Scanning Calorimetry (DSC)Document32 pages02.8 Differential Scanning Calorimetry (DSC)Lendel Mariz O. CepilloNo ratings yet
- Analytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryFrom EverandAnalytical Chemistry of Zirconium and Hafnium: International Series of Monographs in Analytical ChemistryNo ratings yet
- WS 2012 SM1 Worksheet2Document11 pagesWS 2012 SM1 Worksheet2Luis M. MolinaNo ratings yet
- ChisteDocument2 pagesChisteLuis M. MolinaNo ratings yet
- ProgrammeDocument4 pagesProgrammeLuis M. MolinaNo ratings yet
- Ou 2015Document7 pagesOu 2015Luis M. MolinaNo ratings yet
- Aguado 1999Document11 pagesAguado 1999Luis M. MolinaNo ratings yet
- The Quantum Sutton-Chen Many-Body Potential For PRDocument31 pagesThe Quantum Sutton-Chen Many-Body Potential For PRLuis M. MolinaNo ratings yet
- Aguado 2001Document9 pagesAguado 2001Luis M. MolinaNo ratings yet
- Sutton 1990Document9 pagesSutton 1990Luis M. MolinaNo ratings yet
- APS 2021 X10 Al2O3 CatalysisDocument15 pagesAPS 2021 X10 Al2O3 CatalysisLuis M. MolinaNo ratings yet
- CMS 2015 Pt3M CO OxidationDocument9 pagesCMS 2015 Pt3M CO OxidationLuis M. MolinaNo ratings yet
- Catalysis Ot ClustersDocument8 pagesCatalysis Ot ClustersLuis M. MolinaNo ratings yet
- Texlive en PDFDocument42 pagesTexlive en PDFLuis M. MolinaNo ratings yet
- Machine Learning For Interatomic Potential Models: Articles You May Be Interested inDocument14 pagesMachine Learning For Interatomic Potential Models: Articles You May Be Interested inLuis M. MolinaNo ratings yet
- DFT Study of Propene Epoxidation Mechanisms at Silver NanoparticlesDocument11 pagesDFT Study of Propene Epoxidation Mechanisms at Silver NanoparticlesLuis M. MolinaNo ratings yet
- AuN Plus Benz 1Document1 pageAuN Plus Benz 1Luis M. MolinaNo ratings yet
- Molecular Orbital of Chemisorbed Carbon Monoxide: GeohgeDocument6 pagesMolecular Orbital of Chemisorbed Carbon Monoxide: GeohgeLuis M. MolinaNo ratings yet
- Figure 2Document1 pageFigure 2Luis M. MolinaNo ratings yet
- Figure 1Document1 pageFigure 1Luis M. MolinaNo ratings yet
- Intercalation of Transition Metals Into Stacked Benzene Rings: A Model Study of The Intercalation of Transition Metals Into Bilayered GrapheneDocument7 pagesIntercalation of Transition Metals Into Stacked Benzene Rings: A Model Study of The Intercalation of Transition Metals Into Bilayered GrapheneLuis M. MolinaNo ratings yet
- Hydrocarbon Dew Point SpecificationDocument17 pagesHydrocarbon Dew Point Specificationargentino_ar01No ratings yet
- Review of Date FruitsDocument11 pagesReview of Date FruitsAiysah ArisNo ratings yet
- CHE1503-102 2016 2 B PDFDocument16 pagesCHE1503-102 2016 2 B PDFsal27adamNo ratings yet
- LSI Heritage Series Spec Sheet 1987Document6 pagesLSI Heritage Series Spec Sheet 1987Alan MastersNo ratings yet
- An Introduction To Density: by Helen Hanson & John MacalusoDocument23 pagesAn Introduction To Density: by Helen Hanson & John MacalusoMUHAMMAD AKRAMNo ratings yet
- Dispersion and Resolving Power of The Prism and Grating SpectroscopeDocument8 pagesDispersion and Resolving Power of The Prism and Grating SpectroscopeSanoj SebastianNo ratings yet
- AzollaDocument14 pagesAzollatunjungda100% (2)
- 756S PDFDocument248 pages756S PDFShahzad FidaNo ratings yet
- Delvocrete Stabiliser v6Document2 pagesDelvocrete Stabiliser v6Ashutosh RawatNo ratings yet
- ICSS YCChuangDocument3 pagesICSS YCChuangyi-chun.chuangNo ratings yet
- Module 6Document25 pagesModule 6ABIGAIL OLAJUMOKE JOSEPHNo ratings yet
- PCV - Ch1Document27 pagesPCV - Ch1Yathin gowda 8971095275No ratings yet
- 10 5923 S Ijee 201601 04Document9 pages10 5923 S Ijee 201601 04Guillermo TubillaNo ratings yet
- CH 150 Tutorial 4Document2 pagesCH 150 Tutorial 4DechenPemaNo ratings yet
- Process Simulation in Refineries Sampler PDFDocument30 pagesProcess Simulation in Refineries Sampler PDFNguyễn Tiến Dũng100% (2)
- Uniflow Copper Tubes TDSDocument7 pagesUniflow Copper Tubes TDSFilorNo ratings yet
- 3026 2091P100Filter PDFDocument2 pages3026 2091P100Filter PDFZeckNo ratings yet
- Connection - IDEA StatiCa PDFDocument18 pagesConnection - IDEA StatiCa PDFDaniel TuerosNo ratings yet
- Spiraxsarco Pipe SizingDocument52 pagesSpiraxsarco Pipe SizingJan Van PeteghemNo ratings yet
- Bose-Einstein, Fermi-Dirac and Maxwell-Boltzman DistributionDocument5 pagesBose-Einstein, Fermi-Dirac and Maxwell-Boltzman DistributionElumalaiNo ratings yet
- Guidelines On The Procedures and Technical Requirements For The Issuance of A Certification Allowing The Safe Re-Use of Wastewater For Purposes of Irrigation and Other Agricultural UsesDocument28 pagesGuidelines On The Procedures and Technical Requirements For The Issuance of A Certification Allowing The Safe Re-Use of Wastewater For Purposes of Irrigation and Other Agricultural Usesgabinuang2No ratings yet
- Fkarimi,+Journal+Manager,+3 DrBadawyDocument10 pagesFkarimi,+Journal+Manager,+3 DrBadawyLina WinartiNo ratings yet
- GENERAL-CHEMISTRY-2 - Q3 - M1-Intermolecular ForcesDocument20 pagesGENERAL-CHEMISTRY-2 - Q3 - M1-Intermolecular ForcesJaime DimariaNo ratings yet
- Paper 2 SetB 090410 KeyDocument12 pagesPaper 2 SetB 090410 KeyNataraj Singh SardarNo ratings yet
- RTS PS 2024ref2023ftsDocument3 pagesRTS PS 2024ref2023ftsmohitabochare2039No ratings yet
- Page 1 of 17 Sacred Heart of Mary Girls' SchoolDocument17 pagesPage 1 of 17 Sacred Heart of Mary Girls' SchoolAngeleena ANTONo ratings yet
- 01-02. The Chemical Context of LifeDocument4 pages01-02. The Chemical Context of LifeDaniel Angelo MiradorNo ratings yet
- 06 - Thermodynamic - Cycles - (Frigo) 1 PDFDocument44 pages06 - Thermodynamic - Cycles - (Frigo) 1 PDFAntonio Di FioreNo ratings yet