Professional Documents
Culture Documents
2019 Y5 Work Book 2A (Practical 5-1) Tutor - 260619
2019 Y5 Work Book 2A (Practical 5-1) Tutor - 260619
Uploaded by
ChenluyingCopyright:
Available Formats
You might also like
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- Project Plan Simple Electrical CircuitDocument3 pagesProject Plan Simple Electrical CircuitDesyrie Joy Soriano Diray100% (1)
- Final Revision Guide Paper 6Document67 pagesFinal Revision Guide Paper 6Fares Tamer100% (1)
- Jar Test ReportDocument8 pagesJar Test ReportHeLmi Hendrix75% (4)
- Instagram Creators Handbook - IGTV PDFDocument48 pagesInstagram Creators Handbook - IGTV PDFAndrei Neațu100% (1)
- Manual Vindusviskere Ocean Operator's Manual Straight Line Wiper OceanDocument10 pagesManual Vindusviskere Ocean Operator's Manual Straight Line Wiper OceantylerdurdaneNo ratings yet
- F10 Taps in Auto Transformers PaperDocument55 pagesF10 Taps in Auto Transformers Paperverma210No ratings yet
- Airbus vs. Boeing NotesDocument4 pagesAirbus vs. Boeing NotesEsteban AguirreNo ratings yet
- Rate of Reaction of Sodium Thiosulfate and Hydrochloric AcidDocument5 pagesRate of Reaction of Sodium Thiosulfate and Hydrochloric AcidTeacher AlexNo ratings yet
- Physical Exp1Document4 pagesPhysical Exp1shielasamvuraNo ratings yet
- 2019 Y5 Work Book 1 (Practical 3) SolutionsDocument9 pages2019 Y5 Work Book 1 (Practical 3) SolutionsChenluyingNo ratings yet
- 2019 Y5 Work Book 1 (Practical 4) SolutionsDocument9 pages2019 Y5 Work Book 1 (Practical 4) SolutionsChenluyingNo ratings yet
- 9701 s09 QP 31 PDFDocument12 pages9701 s09 QP 31 PDFtess_15No ratings yet
- Fourth Cycle PracticalsDocument8 pagesFourth Cycle PracticalsMohammad Bin MahmoodNo ratings yet
- 1 PR QJJB KYNWDGW1 N SGN EDocument3 pages1 PR QJJB KYNWDGW1 N SGN EPurnima ENo ratings yet
- Laboratory Experiment No. 2 - Instructional MaterialDocument3 pagesLaboratory Experiment No. 2 - Instructional MaterialsgagustinNo ratings yet
- 12.1 Rates of Reaction Worksheet 12.1.1 Following The Rate of A ReactionDocument2 pages12.1 Rates of Reaction Worksheet 12.1.1 Following The Rate of A ReactionAla' ShehadehNo ratings yet
- Gabrielle Robinson - 601 Labs 2021Document13 pagesGabrielle Robinson - 601 Labs 2021Gabrielle RobinsonNo ratings yet
- IGCSE Chemistry 0620 - 2018 Ques PaperDocument12 pagesIGCSE Chemistry 0620 - 2018 Ques PaperMinakshiNo ratings yet
- Week 3 (2) - HYDROMETER (Level 0)Document5 pagesWeek 3 (2) - HYDROMETER (Level 0)Mohd YusriNo ratings yet
- Effect of (Substrate) ConcentrationDocument2 pagesEffect of (Substrate) ConcentrationrawldabishopNo ratings yet
- Chem. Lab 1Document4 pagesChem. Lab 1Sofiia BentsaNo ratings yet
- 2024 H2 Reaction Kinetics Tutorial QnsDocument12 pages2024 H2 Reaction Kinetics Tutorial Qnsjrwayne1709No ratings yet
- Experiment 4: Water Analysis Solids Gallardo, Hans Tristan MDocument7 pagesExperiment 4: Water Analysis Solids Gallardo, Hans Tristan Mjamila milanoNo ratings yet
- Cambridge International AS & A Level: CHEMISTRY 9701/34Document16 pagesCambridge International AS & A Level: CHEMISTRY 9701/34Sonal SinglaNo ratings yet
- Geomatics Engineering: University of TechnologyDocument7 pagesGeomatics Engineering: University of TechnologyEilya Al-mafrajjeNo ratings yet
- Aisyah - Practical Activity On The Rate Law of ReactionDocument5 pagesAisyah - Practical Activity On The Rate Law of ReactionAisyah AlkatiriNo ratings yet
- Relatório de Aula Prática: Medição de Vazão Com Traçadores LíquidosDocument10 pagesRelatório de Aula Prática: Medição de Vazão Com Traçadores LíquidosRafael MurbakNo ratings yet
- Rate of Reaction PDFDocument6 pagesRate of Reaction PDFTan Yan YingNo ratings yet
- My TestDocument33 pagesMy TestqusaielnoorNo ratings yet
- 2023 M5W8 - Mock Chemistry Practical Examination 2 (Part 2)Document6 pages2023 M5W8 - Mock Chemistry Practical Examination 2 (Part 2)shakthee sivakumarNo ratings yet
- 9701 s06 QP 3Document8 pages9701 s06 QP 3Hubbak KhanNo ratings yet
- Chem Sba#13Document3 pagesChem Sba#13Ridhi ParwaniNo ratings yet
- Chem 1Document12 pagesChem 1zaeemhussain665No ratings yet
- Lab 4 - Hydrometer Testnvxjkcvbcxckvbckjvjkvknvck.Document6 pagesLab 4 - Hydrometer Testnvxjkcvbcxckvbckjvjkvknvck.Amirah ShafeeraNo ratings yet
- Unit 1 Manual 2019Document18 pagesUnit 1 Manual 2019JozelleNo ratings yet
- JURNAL8 Ayu 011Document7 pagesJURNAL8 Ayu 011Ayu SuwarniNo ratings yet
- Sulit 4531/3 1: Jawab Semua Soalan Dalam Bahagian IniDocument10 pagesSulit 4531/3 1: Jawab Semua Soalan Dalam Bahagian IniZkria AbdullahNo ratings yet
- (M1.2) REVIEW & COMPLETE - Practical 4.1 The Kinetics of The Reaction Between CaCO3 and HCLDocument3 pages(M1.2) REVIEW & COMPLETE - Practical 4.1 The Kinetics of The Reaction Between CaCO3 and HCLSalma ShakiraNo ratings yet
- Rate of ReactionDocument9 pagesRate of ReactionShamshul Didarelly0% (1)
- Experiment Number: 1a: Gazi University Chemical Engineering Department KM 380E Chemical Engineering Laboratory 1Document6 pagesExperiment Number: 1a: Gazi University Chemical Engineering Department KM 380E Chemical Engineering Laboratory 1ze usNo ratings yet
- 2019 Y5 Work Book 1 (Practical 1) Suggested SolutionsDocument6 pages2019 Y5 Work Book 1 (Practical 1) Suggested SolutionsChenluyingNo ratings yet
- Exp 01Document4 pagesExp 01Hasun MadurangaNo ratings yet
- ES550-Geomorphology LAB 3: Weathering: NameDocument14 pagesES550-Geomorphology LAB 3: Weathering: NamePamela Nicole DomingoNo ratings yet
- Drainage Time of PulpDocument13 pagesDrainage Time of PulpAndrei MurillonNo ratings yet
- Module Form 5 .Rate of ReactionDocument8 pagesModule Form 5 .Rate of ReactionChew Gee Lan100% (1)
- Rates of ReactionDocument2 pagesRates of Reactionlee (nyto)No ratings yet
- Practical Paper - 20-09-22Document3 pagesPractical Paper - 20-09-22FirestoneGamer 182No ratings yet
- Rates of Reaction LabDocument1 pageRates of Reaction LabKyeNo ratings yet
- Zeynep Yılmaz 2371359 HydrometerDocument9 pagesZeynep Yılmaz 2371359 HydrometerEmre UgurNo ratings yet
- Sedimentation Practicum - Unit Operation in Enviornmental EngineeringDocument23 pagesSedimentation Practicum - Unit Operation in Enviornmental EngineeringNaufal BariqueNo ratings yet
- Chemistry Mock IADocument12 pagesChemistry Mock IAYAMAMOTO KeijiNo ratings yet
- Fluids Lab Manual 1Document6 pagesFluids Lab Manual 1اسامة نعمة جبارNo ratings yet
- Bio122 Lab 1Document8 pagesBio122 Lab 1Sano YamikoNo ratings yet
- A Levels Chemistry November 2012 Question Paper 31Document16 pagesA Levels Chemistry November 2012 Question Paper 31Dhakal SauhardaNo ratings yet
- Measuring The Rate of A ReactionDocument2 pagesMeasuring The Rate of A ReactionShahid Ur RehmanNo ratings yet
- Spectrophotometric Determination of Hydrogen Sulfide PDFDocument3 pagesSpectrophotometric Determination of Hydrogen Sulfide PDFVictor HugoNo ratings yet
- 9701 w03 QP 3Document8 pages9701 w03 QP 3Hubbak KhanNo ratings yet
- Jar Test ReportDocument11 pagesJar Test ReportHalimi Honan100% (1)
- Chem GauravDocument11 pagesChem GauravKaran YadavNo ratings yet
- Homework 1Document3 pagesHomework 1Study StudyNo ratings yet
- Soil TextureDocument5 pagesSoil TextureJohn-Paul MollineauxNo ratings yet
- O Level Biology Practice For Structured Questions Movement Of SubstancesFrom EverandO Level Biology Practice For Structured Questions Movement Of SubstancesNo ratings yet
- 2019 Y5 Work Book 1 (Practical 3) SolutionsDocument9 pages2019 Y5 Work Book 1 (Practical 3) SolutionsChenluyingNo ratings yet
- 2019 Y5 Work Book 1 (Practical 4) SolutionsDocument9 pages2019 Y5 Work Book 1 (Practical 4) SolutionsChenluyingNo ratings yet
- 2019 Y5 Work Book 2A (Practical 1-1) Tutor - 030519Document8 pages2019 Y5 Work Book 2A (Practical 1-1) Tutor - 030519ChenluyingNo ratings yet
- 2019 Y5 Work Book 1 (Practical 1) Suggested SolutionsDocument6 pages2019 Y5 Work Book 1 (Practical 1) Suggested SolutionsChenluyingNo ratings yet
- Pre-Lab Assignment:: 2019 DHS Year 5Document14 pagesPre-Lab Assignment:: 2019 DHS Year 5ChenluyingNo ratings yet
- 6 - Portal Frame Method PDFDocument62 pages6 - Portal Frame Method PDFMohamad DuhokiNo ratings yet
- Introduction To Malware Techniques and Logics Part 1 by GuntherDocument38 pagesIntroduction To Malware Techniques and Logics Part 1 by Guntherspeed22slowNo ratings yet
- G484 Jan 11Document12 pagesG484 Jan 11samy9387No ratings yet
- Code Vision AVRDocument3 pagesCode Vision AVRMohammed AsharNo ratings yet
- FB2 - RB2Document15 pagesFB2 - RB2Sajida QadeerNo ratings yet
- Worls First CNG Ship in Indonesia Presentation by Bima PSDocument20 pagesWorls First CNG Ship in Indonesia Presentation by Bima PSAnonymous icnhaNsF100% (2)
- Business Analyses CourseDocument6 pagesBusiness Analyses Coursetangwanlu9177No ratings yet
- KPP 2020Document1 pageKPP 2020Dino BajloNo ratings yet
- DD Env 1993-1-2-2001Document81 pagesDD Env 1993-1-2-2001Zac Francis DaymondNo ratings yet
- Understanding DSP's Frequency Domain, Part 1: by Richard LyonsDocument4 pagesUnderstanding DSP's Frequency Domain, Part 1: by Richard LyonsraviNo ratings yet
- RDM073 - Kilimanjaro VII - RDM ConstructionDocument7 pagesRDM073 - Kilimanjaro VII - RDM ConstructionNico LomibaoNo ratings yet
- Handbook On Achieving Thermal Comfort Within Built EnvironmentDocument56 pagesHandbook On Achieving Thermal Comfort Within Built EnvironmentvandniNo ratings yet
- 2449 Ex4Document15 pages2449 Ex4JC ZuletNo ratings yet
- Microsoft PowerPoint - Lecture-1 - IntroDocument33 pagesMicrosoft PowerPoint - Lecture-1 - IntroIndhujaTNo ratings yet
- Caja de Cambio Caracteristicas T318 PDFDocument2 pagesCaja de Cambio Caracteristicas T318 PDFLenny Virgo100% (1)
- Modullation PPT NewDocument58 pagesModullation PPT NewMayank TripathiNo ratings yet
- Barden Speciality Products Us en PDFDocument73 pagesBarden Speciality Products Us en PDFjjcadena2000No ratings yet
- CHE 260 Week 11 Solutions-1Document4 pagesCHE 260 Week 11 Solutions-1erfanyeganehfarNo ratings yet
- Introduction To Geology - MCQsDocument2 pagesIntroduction To Geology - MCQsmohan kumar100% (1)
- TIKODocument88 pagesTIKOLembaga Sertifikasi ProfesiNo ratings yet
- Carbon Steel Electrodes: Safety Data SheetDocument10 pagesCarbon Steel Electrodes: Safety Data SheetOmar DSNo ratings yet
- Saudi Arabian Oil Company: Raw SewageDocument2 pagesSaudi Arabian Oil Company: Raw SewageAdnanNo ratings yet
- Recombinant Therapeutic Protein Production in Cultivated Mammalian Cells: Current Status and Future ProspectsDocument6 pagesRecombinant Therapeutic Protein Production in Cultivated Mammalian Cells: Current Status and Future ProspectsGryseldaNo ratings yet
- Astm C 582Document7 pagesAstm C 582Tanktech TanktechNo ratings yet
- A Wide-Range, High-Resolution, Compact, CMOS Time To Digital ConverterDocument6 pagesA Wide-Range, High-Resolution, Compact, CMOS Time To Digital ConverterShruti KalraNo ratings yet
2019 Y5 Work Book 2A (Practical 5-1) Tutor - 260619
2019 Y5 Work Book 2A (Practical 5-1) Tutor - 260619
Uploaded by
ChenluyingOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2019 Y5 Work Book 2A (Practical 5-1) Tutor - 260619
2019 Y5 Work Book 2A (Practical 5-1) Tutor - 260619
Uploaded by
ChenluyingCopyright:
Available Formats
2019 DHS Year 5
Practical 5–1: [Kinetics]
To investigate the kinetics of the reaction between thiosulfate ions and acid
Aim of To investigate the kinetics of the reaction between thiosulfate ions and acid
Experiment:
Skills: After this experiment, you should be able to:
• deduce volume proportional to concentration;
• analyse data graphically or with the use of logical reasoning to find orders of
reaction;
• conduct a clock reaction/ experiment;
• use initial rate method to determine order of reaction.
Description: When aqueous sodium thiosulfate is mixed with hydrochloric acid, a suspension
of solid sulfur is formed.
Na2S2O3 (aq) + 2HCl (aq) → 2NaCl (aq) + SO2 (g) + S (s) + H2O (l)
Using FA 1, FA 2 and distilled water, you are to perform experiments to investigate
the kinetics of the reaction between thiosulfate ions and acid.
Pre–Lab You are required to view the LabSkills module and attempt the interactive
Assignment: exercises on Reaction Rate under ‘Lab Calculations_Reaction Rates’ &
Common Experiments_Iodine Clock under ‘Lab Calculations_Reaction
Rates’ before this practical lesson.
You are also required to read the following pages in Practical Handbook 2:
Clock Reaction
Keeping Total Volume of Mixture Constant
© Dunman High School 1
2019 DHS Year 5
Chemicals & You are provided with
Apparatus:
FA 1 is 1.00 mol dm–3 aqueous hydrochloric acid, HCl
FA 2 is 0.10 mol dm–3 aqueous sodium thiosulfate, Na2S2O3
S/N Apparatus Quantity per student
2
plastic measuring
1 100 cm3 measuring cylinder
cylinder to measure
water
2 50 cm3 measuring cylinder 1
3 250 cm3 beaker (clean & dry) 3
4 stop watch 1
5 plastic dropper 2
6 distilled bottle 1
7 card with X 1
Procedure: 1. Using an appropriate measuring cylinder, transfer 20.0 cm3 of FA 1 into a
clean, dry 250 cm3 beaker. Stand the beaker over the printed insert.
2. Transfer 40.0 cm3 of distilled water using another measuring cylinder into
the beaker containing FA 1.
3. By means of another measuring cylinder, pour 50.0 cm3 of FA 2 into the
beaker containing FA 1 and simultaneously start the stopwatch. Swirl the
beaker quickly to allow the reagents to mix, taking care not to spill the
contents. Allow the mixture to stand.
4. Note the time taken, to the nearest second, for the printed insert to
just become completely obscured by sulfur produced in the reaction.
Record your readings as mixture 3 as indicated in the table on the
next page.
5. Calculate the value of the relative rate (given as the reciprocal of the time
taken).
6. Rinse the beaker thoroughly and dry it.
7. Repeat the experiment using four other different volumes of FA 2 and
distilled water. Choose two volumes of FA 2 higher, and two other volumes
lower than 50 cm3, such that there are five volumes of FA 2 between 30 –
70 cm3 in total. Vary the volume of distilled water such that the total volume
of the mixture is kept the same for all five mixtures. Record the time taken
and calculate the values of the relative rate for each mixture.
© Dunman High School 2
2019 DHS Year 5
Results
Volume of Volume of Volume of Relative rate
Mixture FA 1 / cm3 Water / cm3 FA 2 / cm3 Time, t / s 1
( ) / s–1
t
1 20.0 20.0 70.0 32 0.0313
2 20.0 30.0 60.0 38 0.0263
3 20.0 40.0 50.0 48 0.0208
4 20.0 50.0 40.0 60 0.0167
5 20.0 60.0 30.0 89 0.0112
All time readings to nearest 1s [1]
Correctly calculated relative rate, to 3 s.f [1]
Volume of FA 2 chosen are between 30 – 70 cm3 and
total volume of each mixture = 110 cm3 [1]
[3]
© Dunman High School 3
2019 DHS Year 5
Questions:
(a) Explain whether it is valid to assume that the concentration of S2O32– can be represented by
the volume of S2O32– across all 5 experiments
It is valid to assume that concentration of S2O32– can be represented by the volume of S2O32–
in the conducted experiments as
S2O3 2 S2O3
2
vol of
S2O3
2
FA 2 [1]
mixture total volume of mixture
Since the total volume of each mixture is the same at 110 cm3, with the addition of varying
volume of water, and S2O3
2
is also constant [1],
FA 2
[S2O32–]mixture = k (Volume of S2O32–.)
[S2O32–]mixture Volume of S2O32–.
This ensures that [S2O32–]mixture Volume of S2O32–. [1]
[3]
© Dunman High School 4
2019 DHS Year 5
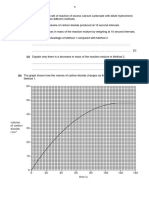
(bi) Plot a graph on the grid provided below to find order of reaction with respect to S2O32-.
Correct axes and labelling of axes with units [1]
Dependent variable: relative rate & independent variable: volume of FA2; wrong
axes [0]
Appropriate scale (occupy at least half the graph grid) [1]
Correct plotting of all 5 points [1]
Straight line graph [1]
All 5 points are no further than one small square away from line in either direction
(ie. best–fit) [1]
[5]
© Dunman High School 5
2019 DHS Year 5
(bii) Based on the graph plotted in (b)(i), determine, with reasoning, the order of reaction with
respect to thiosulfate ions.
[2]
The volume of HCl used was kept constant at 20.0 cm3 and volume of Na2S2O3 is
proportional to concentration of Na2S2O3 in a constant total volume mixture.
Since the graph obtained shows a straight upward sloping line, it indicates that the rate is
directly proportional to the concentration of Na2S2O3. [1]
Thus, the reaction is first order with respect to thiosulfate ions. [1]
(c) Using your graph in (b)(i), determine the time taken for the sulfur to appear when
58 cm3 of FA 2 is mixed with 20 cm3 of FA 1 and 32 cm3 of water.
[2]
From the graph,
for 58 cm3 of FA 2, 1/t = 0.0253 s–1 [1] – with working shown on graph
t, time taken for sulfur to appear = 39.5 s [1]
© Dunman High School 6
You might also like
- O Level Biology Practice Questions And Answers EnzymesFrom EverandO Level Biology Practice Questions And Answers EnzymesRating: 5 out of 5 stars5/5 (1)
- Project Plan Simple Electrical CircuitDocument3 pagesProject Plan Simple Electrical CircuitDesyrie Joy Soriano Diray100% (1)
- Final Revision Guide Paper 6Document67 pagesFinal Revision Guide Paper 6Fares Tamer100% (1)
- Jar Test ReportDocument8 pagesJar Test ReportHeLmi Hendrix75% (4)
- Instagram Creators Handbook - IGTV PDFDocument48 pagesInstagram Creators Handbook - IGTV PDFAndrei Neațu100% (1)
- Manual Vindusviskere Ocean Operator's Manual Straight Line Wiper OceanDocument10 pagesManual Vindusviskere Ocean Operator's Manual Straight Line Wiper OceantylerdurdaneNo ratings yet
- F10 Taps in Auto Transformers PaperDocument55 pagesF10 Taps in Auto Transformers Paperverma210No ratings yet
- Airbus vs. Boeing NotesDocument4 pagesAirbus vs. Boeing NotesEsteban AguirreNo ratings yet
- Rate of Reaction of Sodium Thiosulfate and Hydrochloric AcidDocument5 pagesRate of Reaction of Sodium Thiosulfate and Hydrochloric AcidTeacher AlexNo ratings yet
- Physical Exp1Document4 pagesPhysical Exp1shielasamvuraNo ratings yet
- 2019 Y5 Work Book 1 (Practical 3) SolutionsDocument9 pages2019 Y5 Work Book 1 (Practical 3) SolutionsChenluyingNo ratings yet
- 2019 Y5 Work Book 1 (Practical 4) SolutionsDocument9 pages2019 Y5 Work Book 1 (Practical 4) SolutionsChenluyingNo ratings yet
- 9701 s09 QP 31 PDFDocument12 pages9701 s09 QP 31 PDFtess_15No ratings yet
- Fourth Cycle PracticalsDocument8 pagesFourth Cycle PracticalsMohammad Bin MahmoodNo ratings yet
- 1 PR QJJB KYNWDGW1 N SGN EDocument3 pages1 PR QJJB KYNWDGW1 N SGN EPurnima ENo ratings yet
- Laboratory Experiment No. 2 - Instructional MaterialDocument3 pagesLaboratory Experiment No. 2 - Instructional MaterialsgagustinNo ratings yet
- 12.1 Rates of Reaction Worksheet 12.1.1 Following The Rate of A ReactionDocument2 pages12.1 Rates of Reaction Worksheet 12.1.1 Following The Rate of A ReactionAla' ShehadehNo ratings yet
- Gabrielle Robinson - 601 Labs 2021Document13 pagesGabrielle Robinson - 601 Labs 2021Gabrielle RobinsonNo ratings yet
- IGCSE Chemistry 0620 - 2018 Ques PaperDocument12 pagesIGCSE Chemistry 0620 - 2018 Ques PaperMinakshiNo ratings yet
- Week 3 (2) - HYDROMETER (Level 0)Document5 pagesWeek 3 (2) - HYDROMETER (Level 0)Mohd YusriNo ratings yet
- Effect of (Substrate) ConcentrationDocument2 pagesEffect of (Substrate) ConcentrationrawldabishopNo ratings yet
- Chem. Lab 1Document4 pagesChem. Lab 1Sofiia BentsaNo ratings yet
- 2024 H2 Reaction Kinetics Tutorial QnsDocument12 pages2024 H2 Reaction Kinetics Tutorial Qnsjrwayne1709No ratings yet
- Experiment 4: Water Analysis Solids Gallardo, Hans Tristan MDocument7 pagesExperiment 4: Water Analysis Solids Gallardo, Hans Tristan Mjamila milanoNo ratings yet
- Cambridge International AS & A Level: CHEMISTRY 9701/34Document16 pagesCambridge International AS & A Level: CHEMISTRY 9701/34Sonal SinglaNo ratings yet
- Geomatics Engineering: University of TechnologyDocument7 pagesGeomatics Engineering: University of TechnologyEilya Al-mafrajjeNo ratings yet
- Aisyah - Practical Activity On The Rate Law of ReactionDocument5 pagesAisyah - Practical Activity On The Rate Law of ReactionAisyah AlkatiriNo ratings yet
- Relatório de Aula Prática: Medição de Vazão Com Traçadores LíquidosDocument10 pagesRelatório de Aula Prática: Medição de Vazão Com Traçadores LíquidosRafael MurbakNo ratings yet
- Rate of Reaction PDFDocument6 pagesRate of Reaction PDFTan Yan YingNo ratings yet
- My TestDocument33 pagesMy TestqusaielnoorNo ratings yet
- 2023 M5W8 - Mock Chemistry Practical Examination 2 (Part 2)Document6 pages2023 M5W8 - Mock Chemistry Practical Examination 2 (Part 2)shakthee sivakumarNo ratings yet
- 9701 s06 QP 3Document8 pages9701 s06 QP 3Hubbak KhanNo ratings yet
- Chem Sba#13Document3 pagesChem Sba#13Ridhi ParwaniNo ratings yet
- Chem 1Document12 pagesChem 1zaeemhussain665No ratings yet
- Lab 4 - Hydrometer Testnvxjkcvbcxckvbckjvjkvknvck.Document6 pagesLab 4 - Hydrometer Testnvxjkcvbcxckvbckjvjkvknvck.Amirah ShafeeraNo ratings yet
- Unit 1 Manual 2019Document18 pagesUnit 1 Manual 2019JozelleNo ratings yet
- JURNAL8 Ayu 011Document7 pagesJURNAL8 Ayu 011Ayu SuwarniNo ratings yet
- Sulit 4531/3 1: Jawab Semua Soalan Dalam Bahagian IniDocument10 pagesSulit 4531/3 1: Jawab Semua Soalan Dalam Bahagian IniZkria AbdullahNo ratings yet
- (M1.2) REVIEW & COMPLETE - Practical 4.1 The Kinetics of The Reaction Between CaCO3 and HCLDocument3 pages(M1.2) REVIEW & COMPLETE - Practical 4.1 The Kinetics of The Reaction Between CaCO3 and HCLSalma ShakiraNo ratings yet
- Rate of ReactionDocument9 pagesRate of ReactionShamshul Didarelly0% (1)
- Experiment Number: 1a: Gazi University Chemical Engineering Department KM 380E Chemical Engineering Laboratory 1Document6 pagesExperiment Number: 1a: Gazi University Chemical Engineering Department KM 380E Chemical Engineering Laboratory 1ze usNo ratings yet
- 2019 Y5 Work Book 1 (Practical 1) Suggested SolutionsDocument6 pages2019 Y5 Work Book 1 (Practical 1) Suggested SolutionsChenluyingNo ratings yet
- Exp 01Document4 pagesExp 01Hasun MadurangaNo ratings yet
- ES550-Geomorphology LAB 3: Weathering: NameDocument14 pagesES550-Geomorphology LAB 3: Weathering: NamePamela Nicole DomingoNo ratings yet
- Drainage Time of PulpDocument13 pagesDrainage Time of PulpAndrei MurillonNo ratings yet
- Module Form 5 .Rate of ReactionDocument8 pagesModule Form 5 .Rate of ReactionChew Gee Lan100% (1)
- Rates of ReactionDocument2 pagesRates of Reactionlee (nyto)No ratings yet
- Practical Paper - 20-09-22Document3 pagesPractical Paper - 20-09-22FirestoneGamer 182No ratings yet
- Rates of Reaction LabDocument1 pageRates of Reaction LabKyeNo ratings yet
- Zeynep Yılmaz 2371359 HydrometerDocument9 pagesZeynep Yılmaz 2371359 HydrometerEmre UgurNo ratings yet
- Sedimentation Practicum - Unit Operation in Enviornmental EngineeringDocument23 pagesSedimentation Practicum - Unit Operation in Enviornmental EngineeringNaufal BariqueNo ratings yet
- Chemistry Mock IADocument12 pagesChemistry Mock IAYAMAMOTO KeijiNo ratings yet
- Fluids Lab Manual 1Document6 pagesFluids Lab Manual 1اسامة نعمة جبارNo ratings yet
- Bio122 Lab 1Document8 pagesBio122 Lab 1Sano YamikoNo ratings yet
- A Levels Chemistry November 2012 Question Paper 31Document16 pagesA Levels Chemistry November 2012 Question Paper 31Dhakal SauhardaNo ratings yet
- Measuring The Rate of A ReactionDocument2 pagesMeasuring The Rate of A ReactionShahid Ur RehmanNo ratings yet
- Spectrophotometric Determination of Hydrogen Sulfide PDFDocument3 pagesSpectrophotometric Determination of Hydrogen Sulfide PDFVictor HugoNo ratings yet
- 9701 w03 QP 3Document8 pages9701 w03 QP 3Hubbak KhanNo ratings yet
- Jar Test ReportDocument11 pagesJar Test ReportHalimi Honan100% (1)
- Chem GauravDocument11 pagesChem GauravKaran YadavNo ratings yet
- Homework 1Document3 pagesHomework 1Study StudyNo ratings yet
- Soil TextureDocument5 pagesSoil TextureJohn-Paul MollineauxNo ratings yet
- O Level Biology Practice For Structured Questions Movement Of SubstancesFrom EverandO Level Biology Practice For Structured Questions Movement Of SubstancesNo ratings yet
- 2019 Y5 Work Book 1 (Practical 3) SolutionsDocument9 pages2019 Y5 Work Book 1 (Practical 3) SolutionsChenluyingNo ratings yet
- 2019 Y5 Work Book 1 (Practical 4) SolutionsDocument9 pages2019 Y5 Work Book 1 (Practical 4) SolutionsChenluyingNo ratings yet
- 2019 Y5 Work Book 2A (Practical 1-1) Tutor - 030519Document8 pages2019 Y5 Work Book 2A (Practical 1-1) Tutor - 030519ChenluyingNo ratings yet
- 2019 Y5 Work Book 1 (Practical 1) Suggested SolutionsDocument6 pages2019 Y5 Work Book 1 (Practical 1) Suggested SolutionsChenluyingNo ratings yet
- Pre-Lab Assignment:: 2019 DHS Year 5Document14 pagesPre-Lab Assignment:: 2019 DHS Year 5ChenluyingNo ratings yet
- 6 - Portal Frame Method PDFDocument62 pages6 - Portal Frame Method PDFMohamad DuhokiNo ratings yet
- Introduction To Malware Techniques and Logics Part 1 by GuntherDocument38 pagesIntroduction To Malware Techniques and Logics Part 1 by Guntherspeed22slowNo ratings yet
- G484 Jan 11Document12 pagesG484 Jan 11samy9387No ratings yet
- Code Vision AVRDocument3 pagesCode Vision AVRMohammed AsharNo ratings yet
- FB2 - RB2Document15 pagesFB2 - RB2Sajida QadeerNo ratings yet
- Worls First CNG Ship in Indonesia Presentation by Bima PSDocument20 pagesWorls First CNG Ship in Indonesia Presentation by Bima PSAnonymous icnhaNsF100% (2)
- Business Analyses CourseDocument6 pagesBusiness Analyses Coursetangwanlu9177No ratings yet
- KPP 2020Document1 pageKPP 2020Dino BajloNo ratings yet
- DD Env 1993-1-2-2001Document81 pagesDD Env 1993-1-2-2001Zac Francis DaymondNo ratings yet
- Understanding DSP's Frequency Domain, Part 1: by Richard LyonsDocument4 pagesUnderstanding DSP's Frequency Domain, Part 1: by Richard LyonsraviNo ratings yet
- RDM073 - Kilimanjaro VII - RDM ConstructionDocument7 pagesRDM073 - Kilimanjaro VII - RDM ConstructionNico LomibaoNo ratings yet
- Handbook On Achieving Thermal Comfort Within Built EnvironmentDocument56 pagesHandbook On Achieving Thermal Comfort Within Built EnvironmentvandniNo ratings yet
- 2449 Ex4Document15 pages2449 Ex4JC ZuletNo ratings yet
- Microsoft PowerPoint - Lecture-1 - IntroDocument33 pagesMicrosoft PowerPoint - Lecture-1 - IntroIndhujaTNo ratings yet
- Caja de Cambio Caracteristicas T318 PDFDocument2 pagesCaja de Cambio Caracteristicas T318 PDFLenny Virgo100% (1)
- Modullation PPT NewDocument58 pagesModullation PPT NewMayank TripathiNo ratings yet
- Barden Speciality Products Us en PDFDocument73 pagesBarden Speciality Products Us en PDFjjcadena2000No ratings yet
- CHE 260 Week 11 Solutions-1Document4 pagesCHE 260 Week 11 Solutions-1erfanyeganehfarNo ratings yet
- Introduction To Geology - MCQsDocument2 pagesIntroduction To Geology - MCQsmohan kumar100% (1)
- TIKODocument88 pagesTIKOLembaga Sertifikasi ProfesiNo ratings yet
- Carbon Steel Electrodes: Safety Data SheetDocument10 pagesCarbon Steel Electrodes: Safety Data SheetOmar DSNo ratings yet
- Saudi Arabian Oil Company: Raw SewageDocument2 pagesSaudi Arabian Oil Company: Raw SewageAdnanNo ratings yet
- Recombinant Therapeutic Protein Production in Cultivated Mammalian Cells: Current Status and Future ProspectsDocument6 pagesRecombinant Therapeutic Protein Production in Cultivated Mammalian Cells: Current Status and Future ProspectsGryseldaNo ratings yet
- Astm C 582Document7 pagesAstm C 582Tanktech TanktechNo ratings yet
- A Wide-Range, High-Resolution, Compact, CMOS Time To Digital ConverterDocument6 pagesA Wide-Range, High-Resolution, Compact, CMOS Time To Digital ConverterShruti KalraNo ratings yet