Professional Documents
Culture Documents
Chapter 6 The Biochemical Basis of Life
Chapter 6 The Biochemical Basis of Life
Uploaded by
Jhane Kimberly A. RustiaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 6 The Biochemical Basis of Life
Chapter 6 The Biochemical Basis of Life
Uploaded by
Jhane Kimberly A. RustiaCopyright:
Available Formats
Burton’s Microbiology
for the Health Sciences
Section III.
Chemical and Genetic Aspects
of Microorganisms
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Burton’s Microbiology
for the Health Sciences
Chapter 6. The Biochemical Basis of
Life
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Chapter 6 Outline
• Introduction
• Organic Chemistry
– Carbon Bonds
– Cyclic Compounds
• Biochemistry
– Carbohydrates
– Lipids
– Proteins
– Nucleic Acids
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Introduction
• A microorganism can be thought of as a “bag” of
chemicals that interact with each other in a variety of
ways; even the bag itself is composed of chemicals.
• Everything a microorganism is and does is related to
chemistry.
• Organic chemistry is the study of compounds that contain
carbon.
• Inorganic chemistry involves all other chemical reactions.
• Biochemistry is the chemistry of living cells⎯the
chemistry of life.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Organic Chemistry
• Organic compounds contain carbon.
• Organic chemistry is the branch of science that studies
organic compounds.
• Organic compounds are not necessarily related to living
organisms; although some organic compounds are
associated with living organisms, many are not.
• Organic chemistry involves fossil fuels, dyes, drugs,
paper, ink, paints, plastics, gasoline, rubber tires, food,
and clothing.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Organic Chemistry
Carbon Bonds
• Carbon atoms have a valence of 4, meaning that they
can bond to four other atoms.
• There are three ways in which carbon atoms can bond to
each other: single bond, double bond, and triple bond.
• A covalent bond is one in which a pair of electrons is
shared.
• When atoms of other elements attach to available carbon
bonds, compounds are formed.
• A series of carbon atoms bonded together is referred to
as a chain.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Organic Chemistry
Carbon Bonds (cont.)
• If only hydrogen atoms are bonded to the available carbon
bonds, hydrocarbons are formed.
• Therefore, a hydrocarbon is an organic molecule that contains
only carbon and hydrogen atoms; some examples of simple
hydrocarbons are shown here:
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Organic Chemistry
Cyclic Compounds
• When carbon atoms link to
other carbon atoms to close
a chain, they form rings or
cyclic compounds.
• Benzene is a cyclic
compound with six carbons
and six hydrogens.
The benzene ring
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Biochemistry
• Biochemistry is the study of biology at the molecular
level; it is the chemistry of living organisms.
• Biochemistry involves the study of biomolecules present
within living organisms; biomolecules in living organisms
are usually large molecules called macromolecules.
• Macromolecules include carbohydrates, lipids, proteins,
and nucleic acids.
– Other examples: vitamins, enzymes, hormones, and
energy-carrying molecules such as adenosine
triphosphate (ATP).
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Biochemistry (cont.)
• Humans obtain their nutrients from the foods they eat.
– Carbohydrates, fats, nucleic acids, and proteins
contained in foods are digested; their components
are absorbed and carried to every cell in the body,
where they are broken down and rearranged.
• Microorganisms also absorb essential nutrients into the
cell by various means.
• The nutrients are then used in metabolic reactions as
sources of energy and as “building blocks” for enzymes,
structural macromolecules, and genetic materials.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Biochemistry
Carbohydrates
• Carbohydrates are biomolecules composed of carbon,
hydrogen, and oxygen (in the ratio 1:2:1).
• Examples include glucose, fructose, sucrose, lactose,
maltose, starch, cellulose, and glycogen.
• Categories of carbohydrates include monosaccharides,
disaccharides, and polysaccharides.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Carbohydrates
Monosaccharides
• Monosaccharides are the smallest and simplest of the
carbohydrates. Mono means one, referring to the number
of rings in the structure.
– Glucose (C6H12O6) is the most important
monosaccharide in nature; it may occur as a chain or
in alpha or beta ring configurations.
• Monosaccharides contain two to nine carbon atoms –
most contain five to six.
– A three-carbon monosaccharide is called a triose; a
four-carbon one is a tetrose; a five-carbon one is a
pentose; a six-carbon one is a hexose; a seven-
carbon is a heptose; and so on.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Carbohydrates
Monosaccharides (cont.)
• The main source of energy for body cells is glucose.
– The three forms of glucose are shown above.
– In humans, glucose is carried in the blood to cells where it
is oxidized to produce energy-carrying ATP. ATP is the
main energy source used to drive most metabolic
reactions.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Carbohydrates
Disaccharides
• “Di” means two; disaccharides are double-ringed sugars
that result from the combination of two monosaccharides
(following removal of a water molecule). This is known as
a dehydration synthesis reaction.
– Sucrose (table sugar), lactose, and maltose are
examples of disaccharides.
• Disaccharides react with water in a process called a
hydrolysis reaction, which causes them to break down
into two monosaccharides.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
The Dehydration Synthesis
and Hydrolysis of Sucrose
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Carbohydrates
Disaccharides (cont.)
• Recall that peptidoglycan is found in the cell walls of
all members of the Domain Bacteria; peptidoglycan is
a repeating disaccharide attached by proteins to form
a lattice that surrounds and protects the bacterial cell.
• Carbohydrates composed of three monosaccharides
are called trisaccharides; those composed of four are
called tetrasaccharides; those composed of five are
called pentasaccharides, and so on, until we come to
polysaccharides.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Carbohydrates
Polysaccharides
• The definition of a polysaccharide varies from one reference
book to another. In this book, polysaccharides are defined as
carbohydrates that are composed of many monosaccharides.
Most contain hundreds (e.g., starch and glycogen).
• Polysaccharides serve two main functions:
– Store of energy (e.g., glycogen in animal cells and starch
in plant cells)
– Provide a “tough” molecule for structural support and
protection (e.g., bacterial capsules)
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Carbohydrates
Polysaccharides (cont.)
• Polysaccharides are examples of polymers⎯molecules that
consist of many similar subunits.
• In the presence of the proper enzymes or acids,
polysaccharides may be hydrolyzed or broken down into
disaccharides, and then into monosaccharides.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Carbohydrates
Polysaccharides (cont.)
• Some bacteria produce polysaccharide capsules for
protection from phagocytes.
• Plant and algal cells have cellulose (a polysaccharide) cell
walls to provide support.
• Some protozoa, fungi, and bacteria have enzymes that
can break down cellulose.
• When polysaccharides combine with other chemical
groups (amines, lipids, and amino acids), complex
macromolecules are formed.
– For example, chitin, the main component of the hard
outer covering of insects, spiders, and crabs, is found
in the cell walls of fungi.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Lipids
• An important class of biomolecules.
• Most lipids are insoluble in water, but soluble in fat
solvents, such as ether, chloroform, and benzene.
• Lipids are essential constituents of most living cells.
• Lipids can be classified into the following categories:
-Waxes -Glycolipids
-Fats and oils -Steroids
-Phospholipids -Prostaglandins and leukotrienes
Copyright © 2015 Wolters Kluwer • All Rights Reserved
The General Structure of Some Categories
of Lipids
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Lipids
Fatty Acids
• Fatty acids are the building blocks of lipids; they are
long-chain carboxylic acids that are insoluble in water.
• Saturated fatty acids contain one single bond between
carbon atoms; they are solid at room temperature.
• Monounsaturated fatty acids have one double bond in the
carbon chain and are found in butter, olives, and
peanuts.
• Polyunsaturated fatty acids contain two or more double
bonds and are found in soybeans, safflowers, and corn.
• Essential fatty acids cannot be synthesized in the human
body and must be provided in the diet.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Lipids
Waxes
• A wax consists of a saturated fatty acid and a long-chain
alcohol.
– Examples: the wax coating on fruits, leaves, skin,
fur, and feathers of animals.
– The cell wall of Mycobacterium tuberculosis (the
causative agent of tuberculosis) contains waxes.
• These waxes protect M. tuberculosis from
digestion following phagocytosis by white blood
cells.
• These waxes make M. tuberculosis difficult to stain
and destain; this explains why M. tuberculosis is
acid-fast.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Lipids
Fats and Oils
• Fats and oils are the most common types of lipids. They are
also known as triglycerides because they are composed of
glycerol and three fatty acids.
• Most fats come from animal sources (e.g., beef); most oils
come from plant sources (e.g., olive oil).
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Lipids
Phospholipids
• Phospholipids contain glycerol, fatty acids, a phosphate
group, and an alcohol. There are two types:
– Glycerophospholipids (also known as
phosphoglycerides)
– Sphingolipids
• Glycerophospholipids are the most abundant lipids in cell
membranes.
– A cell membrane is a lipid bilayer, consisting of two
rows of phospholipids, arranged tail-to-tail.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
The Lipid Bilayer Structure
of Cell Membranes
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Lipids
Phospholipids, cont.
• The outer membrane of Gram-negative bacterial cell
walls contains lipoproteins and lipopolysaccharide (LPS).
– LPS consists of a lipid and a polysaccharide portion.
• The cell walls of Gram-positive organisms do not contain
LPS.
• Lecithins and cephalins are glycerophospholipids found in
brain and nerve tissues, as well as egg yolks.
• Sphingolipids are phospholipids that contain sphingosine
rather than glycerol. Sphingolipids are found in brain and
nerve tissues. Sphingomyelin is one of the most
abundant sphingolipids, and makes up the myelin sheath
that coats nerve cells.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Lipids
Glycolipids, Steroids, Prostaglandins,and
Leukotrienes
• Glycolipids are abundant in the brain and in the myelin
sheath of nerves.
• Steroids are complex, four-ringed structures; examples
are cholesterol, bile salts, steroid hormones, and fat-
soluble vitamins (A, D, E, and K).
• Prostaglandins and leukotrienes are derived from a fatty
acid called arachidonic acid.
– Both have a wide variety of effects on body
chemistry such as controlling blood pressure or
hormones; leukotrienes can produce long-lasting
muscle contractions.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Proteins
• Proteins are the most essential chemicals in all living
cells; they are considered “the substance of life.”
• Some proteins are the structural components of
membranes, cells, and tissues; others are enzymes and
hormones.
• All proteins are polymers of amino acids.
• All proteins contain carbon, hydrogen, oxygen, and
nitrogen (and sometimes sulfur).
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Proteins
Amino Acids
• Amino acids contain carbon, hydrogen, oxygen, and nitrogen;
some also have sulfur in the molecule.
• Humans can synthesize certain amino acids, but not others.
• The thousands of different proteins in the human body are
composed of a wide variety of amino acids in various quantities
and arrangements.
• The basic structure of an amino acid is shown here:
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Proteins
Protein Structure
• Amino acids are linked together to form proteins via
covalent bonds referred to as peptide bonds; there are
dipeptides, tripeptides, and polypeptides.
• The linear sequence of amino acids is referred to as the
primary protein structure.
• The twisting or coiling of the chain of amino acids is
referred to as the secondary protein structure.
• The folding or entwining of the chain is the tertiary
protein structure.
• The bonding of two or more polypeptide chains is known
as the quaternary protein structure.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
The Formation of a Dipeptide
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Protein Structure
A.Primary structure
B.Secondary structure
C.Tertiary (globular)
structure
D.Quaternary structure
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Proteins
Enzymes
• Enzymes are specialized protein molecules produced by
living cells. They are known as biological catalysts; that
is, they catalyze metabolic reactions.
– A catalyst is an agent that speeds up a chemical
reaction without being consumed in the reaction.
• Almost every chemical reaction in a cell requires a
specific enzyme.
• Some protein molecules function as enzymes by
themselves; other proteins, called apoenzymes, only
function when linked with a nonprotein cofactor (such as
Ca2+, Fe2+, Mg2+, Cu2+) or a coenzyme.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Proteins
Enzymes (cont.)
• Some apoenzymes require vitamin-type compounds
called coenzymes; examples are vitamin C, flavin-
adenine dinucleotide (FAD), and nicotinamide-adenine
dinucleotide (NAD).
• The combination of an apoenzyme plus a cofactor is
called a holoenzyme (i.e., a “whole” enzyme).
• Enzymes are usually named by adding the ending “-ase”
to the word. Hemolysins and lysozyme are examples of
enzymes not ending in “ase.”
• The specific molecule on which an enzyme acts is
referred to as that enzyme’s substrate.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Nucleic Acids
Function
• DNA and RNA form the fourth major group of
biomolecules in living cells.
• DNA and RNA are critical to proper functioning of a cell.
• DNA is the “hereditary molecule”⎯the molecule that
contains the genes and genetic code.
– Information in DNA must flow to the rest of the cell
for the cell to function properly⎯the flow is
accomplished by RNA.
• RNA molecules participate in the conversion of the
genetic code into proteins and other gene products.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Nucleic Acids
Structure
• In addition to the elements C, H, O, and N, DNA and RNA
also contain phosphorus, P.
• The building blocks of nucleic acid polymers are called
nucleotides.
– Nucleotides are more complex monomers than amino
acids.
• The building blocks of DNA are called DNA nucleotides.
• The building blocks of RNA are called RNA nucleotides.
• DNA contains dexoyribose as its pentose, whereas RNA
contains ribose as it pentose.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Two Nucleotides, Each Consisting of a
Nitrogenous Base (A or T), a Five-carbon Sugar
(S), and a Phosphate Group (P)
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Nucleic Acids
Structure (cont.)
• There are three types of RNA, named for their function:
– Messenger RNA (mRNA)
– Ribosomal RNA (rRNA)
– Transfer RNA (tRNA)
• The five nitrogenous bases in nucleic acids are adenine
(A), guanine (G), thymine (T), cytosine (C), and uracil
(U). A and G are purines; T, C, and U are pyrimidines.
– Thymine is found in DNA but not in RNA. Uracil is
found in RNA, but not in DNA. The other three bases
are found in both.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
The Pyrimidines and Purines
Found in DNA and RNA
Copyright © 2015 Wolters Kluwer • All Rights Reserved
DNA Structure
• For a double-stranded DNA molecule to form, the
nitrogenous bases on the two separate strands must
bond together.
– A always bonds with T via two hydrogen bonds.
– G always bonds with C via three hydrogen bonds.
– A–T and G–C are known as “base pairs.”
• The bonding forces of the double-stranded polymer cause
it to assume the shape of a double -helix, similar to a
right-handed spiral staircase.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
A Section of a Nucleic Acid Polymer
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Base Pairs That Occur in Double-stranded
DNA Molecules
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Double-Stranded DNA Molecule, Also
Known as a Double Helix
Copyright © 2015 Wolters Kluwer • All Rights Reserved
DNA Replication
• When a cell is preparing to divide, all DNA molecules in
the chromosomes of the cell must duplicate, thereby
ensuring that the same genetic information is passed on
to both daughter cells. This is called DNA replication.
• DNA replication occurs by separation of the two DNA
strands and the building of complementary strands by
the addition of the correct DNA nucleotides.
• DNA polymerase (also known as DNA-dependent DNA
polymerase) is the most important enzyme required for
DNA replication.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
DNA Replication
Copyright © 2015 Wolters Kluwer • All Rights Reserved
DNA Replication
Gene Expression
• A gene is a particular segment of a DNA molecule or
chromosome.
– A gene contains the blueprint that will enable a cell
to make what is known as a gene product.
• It is the sequence of the four nitrogenous bases of DNA
(i.e., A, G, C, and T) that spell out the instructions for a
particular gene product.
• Although most genes code for proteins, some code for
rRNA and tRNA.
• Some genes code for more than one gene product.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
DNA Replication
Gene Expression (cont.)
• The Central Dogma explains the flow of genetic
information within a cell (proposed by Francis Crick in
1957).
– DNA mRNA protein.
– Also known as the “one gene–one protein
hypothesis.”
– One gene of a DNA molecule is used to make one
molecule of mRNA by a process known as
transcription.
– The genetic information in the mRNA is then used to
make one or more protein by a process known as
translation.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
DNA Replication
Gene Expression (cont.)
• All genes on a chromosome are not being expressed at
any given time. It would not be logical for a cell to
produce a particular enzyme if it was not needed.
– Genes that are only expressed when the gene
products are needed are called inducible genes.
– Genes that are expressed at all times are called
constitutive genes.
• The process by which the genetic code within the DNA
molecule is transcribed to produce an mRNA molecule is
called transcription.
– The primary enzyme involved is RNA polymerase.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
DNA Replication
Gene Expression (cont.)
• In eukaryotes, transcription occurs within the nucleus;
the newly formed mRNA molecules then travel out
through the pores of the nuclear membrane into the
cytoplasm, where they are used to produce proteins.
• In prokaryotes, transcription occurs in the cytoplasm;
ribosomes attach to the mRNA molecules as they are
being transcribed at the DNA; thus both transcription and
translation may occur simultaneously.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Transcription
Copyright © 2015 Wolters Kluwer • All Rights Reserved
DNA Replication
Gene Expression (cont.)
• The process of translating the message carried by mRNA,
whereby particular tRNAs carry amino acids to be bound
together in the proper sequence to make a protein, is
called translation.
• The base sequence of the mRNA molecule is read in
groups of three bases, called codons.
• The three-base sequence codon can be read by a
complementary three-base sequence (the anticodon) on
a tRNA molecule.
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Chart to Illustrate the Sequence of Three Bases (GGC) in the DNA
Template That Codes for a Particular Codon (CCG) in mRNA,
Which in Turn Attracts a Particular Anticodon (GGC) on the tRNA
Carrying an Amino Acid (Proline in This Case)
DNA mRNA tRNA Amino
Template (Codon) (Anticodon) Acid
G C G
Proline
G C G
C G C
Copyright © 2015 Wolters Kluwer • All Rights Reserved
Translation (Protein Synthesis)
Copyright © 2015 Wolters Kluwer • All Rights Reserved
You might also like
- Protein Assay by The Bradford MethodDocument10 pagesProtein Assay by The Bradford MethodMichelle79% (14)
- Exam 1 Study Guide For NUTR 100Document5 pagesExam 1 Study Guide For NUTR 100marc100% (1)
- Biology Chapter 1 STPM Sem1Document12 pagesBiology Chapter 1 STPM Sem1Jia Hui100% (4)
- Chapter 09 Controlling Microbial Growth in Vivo Using Antimicrobial AgentsDocument49 pagesChapter 09 Controlling Microbial Growth in Vivo Using Antimicrobial AgentsSherinne Jane Cariazo100% (1)
- Chapter 6Document22 pagesChapter 6Marlop CasicasNo ratings yet
- Week 1 - Burton'S - LWW Introduction To MicrobiologyDocument21 pagesWeek 1 - Burton'S - LWW Introduction To MicrobiologyCathy Lago100% (1)
- Burton's Microbiology For The Health Sciences: Chapter 2. Viewing The Microbial WorldDocument23 pagesBurton's Microbiology For The Health Sciences: Chapter 2. Viewing The Microbial WorldMrkTrstn99No ratings yet
- Burton's Microbiology For The Health Sciences: Diagnosing Infectious DiseasesDocument35 pagesBurton's Microbiology For The Health Sciences: Diagnosing Infectious DiseasesJehu C LanieNo ratings yet
- A Brief History of Microbiology Chapter 1Document10 pagesA Brief History of Microbiology Chapter 1eyearenaNo ratings yet
- Virtual Lab Report by Honey Noreen Belnas: The Gram Stain: Identify and DifferentiateDocument16 pagesVirtual Lab Report by Honey Noreen Belnas: The Gram Stain: Identify and DifferentiateJihil KishaNo ratings yet
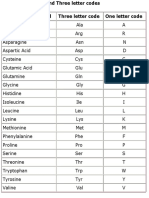
- Amino Acid CodesDocument1 pageAmino Acid Codesmorzan ortizNo ratings yet
- Chapter 7 Microbial Physiology and GeneticsDocument47 pagesChapter 7 Microbial Physiology and GeneticsJhane Kimberly A. RustiaNo ratings yet
- Controlling Micriobial GrowthDocument47 pagesControlling Micriobial GrowthasiNo ratings yet
- Ncm101 Microbiology Prelims (Lecture)Document31 pagesNcm101 Microbiology Prelims (Lecture)k78cwhs6v7No ratings yet
- Introduction To Biochemistry TransesDocument2 pagesIntroduction To Biochemistry TransesJenny Ruth TubanNo ratings yet
- MICROBIODocument158 pagesMICROBIOJoyce VillanuevaNo ratings yet
- Chapter 4: Microbial Diversity: Part 1: Acelluar and Prokaryotic MicrobesDocument48 pagesChapter 4: Microbial Diversity: Part 1: Acelluar and Prokaryotic MicrobesMudarab AliNo ratings yet
- Kami Export - Chem 216 (Lec & Lab) SyllabusDocument21 pagesKami Export - Chem 216 (Lec & Lab) SyllabusMAYNARD E. ABONADORNo ratings yet
- Microbial Diversity Part 1: Acellular and Prokaryotic MicrobesDocument53 pagesMicrobial Diversity Part 1: Acellular and Prokaryotic MicrobesSedqamNo ratings yet
- Bustalino Jill C Activity 3 MicroparaDocument5 pagesBustalino Jill C Activity 3 MicroparaJill Cabasag BustaliñoNo ratings yet
- Biochem IntroDocument6 pagesBiochem IntroAlliah CasingNo ratings yet
- Unit 4 Infectious Disease - Modes of TransmissionDocument26 pagesUnit 4 Infectious Disease - Modes of TransmissionEvet Vaxbm100% (1)
- BIOCHEMISTRY - Week 1Document12 pagesBIOCHEMISTRY - Week 1Cherry Mae TorresNo ratings yet
- Prelims Micropara LecDocument14 pagesPrelims Micropara LecBSN1F- JACILDO, KUH KYLA C.No ratings yet
- 14 Fungal and Parasitic Infections of Integumentary CNS and Sense OrgansDocument30 pages14 Fungal and Parasitic Infections of Integumentary CNS and Sense OrgansNea Therese AmbidaNo ratings yet
- Chapter 1-HADocument21 pagesChapter 1-HASHELLAH MARIE MAMAWAGNo ratings yet
- Controlling Microbial Growth in VitroDocument30 pagesControlling Microbial Growth in VitroJhon Lapuz100% (1)
- Food Microbiology and ParasitologyDocument23 pagesFood Microbiology and ParasitologyStephen Kariuki67% (3)
- Chap 01Document35 pagesChap 01Agita RakaNo ratings yet
- Control of Microbial GrowthDocument35 pagesControl of Microbial GrowthVincent ManganaanNo ratings yet
- Microbiology and Parasitology Presentation by W KakumuraDocument14 pagesMicrobiology and Parasitology Presentation by W KakumurahamiltonNo ratings yet
- Pathogenesis and Host Defense Mechanism: Instructor: Bertha Escobar-Poni, MDDocument32 pagesPathogenesis and Host Defense Mechanism: Instructor: Bertha Escobar-Poni, MDGelvia AwaehNo ratings yet
- 5enzymes and Vitamins PDFDocument48 pages5enzymes and Vitamins PDFRomelyn AngelNo ratings yet
- Microbiology For The Health Sciences: Chapter 2. MicrosDocument23 pagesMicrobiology For The Health Sciences: Chapter 2. MicrosHashim Ghazo100% (1)
- The Philippine Society For Microbiology Inc. It Is An Organization Founded by 20Document3 pagesThe Philippine Society For Microbiology Inc. It Is An Organization Founded by 20MariaSenaSecretoErtaNo ratings yet
- Tissues ReviewerDocument5 pagesTissues ReviewerJoannah MarieNo ratings yet
- Microbiology ReviewerDocument16 pagesMicrobiology ReviewerAnna CastroNo ratings yet
- Burton's Microbiology For The Health Sciences: Chapter 10. Microbial Ecology and Microbial BiotechnologyDocument20 pagesBurton's Microbiology For The Health Sciences: Chapter 10. Microbial Ecology and Microbial BiotechnologyKenj PereñaNo ratings yet
- Hang Drop PDFDocument2 pagesHang Drop PDFIndhumathiNo ratings yet
- Microbiology For The Health Sciences: Chapter 4. Diversity of MicroorganismsDocument59 pagesMicrobiology For The Health Sciences: Chapter 4. Diversity of MicroorganismsHiba Abu-jumahNo ratings yet
- Micropara Lec Unit 1 LaDocument1 pageMicropara Lec Unit 1 LaGrace HernandezNo ratings yet
- 1microbiology PRELIM-merged PDFDocument134 pages1microbiology PRELIM-merged PDFHelping HandsNo ratings yet
- Clinical LaboratoryDocument41 pagesClinical LaboratoryBalaji GandhiNo ratings yet
- Carbohydrate Reading ModuleDocument33 pagesCarbohydrate Reading ModuleSebastian Smythe100% (1)
- Chapter 10Document5 pagesChapter 10Apryll DarlineNo ratings yet
- Lecture - Microbial Diversity The Eukaryotic Microbes - Chapter 4 - BSEDDocument5 pagesLecture - Microbial Diversity The Eukaryotic Microbes - Chapter 4 - BSEDMaden betoNo ratings yet
- HES007 ReviewerDocument9 pagesHES007 ReviewerAira Andrada100% (1)
- BIOCHEMISTRYDocument95 pagesBIOCHEMISTRYROSHAN JUSTICE GALLOSNo ratings yet
- Week 3 Lec. NCMA110. Overview of Theory in NursingDocument2 pagesWeek 3 Lec. NCMA110. Overview of Theory in NursingMai Dei100% (1)
- Defining The MT ProfessionDocument26 pagesDefining The MT ProfessionLady Juliana MarieNo ratings yet
- VIBAR, Justine - Biochem LE 1&2Document3 pagesVIBAR, Justine - Biochem LE 1&2JustineNo ratings yet
- By: Catherine M. Souribio, R.NDocument40 pagesBy: Catherine M. Souribio, R.NIsrael AgrisNo ratings yet
- Introduction To BiologyDocument6 pagesIntroduction To BiologyFransiel B. AkistoyNo ratings yet
- Streak Plate Technique For Isolating BacteriaDocument3 pagesStreak Plate Technique For Isolating BacteriaHimanshu tripathiNo ratings yet
- Microbial DiversityDocument4 pagesMicrobial DiversityArun SendhilNo ratings yet
- Quiz#1 For 1A (NCM 100 1A) : PointsDocument3 pagesQuiz#1 For 1A (NCM 100 1A) : PointsbrylleNo ratings yet
- Health Economics: Teacher: Teresita Balgos Reporter: Group 4 MLS-II FDocument19 pagesHealth Economics: Teacher: Teresita Balgos Reporter: Group 4 MLS-II FThyrealle Frances Sorongon100% (1)
- Nueva Ecija University of Science and Technology: Republic of The Philippines Cabanatuan CityDocument3 pagesNueva Ecija University of Science and Technology: Republic of The Philippines Cabanatuan CityKazuhero LagranaNo ratings yet
- Basic Biology: 1. Cellular StructureDocument3 pagesBasic Biology: 1. Cellular StructureAbhay VishwakarmaNo ratings yet
- Controlling Microbial Growth in Vivo Using Antimicrobial AgentsDocument30 pagesControlling Microbial Growth in Vivo Using Antimicrobial AgentsJen PanganibanNo ratings yet
- The politics of hunger: Protest, poverty and policy in England, <i>c.</i> 1750–<i>c.</i> 1840From EverandThe politics of hunger: Protest, poverty and policy in England, <i>c.</i> 1750–<i>c.</i> 1840No ratings yet
- Humans in The World of Biology Humans in The World of BiologyDocument32 pagesHumans in The World of Biology Humans in The World of BiologyJEANNo ratings yet
- Aminoacid and Protein Structure (I)Document24 pagesAminoacid and Protein Structure (I)Game OverNo ratings yet
- Lecture On Transcription and TranslationDocument47 pagesLecture On Transcription and TranslationAnna Beatrice BautistaNo ratings yet
- Enzymes Models of ActionDocument29 pagesEnzymes Models of ActionJeison Vara ValenzuelaNo ratings yet
- Physical Science SHS 8.1 ProteinsDocument42 pagesPhysical Science SHS 8.1 ProteinsDave RovicNo ratings yet
- Midterm Exam For BIOLOGY RogersDocument2 pagesMidterm Exam For BIOLOGY RogersGarde PiaoNo ratings yet
- MANAS, Biochem Lab June 29 PDFDocument2 pagesMANAS, Biochem Lab June 29 PDFlovelykissNo ratings yet
- Pex 08 03Document4 pagesPex 08 03Adriana Anaya GaribaldiNo ratings yet
- AP Bio Gene Expression & Regulation MC (5 Steps To A 5)Document7 pagesAP Bio Gene Expression & Regulation MC (5 Steps To A 5)Help Me Study TutoringNo ratings yet
- The Physiology and Habitat of The Last Universal Common AncestorDocument18 pagesThe Physiology and Habitat of The Last Universal Common AncestorJose MaurtuaNo ratings yet
- DNA PackagingDocument25 pagesDNA PackagingChamara ChathurangaNo ratings yet
- Smart Note Enzyme Bio f4Document2 pagesSmart Note Enzyme Bio f4Cik PezzanNo ratings yet
- Concept Map BotanyDocument1 pageConcept Map BotanyCindy AlvarezNo ratings yet
- Prof Rika - Konsep Dasar Biologi Sel Dan MolekularDocument33 pagesProf Rika - Konsep Dasar Biologi Sel Dan MolekularTiaradestiana AbeeNo ratings yet
- Pattern Matching: Classifying Organic MoleculesDocument6 pagesPattern Matching: Classifying Organic MoleculesSeungju HanNo ratings yet
- BCM 3521 L3-MinDocument17 pagesBCM 3521 L3-MinLutendo Assurance MadzivhaaNo ratings yet
- Amino Acids - Biochemistry Questions and Answers - SanfoundryDocument1 pageAmino Acids - Biochemistry Questions and Answers - SanfoundryAli HassanNo ratings yet
- 20 Common Amino Acids v2 PDFDocument1 page20 Common Amino Acids v2 PDFRenaldy NugrahaNo ratings yet
- Second Messengers in Signal TrasductionDocument4 pagesSecond Messengers in Signal TrasductionAlice RheeNo ratings yet
- MD Tutorial Using GromacsDocument22 pagesMD Tutorial Using GromacsSeagal AsjaliNo ratings yet
- Essay Question For Protein SynthesisDocument8 pagesEssay Question For Protein SynthesisDoMyPaperForMoneyFargo100% (2)
- Biological MoleculesDocument16 pagesBiological MoleculesThandeka NcubeNo ratings yet
- Zumdahl Biology Tie inDocument2 pagesZumdahl Biology Tie injimmy615615No ratings yet
- Bio Trial BaruDocument3 pagesBio Trial BaruBrandon JudeNo ratings yet
- Decoding DnaDocument2 pagesDecoding DnatheflyingcatsontheairNo ratings yet
- Practical 2: Organic Compositions of The Cell: Student Name: Lê Hà Phương Ly ID: BTBTIU21220Document4 pagesPractical 2: Organic Compositions of The Cell: Student Name: Lê Hà Phương Ly ID: BTBTIU21220Le Phuong LyNo ratings yet
- Metabolic Processes - Pr. PirouziDocument32 pagesMetabolic Processes - Pr. PirouzipirouziNo ratings yet
- Pepsin EnzymeDocument5 pagesPepsin Enzymesaitama12343217No ratings yet