Professional Documents
Culture Documents
Overexpression of Myosin Is Associated With The Development of Uterine Myoma
Overexpression of Myosin Is Associated With The Development of Uterine Myoma
Uploaded by
Muhammad Saad FarooqOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Overexpression of Myosin Is Associated With The Development of Uterine Myoma
Overexpression of Myosin Is Associated With The Development of Uterine Myoma
Uploaded by
Muhammad Saad FarooqCopyright:
Available Formats
bs_bs_banner
doi:10.1111/jog.12461 J. Obstet. Gynaecol. Res. Vol. 40, No. 9: 2051–2057, September 2014
Overexpression of myosin is associated with the
development of uterine myoma
Wenchao Zhang1,2, Zhongping Cheng2, Xiaoyan Qu2, Hong Dai2, Xiaoping Ke2 and
Zijiang Chen1
1
Center for Reproductive Medicine, Provincial Hospital Affiliated to Shandong University, Jinan, and 2Department of
Obstetrics and Gynecology, Yangpu District Central Hospital, Shanghai, China
Abstract
Aim: Myosin is involved in cell contraction and motility, but it is unclear whether it is involved in cell
proliferation in uterine myoma. In this study therefore we aimed to explore the role of myosin in uterine
myoma.
Material and Methods: Immunohistochemistry and real-time polymerase chain reaction were used to deter-
mine the expression of myosin light chain (MLC), myosin heavy chain (MHC) and myosin light chain kinase
(MLCK) in patient uterine myoma and adjacent smooth muscle tissue. Human uterine fibroid cells were
isolated and cultured in vitro, myosin heavy chain 11 (MHC subtype expressed in uterine fibroid cells) was
knocked down by RNA interference to reduce the expression of myosin, then cell proliferation was determined
by the methyl thiazol tetrazolium bromide method. To explore the possible mechanism of reduced cell
proliferation after myosin heavy chain 11 knockdown, the downstream proteins collagen I, insulin-like growth
factor-1, fibronectin and proteoglycans were analyzed.
Results: Expression of MLC, MHC, MLCK and p-MLCK in uterine myoma cells was significantly higher than
in adjacent smooth muscle cells. After knockdown of MHC, smooth muscle cell proliferation decreased, and
the production of collagen I, insulin-like growth factor-1 and fibronectin was also reduced, but proteoglycans
did not show any significant change.
Conclusion: Myosin is overexpressed in uterine myoma, and the overexpression of myosin is associated with
both uterine contraction and tumor development of uterine myoma.
Key words: contraction, myosin heavy chain, myosin light chain, myosin light chain kinase, uterine myoma.
Introduction patients’ uterine cavity pressure using a pressure
sensor, and found that patients with uterine fibroids
Uterine fibroids are the most common benign tumors showed abnormal uterine contraction, possibly leading
in women, with a clinical morbidity rate of 20–40%.1 to infertility.4,5 Subsequently, by observing the ultra-
However, the pathogenesis of uterine fibroids is still structure using electron microscopy they also found
not fully understood. Previously, research on the patho- differences in the density band of myoma cells com-
genesis of uterine fibroids has focused on cell differen- pared to normal uterine smooth muscle cells, indicat-
tiation.2 Recent studies showed that the phenomenon ing that contraction of the myoma cells is abnormal.6
of abnormal contraction was also found in myoma.3 As As is well known, the contraction-related
early as the 1980s–1990s, some researchers monitored pathway and its regulation are important in uterine
Received: November 26 2013.
Accepted: March 29 2014.
Reprint request to: Professor Zhongping Cheng, Department of Obstetrics and Gynecology, Yangpu District Central Hospital,
Tengyue Road 450, Shanghai, China 200090. Email: paperch@sina.com
© 2014 The Authors 2051
Journal of Obstetrics and Gynaecology Research © 2014 Japan Society of Obstetrics and Gynecology
W. Zhang et al.
pathophysiology. The thick myofilaments of the myo- were all diagnosed with intramural myoma, and were
fibrils are composed of myosins, which play an im- excluded if they exhibited other complications, such as
portant role in muscle movement. Myosins form a ovarian cysts or adenomyosis.
structurally and functionally diverse superfamily that
consists of at least 18 distinct members.7 The classic Immunohistochemistry
two-headed myosin is called conventional myosin and The immunohistochemistry was performed using the
represents the myosin II family; the other 17 members StreptAvidin-Biotin Complex method. Briefly, after
are referred to as unconventional myosins.7,8 Myosin is hydration and antigen repair of tissues, the primary
composed of six polypeptide chains, comprising two antibodies were added at the dilutions indicated
myosin heavy chain (MHC) molecules and two pairs of (MLC, 1:200; MHC, 1:100; MLCK, 1:100; p-MLCK,
myosin light chains (MLC). MLC are divided into basic 1:100; all from Abcam) and slides were incubated at
light chains and regulatory light chains. The major role 4°C overnight. The next day, the tissues were stained
of the basic light chains is to stabilize the structure of with biotinylated secondary antibodies, and DAB dye
the heavy chains, while regulatory light chains work to was used for color development. Images were collected
regulate the activity of myosin.9 under a microscope (OLYMPUS BX-51), positive area
Actin and adenosine triphosphate (ATP) binding (brown) was abstracted by negative area (purple) and
sites are located in the head of the MHC molecule. Pairs the area size was measured by micrometer. The average
of spherical MHC heads join up and move rotatively, a optical density (OD) was measured as follows: Unit
conformational change occurs, then the MHC head conversion (total area 351 000 pixels) and positive
binds to actin, hydrolyzing ATP and generating con- area (1 pixel point = 0.095 μm2). Immunohistochemical
traction.10 Myosin heavy chain 11 (MYH11) encodes index was calculated as positive area multiplied by
the smooth muscle MHC, and belongs to the family OD value.
of conventional myosins. MLC are regulated by dual
signals, being activated by myosin light chain kinase
Real-time polymerase chain reaction
(MLCK) signaling, which is dependent on Ca2+
/calmodulin (CaM), and by myosin phosphatase Total RNA was extracted using Trizol reagent
signaling, which is dependent on the RhoA pathway. (Invitrogen). The cDNA synthesis kit and real-time
MLCK is an important regulator of MLC through its polymerase chain reaction (PCR) kit were purchased
phosphorylation and activation.11 Thus, in this study from Takara. cDNA was synthesized as follows:
we analyzed both MLC and MLCK expression in 5 × reverse transcription buffer, oligo dT, dNTPs,
uterine myoma. reverse transcriptase MMLV, DEPC water and RNA
Given the important role of myosin both in cell template. Real-time PCR was then performed in a
contraction and in proliferation, we isolated human mixture of real-time PCR mix, primers and cDNA tem-
uterine fibroid cells and tested cell proliferation after plate in a total volume of 20 μL. PCR amplification
knockdown of myosin. We also analyzed downstream conditions: 95°C preliminary denaturation 5 min; 95°C
signals to reveal the underlying mechanism. denaturation 15 s, 60°C annealing 35 s, 40 cycles. MLC
primers: forward 5′-CGGGTCCTGAAATCTTACTC-3′,
reverse 5-′AGGCTGCTTATGGCAATC-3′; MLCK
Methods primers: forward 5′-CGCCACTTCCAGATAGACTAC-
Patients and samples 3′, reverse 5′-ACCTTCCTCCATCGTTTCC-3′. Glycer-
This study was approved by the medical ethics com- aldehyde 3-phosphate dehydrogenase was used as an
mittee of Yangpu District Central Hospital, Shanghai, internal control, using the primers: forward 5′-ATCAG
China, and informed consent was obtained from each CAATGCCTCCTGCAC-3′, reverse 5′-CGTCAAAGGT
patient. Uterine myoma and adjacent smooth muscle GGAGGAGTGG-3. Gene expression was calculated as
tissue were collected from 30 patients in our hospital the relative gene expression to internal control (△CT).
who underwent surgical treatment in 2011. The
samples were confirmed by pathological examination Human uterine fibroid cells culture
after surgery, with adjacent smooth muscle tissue The uterine fibroid tissues were isolated from patients
defined as tissue at a distance of 0.5 cm from myoma with uterine fibroids, the tissues were cut into small
tissue. The age of the patients ranged between 37 and pieces at 4°C and digested with collagenase II for 2 h at
59 years, with a mean age of 44.5 ± 3.3 years. Patients 37°C. The samples were centrifuged at 1000 rpm for
2052 © 2014 The Authors
Journal of Obstetrics and Gynaecology Research © 2014 Japan Society of Obstetrics and Gynecology
Overexpression of myosin in myoma
10 min after repeated pipetting. After this step, small and the plate was read immediately on a microtiter
pieces of loose tissue were placed in 6-cm plastic plate reader at 450 nm.
culture dishes and cultured in DMEM with 10% fetal
bovine serum (Gibco/Life Technologies). After 2 days, Statistical analysis
shuttle-shaped uterine fibroid cells were observed Data were expressed as the mean ± standard deviation
growing out from the pieces of tissue. or standard error and analyzed by the Mann–Whitney
U-test or Student’s t-test using spss. A P-value <0.05
MYH11 interference was considered statistically significant.
Oligonucleotide fragments were designed to target
the homo sapiens MYH11 smooth muscle 1A Results
(NM_002474) gene sequence, synthesized and Overexpression of myosin and MLCK in
annealed into the double-stranded form. They were uterine myoma
then inserted into the expression vector pcDNA 6.2-
GW/EmGFPmiR (Invitrogen) with the BLOCK-iT In order to explore whether crosstalk occurs between
Pol II miR RNAi Expression Vector Kit. The lentiviral cell contraction and proliferation in uterine myoma,
vector pLENT6.3/V5-SR60_1 was constructed and expression of two important proteins, myosin and its
packaged, and viral titers were determined. Uterine upstream kinase MLCK, was determined in patient
fibroid cells were used for transduction, and a high samples of uterine myoma. As shown in Figure 1, the
transfection efficiency and interference were achieved. expression of MLC, MHC, MLCK and p-MLCK was
detected both in uterine myoma and in adjacent
smooth muscle, with positive expression located
Cell proliferation assay
mainly in the cytoplasm. The expression of MLC,
The cell proliferation rate was tested by methyl thiazol MHC, MLCK, and p-MLCK in uterine myoma was
tetrazolium bromide (MTT) assay. The cells were plated much higher than in smooth muscle tissue (P < 0.05)
into 96-well plates at a concentration of 3000 cells per (Fig. 1 and Table 1).
well and incubated for 72 h, resulting in a final MTT To verify the overexpression of MLC and MLCK
concentration of 0.5 mg/mL. MTT was added to each in myoma, we used real-time PCR to analyze mRNA
well and they were returned to the incubator for a expression. As shown in Figure 2, the mRNA levels
further 4 h. The culture supernatant was then removed of both MLC and MLCK were significantly higher in
from each well, 150 μL of dimethyl sulfoxide was added myoma (P < 0.05).
and the cultures were shaken for 10 min. A wavelength
of 560 nm was used to measure the absorbance of each Knockdown of MHC expression reduces
well using a microplate reader, and the growth rate was cell proliferation
calculated relative to the control group. In order to investigate the effect of myosin to uterine
fibroid cells, we disrupted the function of myosin by
Enzyme-linked immunosorbent assay knockdown of MYH11, which is specifically expressed
Enzyme-linked immunosorbent assay (ELISA) kits for in uterine fibroid cells. Uterine fibroid cells were trans-
collagen I (Genway), insulin-like growth factor (IGF)-1 duced with lentiviral to knockdown the expression of
(Genway), fibronectin (Abcam) and proteoglycans MYH11 (Fig. 3a,b). The interference effect was showed
(antibodies) were used to assay protein secretion in to cause a reduction of 72% of MYH11 at the mRNA
the cell culture supernatants. After blocking non- level (Fig. 3c). In order to evaluate the effect of MHC on
specific binding by adding 200 μL of 1% bovine serum cell proliferation, control cells and MYH11 knockdown
albumin, 100 μL of each culture supernatant was trans- cells were examined using the MTT method to deter-
ferred to the wells of a microtiter plate at different mine their growth rate. As shown in Figure 3d, the
dilutions, and incubated for 2 h at 37°C. After washing, growth rate of MYH11 knockdown cells was signifi-
100 μL of horseradish peroxidase-conjugated second- cantly reduced compared to control cells (P < 0.05).
ary antibody was added to the wells and they were
incubated for 1 h, then the washing step was repeated, Effect of MHC downregulation on uterine
and finally 100 μL of tetramethylbenzidine was added fibroid cells
to each well and they were incubated for 30 min at The knockdown of MHC could change the structure
room temperature. Finally the stop solution was added and distribution of the cytoskeleton. This might result
© 2014 The Authors 2053
Journal of Obstetrics and Gynaecology Research © 2014 Japan Society of Obstetrics and Gynecology
W. Zhang et al.
Figure 1 Expression of myosin
light chain (MLC), myosin heavy
chain (MHC), myosin light chain
kinase (MLCK) and p-MLCK in
uterine myoma as determined
by immunohistochemistry. Rep-
resentative increased expres-
sion of MLC, MHC, MLCK and
p-MLCK in uterine myoma as
compared to smooth muscle
tissue. The expression of MLC,
MHC, MLCK and p-MLCK were
detected by their primary anti-
bodies and biotinylated second-
ary antibodies, and then DAB
dye was used for color deve-
lopment (StreptAvidin-Biotin
Complex, ×100). Strong expres-
sion is shown in brown staining,
while low expression is shown
in blue staining.
Table 1 Comparison of immunohistochemical index of MLC, MLCK, p-MLCK in
myoma and smooth muscle tissue (mean ± standard deviation)
Myoma Smooth muscle P-value*
(n = 30) (n = 30)
MLC 35 635.77 ± 8 575.76 16 161.53 ± 5 846.28 <0.05
MHC 27 724.56 ± 12 306.34 12 316.08 ± 9 058.74 <0.05
MLCK 30 313.73 ± 13 722.96 17 895.93 ± 9 819.17 <0.05
p-MLCK 38 063.57 ± 5 370.23 24 621.70 ± 6 012.04 <0.05
*Analyzed by Mann–Whitney U-test. MHC, myosin heavy chain; MLC, myosin light chain;
MLCK, myosin light chain kinase.
2054 © 2014 The Authors
Journal of Obstetrics and Gynaecology Research © 2014 Japan Society of Obstetrics and Gynecology
Overexpression of myosin in myoma
Figure 2 mRNA expression of myosin light chain (MLC) and myosin light chain kinase (MLCK) expression in myoma as
determined by real-time polymerase chain reaction. Expression of MLC and MLCK were increased in myoma, as
compared to smooth muscle tissues. Gene expression was calculated as the relative gene expression to internal control
(△CT). The bars indicate the mean ± standard error (n = 30) for each group. Data were analyzed by Student’s t-test.
*P < 0.05, as compared to the control group. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MC, myoma cell; SMC,
smooth muscle cell.
Figure 3 Knockdown of myosin
heavy chain expression in
human uterine fibroid cells.
(a) Light microscopic picture
of uterine fibroid cells that
were cultured for 72 h in vitro.
(b) Expression of pLENT6.3/
V5-SR60 (myosin heavy chain
11 [MYH11] RNAi) in uterine
fibroid cells as monitored by
EGFP fluorescence. Scale bar
50 μm. (c) mRNA expression of
MYH11 was detected by real-
time polymerase chain reaction.
(d) Cell proliferation rate was
determined by methyl thiazol
tetrazolium bromide assay. The
bars indicate the mean ± stan-
dard error (n = 3) for each group.
Data were analyzed by Student’s
t-test. *P < 0.05, as compared to
the control group.
in changes in secretion of some factors. In order of which play important roles in uterine fibroid cell
to investigate the effect of MHC downregulation on proliferation. ELISA was performed to detect the
uterine fibroid cells, we analyzed the expression of extracellular secretion of collagen I, IGF-1, fibronectin
collagen I, IGF-1, fibronectin and proteoglycans, all and proteoglycans. The results showed that collagen I
© 2014 The Authors 2055
Journal of Obstetrics and Gynaecology Research © 2014 Japan Society of Obstetrics and Gynecology
W. Zhang et al.
Figure 4 Expression of collagen
I, insulin-like growth factor
(IGF)-1 and fibronectin were
decreased after myosin heavy
chain 11 (MYH11) interference
in human uterine fibroid cells, as
determined by enzyme-linked
immunosorbent assay. (a) Secre-
tion of collagen I. (b) Secretion
of IGF-1. (c) Secretion of fibro-
nectin. (d) Secretion of proteo-
glycans. The bars indicate the
mean ± standard error (n = 3) for
each group. Data were analyzed
by Student’s t-test. *P < 0.05,
as compared to the control
group. , Scramble RNAi;
, MYH11 RNAi.
secretion was maintained at a stable level after MYH11 Myosins are involved in various cellular functions,
interference. In control cells, collagen I production including muscle contraction, cell motility, intracellu-
increased gradually (at 48 and 72 h), compared with lar transport, endocytosis, exocytosis, and, probably,
control group cells, so that over time the secretion of gene expression.7,16 Myosin is a multifunctional protein
collagen I was significantly decreased after MYH11 and plays an important role in muscle contraction.17 In
knockdown (P < 0.05). IGF-1 secretion was also recent years, studies have shown that myosin is closely
reduced after MYH11 knockdown compared to control involved with tumor proliferation, invasion and migra-
cells. In control group cells, IGF-1 remained at a high tion. MLC phosphorylation is necessary for tumor cell
level at 24 and 48 h, after which it decreased gradually, proliferation, because cell division depends on the
but levels remained higher than those in MHY11 RNAi activity of the cytoskeleton, and MLC phosphorylation
cells (P < 0.05). In addition, fibronectin secretion was causes myosin and actin to interact, thus controlling
also reduced after MYH11 knockdown compared to cytoskeleton activity, further influencing cell growth.18
control cells (P < 0.05). In control cells, fibronectin Experiments have shown that treating liver cancer cells
production increased gradually over time. But with an MLCK inhibitor caused a shape change and
proteoglycans did not show any significant difference growth inhibition.19 Bessard showed that MLCK plays
compared to control cells (P > 0.05). Production a key role in cell cycle progression in hepatocytes, pro-
increased gradually in either MHY11 RNAi cells or viding evidence that MLCK regulates S phase entry.20
control group cells (Fig. 4). In this study we found that the expression of myosin,
MLCK and p-MLCK were significantly increased in
Discussion uterine myoma, suggesting that an increase in the
MLCK-myosin pathway contributes to cell prolifera-
Although myomas are common, myoma research has tion in the development of uterine myoma. Fibroids
been slow compared with research on other non- are uterine smooth muscle cell-derived tumors. The
malignant diseases.12,13 However, with much younger collagen components of the uterine fibroids provide for
patients now presenting with this condition, and the a strong, fibrous texture.21,22 Uncontrolled secretion
10-year recurrence rate after myomectomy gradually of growth factors leads to this abnormal growth of
increasing over the years,14,15 an increasing number uterine smooth muscle cells.23,24 Abnormal secretion
of studies has focused on the pathogenesis and mecha- of fibronectin can enhance cell adhesion with the sub-
nisms of uterine fibroids. Most patients with uterine strate. By sticking, fibronectin can regulate cell shape
fibroids have a dysfunction of contraction and endo- and cytoskeleton through the cell signal transduction
crine disorders. pathways. As weak contractions would lead to massive
2056 © 2014 The Authors
Journal of Obstetrics and Gynaecology Research © 2014 Japan Society of Obstetrics and Gynecology
Overexpression of myosin in myoma
bleeding,25,26 we postulated that interference with the 9. Cheng Z, Xie Y, Dai H, Hu L, Zhu Y, Gong J. Unequal tissue
contraction-related protein MYH11 would affect intra- expression of proteins from the PA/PAI system, myoma
necrosis, and uterus survival after uterine artery occlusion.
cellular protein traffic. Myosins are actin-dependent
Int J Gynaecol Obstet 2008; 102: 55–59.
molecular motors that use the energy of ATP hydroly- 10. Huriaux F, Vandewalle P, Focant B. Myosin heavy chain
sis to move along actin filaments.27 We knocked down isoforms in white, red and ventricle muscles of barbel
the expression of MYH11 and observed downstream (Barbus barbus L.). Comp Biochem Physiol B 1991; 100: 309–312.
protein secretion. Our results show that collagen I, 11. Murthy KS. Signaling for contraction and relaxation in
smooth muscle of the gut. Annu Rev Physiol 2006; 68: 345–374.
IGF-1 and fibronectin secretion were reduced after
12. Walker CL, Stewart EA. Uterine fibroids: The elephant in the
MYH11 knockdown, which subsequently may reduce room. Science 2005; 308: 1589–1592.
cell proliferation. But proteoglycans did not show any 13. Fleischer R, Weston GC, Vollenhoven BJ, Rogers PA. Patho-
significant change and this result suggested that the physiology of fibroid disease: Angiogenesis and regulation
secretion of proteoglycans may be influenced by other of smooth muscle proliferation. Best Pract Res Clin Obstet
Gynaecol 2008; 22: 603–614.
factors.
14. Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB.
In conclusion, this study shows that myosin and Recurrence of leiomyomata after myomectomy. Hum Reprod
MLCK are highly expressed in myoma tissues, which Update 2000; 6: 595–602.
suggests that they may be involved in the develop- 15. Marshall LM, Spiegelman D, Barbieri RL et al. Variation in the
ment of myoma. Knockdown of myosin could reduce incidence of uterine leiomyoma among premenopausal
women by age and race. Obstet Gynecol 1997; 90: 967–973.
the expression and secretion of collagen I, IGF-1 and
16. Redowicz MJ. Myosins and pathology: Genetics and biology.
fibronectin, further supporting the important role of Acta Biochim Pol 2002; 49: 789–804.
myosin in myoma. However, the exact role of myosin 17. Higashi-Fujime S, Nakamura A. Cell and molecular biology
in cell contraction and proliferation needs further of the fastest myosins. Int Rev Cell Mol Biol 2009; 276: 301–347.
exploration in future. 18. Petecchia L, Sabatini F, Usai C et al. Mechanisms of
bradykinin-induced contraction in human fetal lung fibro-
blasts. Eur Respir J 2010; 36: 655–664.
Disclosure 19. Zhou X, Liu Y, You J, Zhang H, Zhang X, Ye L. Myosin
light-chain kinase contributes to the proliferation and migra-
No author has any potential conflict of interest. tion of breast cancer cells through cross-talk with activated
ERK1/2. Cancer Lett 2008; 270: 312–327.
20. Bessard A, Coutant A, Rescan C et al. An MLCK-dependent
References window in late G1 controls S phase entry of proliferating
rodent hepatocytes via ERK-p70S6K pathway. Hepatology
1. Cheng ZP, Tao X, Gong J, Dai H, Hu LP, Yang WH. 2006; 44: 152–163.
Early-stage morphological observations of myoma and 21. Adiguzel E, Ahmad PJ, Franco C, Bendeck MP. Collagens
myometrium after laparoscopic uterine artery occlusion in the progression and complications of atherosclerosis. Vasc
treatment. Eur J Obstet Gynecol Reprod Biol 2009; 145: 113– Med 2009; 14: 73–89.
116. 22. Rammeh-Rommani S, Mokni M, Stita W et al. Uterine smooth
2. Draeger A, Graf AH, Staudach A, North AJ, Small JV. Smooth muscle tumors: Retrospective epidemiological and pathologi-
muscle differentiation in human myometrium and uterine cal study of 2760 cases. J Gynecol Obstet Biol Reprod (Paris)
leiomyoma. Virchows Arch B Cell Pathol Incl Mol Pathol 1993; 2005; 34: 568–571.
64: 21–27. 23. Gargett CE, Bucak K, Zaitseva M et al. Estrogen receptor-
3. Neamtu MC, Neamtu RL, Avramescu ET, Vrabete M, Călina alpha and -beta expression in microvascular endothelial cells
LM, Mîndrilă I. Contributions to myometrium study in and smooth muscle cells of myometrium and leiomyoma.
uterine-tubal junction. Rom J Morphol Embryol 2009; 50: 675– Mol Hum Reprod 2002; 8: 770–775.
681. 24. Di X, Yu L, Moore AB et al. A low concentration of genistein
4. Iosif CS, Akerlund M. Fibromyomas and uterine activity. induces estrogen receptor-alpha and insulin-like growth
Acta Obstet Gynecol Scand 1983; 62: 165–167. factor-I receptor interactions and proliferation in uterine leio-
5. Agdi M, Tulandi T. Minimally invasive approach for myo- myoma cells. Hum Reprod 2008; 23: 1873–1883.
mectomy. Semin Reprod Med 2010; 28: 228–234. 25. Parker WH. Etiology, symptomatology, and diagnosis of
6. Degani S, Tamir A, Leibovitz Z, Shapiro I, Gonen R, Ohel G. uterine myomas. Fertil Steril 2007; 87: 725–736.
Three-dimensional power Doppler in the evaluation of 26. Di Lieto A, De Falco M, Pollio F et al. Clinical response, vas-
painful leiomyomas and focal uterine thickening in preg- cular change, and angiogenesis in gonadotropin-releasing
nancy. Int J Gynaecol Obstet 2007; 99: 122–126. hormone analogue-treated women with uterine myomas.
7. Berg JS, Powell BC, Cheney RE. A millennial myosin census. J Soc Gynecol Investig 2005; 12: 123–128.
Mol Biol Cell 2001; 12: 780–794. 27. Applegate D, Pardee JD. Actin-facilitated assembly of smooth
8. Thompson RF, Langford GM. Myosin superfamily evolution- muscle myosin induces formation of actomyosin fibrils. J Cell
ary history. Anat Rec 2002; 268: 276–289. Biol 1992; 117: 1223–1230.
© 2014 The Authors 2057
Journal of Obstetrics and Gynaecology Research © 2014 Japan Society of Obstetrics and Gynecology
You might also like
- The Clear Skin Diet by Nina Nelson PDFDocument370 pagesThe Clear Skin Diet by Nina Nelson PDFmia agustina83% (6)
- S74 Poster Abstracts: Table 1Document1 pageS74 Poster Abstracts: Table 1HanaNo ratings yet
- Cancer-Associated Adipocytes Promote The Invasion and Metastasis in Breast Cancer Through LIFCXCLs Positive Feedback Loop Ayesha MudassarDocument35 pagesCancer-Associated Adipocytes Promote The Invasion and Metastasis in Breast Cancer Through LIFCXCLs Positive Feedback Loop Ayesha MudassarAyesha MudassarNo ratings yet
- 39708-Article Text-251234-3-10-20230319Document7 pages39708-Article Text-251234-3-10-20230319isamatias01No ratings yet
- AMM V 27.07Document138 pagesAMM V 27.07paul_calburean7899No ratings yet
- Huang 2005Document6 pagesHuang 2005Hector Javier BurgosNo ratings yet
- 2152 FullDocument15 pages2152 FullYoana VeselinovaNo ratings yet
- tmp3831 TMPDocument3 pagestmp3831 TMPFrontiersNo ratings yet
- Adipose-Derived Mesenchymal Stem Cells TreatmentsDocument10 pagesAdipose-Derived Mesenchymal Stem Cells TreatmentsTatiana SiqueiraNo ratings yet
- Or 26 1 185 PDFDocument7 pagesOr 26 1 185 PDFRoland_IINo ratings yet
- Quercetin 2013Document4 pagesQuercetin 2013Rahmad DarmawanNo ratings yet
- Acta Obstet Gynecol Scand - 2004 - Mayerhofer - Ki 67 Expression in Patients With Uterine Leiomyomas Uterine Smooth MuscleDocument4 pagesActa Obstet Gynecol Scand - 2004 - Mayerhofer - Ki 67 Expression in Patients With Uterine Leiomyomas Uterine Smooth MuscleMe Me2No ratings yet
- Srep32933 PDFDocument13 pagesSrep32933 PDFCarolina RicárdezNo ratings yet
- NIACINAMIDE 5% BrighteningDocument12 pagesNIACINAMIDE 5% Brighteningproduksi roiNo ratings yet
- ღეროვანი უჯრედებიDocument10 pagesღეროვანი უჯრედებიIrakli GhlontiNo ratings yet
- Microglia Phagocytose Myelin Sheaths To Modify Developmental MyelinationDocument23 pagesMicroglia Phagocytose Myelin Sheaths To Modify Developmental MyelinationCony GSNo ratings yet
- Mitochondrial Gene Replacement in Primate Offspring and Embryonic Stem CellsDocument6 pagesMitochondrial Gene Replacement in Primate Offspring and Embryonic Stem CellsMagdalena AndjelevskiNo ratings yet
- MMP10 NSCLCDocument9 pagesMMP10 NSCLCbhaskarNo ratings yet
- Inhibiting Collagen I Production and Tumor Cell Colonizatio - 2022 - Acta PharmaDocument13 pagesInhibiting Collagen I Production and Tumor Cell Colonizatio - 2022 - Acta PharmaMohammed Shuaib AhmedNo ratings yet
- 53 389Document8 pages53 389Muna FiahNo ratings yet
- Chistine Eichbaum-2011Document9 pagesChistine Eichbaum-2011Gonzalo GonzalezNo ratings yet
- Mitochondrial Energy Metabolism in Baby Hamster Kidney (BHK-21/C13) Cells Treated With Karnozin EXTRA®Document7 pagesMitochondrial Energy Metabolism in Baby Hamster Kidney (BHK-21/C13) Cells Treated With Karnozin EXTRA®Garbuz ElenaNo ratings yet
- Wang 2009Document13 pagesWang 2009Novi ArthaNo ratings yet
- Wound Cancer 5Document4 pagesWound Cancer 5sitilae03No ratings yet
- Ijcep0007 0602Document9 pagesIjcep0007 0602Deedee RenovaldiNo ratings yet
- 2016 - Extracellular Matrix Components Supporting Human Islet Function in Alginate-Based Immunoprotective Microcapsules For Treatment of DiabetesDocument9 pages2016 - Extracellular Matrix Components Supporting Human Islet Function in Alginate-Based Immunoprotective Microcapsules For Treatment of DiabetesAndrés MenesesNo ratings yet
- Synthesis Oxytocin Amnion, Chorion, Influence The Timing of HumanDocument8 pagesSynthesis Oxytocin Amnion, Chorion, Influence The Timing of HumanElissa IsdasariNo ratings yet
- Integrative CUT&Tag-RNA-Seq Analysis of Histone Variant macroH2A1-dependent Orchestration of Human Induced Pluripotent Stem Cell ReprogrammingDocument16 pagesIntegrative CUT&Tag-RNA-Seq Analysis of Histone Variant macroH2A1-dependent Orchestration of Human Induced Pluripotent Stem Cell Reprogrammingniccolo.liorni1No ratings yet
- Campeanu Et Al 2014 PDFDocument8 pagesCampeanu Et Al 2014 PDFBeatriceMihaelaRaduNo ratings yet
- Stromal CD4+ and CD8+ T Cells in Human Breast Carcinomas. Its Correlation With Chemokine Mig/Cxcl9Document5 pagesStromal CD4+ and CD8+ T Cells in Human Breast Carcinomas. Its Correlation With Chemokine Mig/Cxcl9Uum SudrajatNo ratings yet
- Kuze 1997Document8 pagesKuze 1997Sarly FebrianaNo ratings yet
- tmpD776 TMPDocument6 pagestmpD776 TMPFrontiersNo ratings yet
- Human Adipose-Derived Stem Cells Inhibit Bioactivity of Keloid FibroblastsDocument8 pagesHuman Adipose-Derived Stem Cells Inhibit Bioactivity of Keloid FibroblastssesiaNo ratings yet
- Efectos Del Ultrasonido en FibroblastosDocument7 pagesEfectos Del Ultrasonido en FibroblastosErika VillacisNo ratings yet
- Atypical Cyclin P Regulates Cancer Cell Stemness Through Activation of The WNT PathwayDocument14 pagesAtypical Cyclin P Regulates Cancer Cell Stemness Through Activation of The WNT Pathwaygg2179No ratings yet
- Isoprenylcysteine Carboxylmethyltransferase Regulates Ovarian CancerDocument7 pagesIsoprenylcysteine Carboxylmethyltransferase Regulates Ovarian CancerVICTOR MORONo ratings yet
- Association of Mast CellsDocument8 pagesAssociation of Mast CellsJéssica MacêdoNo ratings yet
- Supplemental DataRevisedDocument12 pagesSupplemental DataRevisedCezary TomczykNo ratings yet
- JCMM 15717Document9 pagesJCMM 15717Ioana LețiNo ratings yet
- Inflammatory Myofibroblastic Tumors in The Peculiar Context of The Postpartum Case ReportDocument5 pagesInflammatory Myofibroblastic Tumors in The Peculiar Context of The Postpartum Case ReportIJAR JOURNALNo ratings yet
- Pre ResechDocument20 pagesPre ResechWida MarianeNo ratings yet
- A Preclinical Mouse Model of Osteosarcoma To Define The Extracellular Vesicle-Mediated Communication Between Tumor and Mesenchymal Stem CellsDocument8 pagesA Preclinical Mouse Model of Osteosarcoma To Define The Extracellular Vesicle-Mediated Communication Between Tumor and Mesenchymal Stem CellsRoberto CrepesNo ratings yet
- CXCR2 InhibitorDocument11 pagesCXCR2 InhibitorSaheed AbdulkarimNo ratings yet
- CPG Island Hypermethylation-Associated Silencing of Micrornas Promotes Human Endometrial CancerDocument10 pagesCPG Island Hypermethylation-Associated Silencing of Micrornas Promotes Human Endometrial CancerFerdina NidyasariNo ratings yet
- Janssen 2010Document4 pagesJanssen 2010barti koksNo ratings yet
- Targeting Neddylation Pathway With MLN4924 (Pevonedistat) InducesDocument6 pagesTargeting Neddylation Pathway With MLN4924 (Pevonedistat) InducescarlakerengrNo ratings yet
- Onionin A Inhibits Ovarian Cancer Progression by Suppressing Cancer Cell Proliferation and The Protumour Function of MacrophagesDocument11 pagesOnionin A Inhibits Ovarian Cancer Progression by Suppressing Cancer Cell Proliferation and The Protumour Function of Macrophageskhangkhang151203No ratings yet
- CXCR4Document7 pagesCXCR4opopopopNo ratings yet
- Braga C Cytotherapy 2022 Proteomics of MSC EV Normoxia HypoxiaDocument14 pagesBraga C Cytotherapy 2022 Proteomics of MSC EV Normoxia HypoxiaAlmira AlodiaNo ratings yet
- Cox 2Document11 pagesCox 2Agnes WindyasariNo ratings yet
- C2C12 Weiwei Chu 2016Document7 pagesC2C12 Weiwei Chu 2016Fujiko Saavedra LeivaNo ratings yet
- Interfering With Mitochondrial Dynamics (PINK1) Sensitizes GlioblastomaDocument20 pagesInterfering With Mitochondrial Dynamics (PINK1) Sensitizes GlioblastomaT Smith AndresNo ratings yet
- Research Article: Loratadine, An H Antihistamine, Inhibits Melanogenesis in Human MelanocytesDocument9 pagesResearch Article: Loratadine, An H Antihistamine, Inhibits Melanogenesis in Human MelanocytesZafitri AsrulNo ratings yet
- AMM V 27.07Document138 pagesAMM V 27.07Anonymous 5cHdazK6b100% (1)
- Ijcep0012 2657Document8 pagesIjcep0012 2657Ethel GeanNo ratings yet
- 06-Lu Et AlDocument6 pages06-Lu Et AlKurnia Fitri AprillianaNo ratings yet
- 1 s2.0 S0893395222029118 MainDocument7 pages1 s2.0 S0893395222029118 MainEmanuelly MatosNo ratings yet
- Whole Ovary TransplantationDocument7 pagesWhole Ovary Transplantationari naNo ratings yet
- Kista OvariumDocument6 pagesKista OvariumAde Gustina SiahaanNo ratings yet
- Ertao 2016Document7 pagesErtao 2016chemistpl420No ratings yet
- Neuroendocrine Tumors: Surgical Evaluation and ManagementFrom EverandNeuroendocrine Tumors: Surgical Evaluation and ManagementJordan M. CloydNo ratings yet
- Checklist For Importer Enlistment (Form-6)Document1 pageChecklist For Importer Enlistment (Form-6)Muhammad Saad FarooqNo ratings yet
- 3G StatsDocument4,522 pages3G StatsMuhammad Saad FarooqNo ratings yet
- HUA 3G Capacity OptimizationDocument39 pagesHUA 3G Capacity Optimizationismail_hw91% (11)
- Rams-II (70799) SSV Site Report: Drive Test DateDocument15 pagesRams-II (70799) SSV Site Report: Drive Test DateMuhammad Saad Farooq100% (1)
- 00 WCDMA RNP FundamentalDocument73 pages00 WCDMA RNP FundamentalMuhammad Saad FarooqNo ratings yet
- Haemonetics Cell Saver CS5Document8 pagesHaemonetics Cell Saver CS5zairmendesNo ratings yet
- Passmedicine MRCP Notes-OphthalmologyDocument31 pagesPassmedicine MRCP Notes-OphthalmologyHashim Ahmad50% (2)
- Part VII - Deadly Injection: The Hidden Ingredients in VaccinesDocument19 pagesPart VII - Deadly Injection: The Hidden Ingredients in VaccinesGary Null100% (3)
- Khan Mental Disorder CourseDocument21 pagesKhan Mental Disorder Courseadmin1666No ratings yet
- Add and Mastering It Cheat SheetDocument2 pagesAdd and Mastering It Cheat SheetSoufian CherkiNo ratings yet
- Sample: Patient Safety in Healthcare, Forecast To 2022Document20 pagesSample: Patient Safety in Healthcare, Forecast To 2022Rolando PinchettiNo ratings yet
- Power Point AutismDocument11 pagesPower Point Autismjakolej949954100% (1)
- 834-Food Nutrition and DieteticsDocument11 pages834-Food Nutrition and DieteticsJanvi ShahiNo ratings yet
- 3 7 6Document8 pages3 7 6Mary BassanNo ratings yet
- 5.practice Set SSC-CGL TIER I PDFDocument17 pages5.practice Set SSC-CGL TIER I PDFmanuNo ratings yet
- Next-Generation SequencingDocument6 pagesNext-Generation SequencingvisiniNo ratings yet
- Ambulatory - Care - EditedDocument17 pagesAmbulatory - Care - Editedstephen karikariNo ratings yet
- Adult Assessment: Head To Toe Assessment Is The Baseline and Ongoing Data That Is Needed OnDocument2 pagesAdult Assessment: Head To Toe Assessment Is The Baseline and Ongoing Data That Is Needed OnAldrin NavarroNo ratings yet
- Banana Nutrition Health BenefitsDocument9 pagesBanana Nutrition Health BenefitsUmar PervezNo ratings yet
- Research Article: Aman Singh and Babita PandeyDocument10 pagesResearch Article: Aman Singh and Babita PandeyadityamduttaNo ratings yet
- Genetically Engineered Bacteriocins and Their Potential As The Next Generation of AntimicrobialsDocument9 pagesGenetically Engineered Bacteriocins and Their Potential As The Next Generation of AntimicrobialsValeria VelasquezNo ratings yet
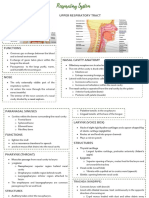
- Respiratory System ReviewerDocument7 pagesRespiratory System ReviewerVictoria Ellex TiomicoNo ratings yet
- AI in HealthcareDocument8 pagesAI in Healthcaresammayekar14No ratings yet
- NHAI Recruitment 2022 Notification and FormDocument30 pagesNHAI Recruitment 2022 Notification and FormVikas SharmaNo ratings yet
- Edited - The Viruses Can Make People Sick, Usually With A Mild To Moderate Upper Respiratory Tract Illness, Similar To A Common ColdDocument1 pageEdited - The Viruses Can Make People Sick, Usually With A Mild To Moderate Upper Respiratory Tract Illness, Similar To A Common ColdDomo BerdaudaraNo ratings yet
- RyaltrisDocument6 pagesRyaltrisNaveen RaghavanNo ratings yet
- Sequencing Primer: 9 Ways To Plan A Yoga Class: Also SeeDocument20 pagesSequencing Primer: 9 Ways To Plan A Yoga Class: Also SeeLaerteNo ratings yet
- Blood Transfusion ProblemsDocument52 pagesBlood Transfusion ProblemsAulia RahmanNo ratings yet
- November 21, 2014 Strathmore TimesDocument28 pagesNovember 21, 2014 Strathmore TimesStrathmore TimesNo ratings yet
- Daftar PustakaDocument2 pagesDaftar PustakaDheaa Fauzantz SalsabilaNo ratings yet
- Cancer Chemotherapy: Acronym of RegimenDocument13 pagesCancer Chemotherapy: Acronym of RegimenVaibhav Bharat100% (1)
- Mechanisms of Ageing and Development: A.U. Trendelenburg, A.C. Scheuren, P. Potter, R. Müller, I. Bellantuono TDocument10 pagesMechanisms of Ageing and Development: A.U. Trendelenburg, A.C. Scheuren, P. Potter, R. Müller, I. Bellantuono TMIGUEL MORENONo ratings yet
- 2-Cystotomy in A SADocument22 pages2-Cystotomy in A SAOsama GhaziNo ratings yet
- Food Microbiology PDFDocument217 pagesFood Microbiology PDFFernando Dias100% (3)