Professional Documents
Culture Documents
Chemical-Bonding Part 01
Chemical-Bonding Part 01
Uploaded by
Sampa SadhukhanCopyright:
Available Formats
You might also like
- Nclex Cheat SheetDocument6 pagesNclex Cheat SheetLeeAnn Marie100% (34)
- 2011 Uaw Ford ContractDocument562 pages2011 Uaw Ford ContractbobologyNo ratings yet
- Donate Electron Accept ElectronDocument2 pagesDonate Electron Accept ElectronPraveen Raj RajamaniNo ratings yet
- Chemical BondingDocument131 pagesChemical BondingAnant VashishtNo ratings yet
- ChemistryDocument20 pagesChemistryFatma SharifNo ratings yet
- Dokumen - Tips Ib Chemistry On VseprDocument14 pagesDokumen - Tips Ib Chemistry On VseprIwona Agata GórnickaNo ratings yet
- Chemical Bonding - Short Notes - Learning Tales 2Document5 pagesChemical Bonding - Short Notes - Learning Tales 2balajibhakte646No ratings yet
- Chemical Bonding Short Notes Raftaar NItesh Devnani PDF CrdownloadDocument5 pagesChemical Bonding Short Notes Raftaar NItesh Devnani PDF Crdownloadaadil0% (1)
- Chemical Bonding Short Notes Nitesh DevnaniDocument5 pagesChemical Bonding Short Notes Nitesh Devnanivrinda11xxNo ratings yet
- Chemical Bonding (Infographic)Document1 pageChemical Bonding (Infographic)pundleturdyyNo ratings yet
- Chemical Bonding One Day One Chapter Nitesh DevnaniDocument41 pagesChemical Bonding One Day One Chapter Nitesh Devnanivrinda11xxNo ratings yet
- Chemical Bonding Reny RosalinaDocument49 pagesChemical Bonding Reny Rosalinawildan ariefNo ratings yet
- Chemical Bonding Reny RosalinaDocument52 pagesChemical Bonding Reny RosalinaViand NugrohoNo ratings yet
- Carbon and Its Compounds - Class - 10Document9 pagesCarbon and Its Compounds - Class - 10Mamta JoshiNo ratings yet
- CAN 1 Ionic Bonding Mat 2Document1 pageCAN 1 Ionic Bonding Mat 2Kev WattsNo ratings yet
- Breakdown in GasesDocument28 pagesBreakdown in GasesGaurav kumarNo ratings yet
- Chemsheet Structure Summary WsDocument1 pageChemsheet Structure Summary WsShiv PatelNo ratings yet
- Structure Types: Monatomic Simple Molecular Giant Covalent Ionic MetallicDocument1 pageStructure Types: Monatomic Simple Molecular Giant Covalent Ionic MetallicZack CurryNo ratings yet
- SARA PEREZ TRUJILLO - Molecules and Bonds 7BDocument4 pagesSARA PEREZ TRUJILLO - Molecules and Bonds 7BApuestas JulanoNo ratings yet
- Electrocoagulation: Program Studi Teknik Pengolahan Limbah - PpnsDocument30 pagesElectrocoagulation: Program Studi Teknik Pengolahan Limbah - PpnsrizkaNo ratings yet
- Enercon Surface Treatment EffectsDocument1 pageEnercon Surface Treatment EffectscavekeNo ratings yet
- Chemical Bonding UssDocument8 pagesChemical Bonding Usstech 2 lifeNo ratings yet
- Biochemistry BasicsDocument22 pagesBiochemistry Basicsjazlamba09No ratings yet
- .Trashed 1702966746 1700327622328Document40 pages.Trashed 1702966746 1700327622328gno667533No ratings yet
- @bohring - Bot 01. Chemical Bonding Syn (1-32)Document32 pages@bohring - Bot 01. Chemical Bonding Syn (1-32)PranavNo ratings yet
- Topic 3: Chemical BondsDocument49 pagesTopic 3: Chemical BondsFlores DavidNo ratings yet
- 1-Kimia Lingkungan 2021Document41 pages1-Kimia Lingkungan 2021Ahmad SyahrulNo ratings yet
- ElectricityDocument20 pagesElectricityJürgen GeermanNo ratings yet
- Ionic BondingDocument2 pagesIonic Bondingdigjhon6No ratings yet
- Chemical Bonding and Molecular StructureDocument1 pageChemical Bonding and Molecular Structuresarthakyedlawar04No ratings yet
- Chemical Bonding and Molecular StructureDocument1 pageChemical Bonding and Molecular StructureRao GootleyNo ratings yet
- Notes On Bonding IDocument38 pagesNotes On Bonding IEdem DumashieNo ratings yet
- Ionic Bonding - FactRecallDocument1 pageIonic Bonding - FactRecallgabby fosterNo ratings yet
- Chapter 8 AssessmentDocument19 pagesChapter 8 AssessmentLeinNo ratings yet
- Notes On Bonding IDocument38 pagesNotes On Bonding INika ReleniNo ratings yet
- 1.3 Revision Guide Bonding AqaDocument8 pages1.3 Revision Guide Bonding AqaRS JNo ratings yet
- Core Bonding - Ionic BondingDocument21 pagesCore Bonding - Ionic BondingMarin PesicNo ratings yet
- Knowledge Organiser: Elements and Compounds Development of The Model of The AtomDocument35 pagesKnowledge Organiser: Elements and Compounds Development of The Model of The AtomOblizinNo ratings yet
- Acids, Bases & Buffers: Ionic BondsDocument4 pagesAcids, Bases & Buffers: Ionic BondsSamantha BolanteNo ratings yet
- Lecture - 05 - Chemical Bonding I Basic ConceptsDocument55 pagesLecture - 05 - Chemical Bonding I Basic ConceptsDuy Do MinhNo ratings yet
- Carbon and Its Compounds - Shobhit NirwanDocument17 pagesCarbon and Its Compounds - Shobhit NirwanBhaskar 8287No ratings yet
- Carbon and Its Compounds CH4Document14 pagesCarbon and Its Compounds CH4SuryaNo ratings yet
- 4A Ionic Bonding Edrolo Study Notes AnnotatedDocument17 pages4A Ionic Bonding Edrolo Study Notes AnnotatedMr FiddleNo ratings yet
- I Graphite As An Electrical Conductor: Si Atoms O AtomsDocument3 pagesI Graphite As An Electrical Conductor: Si Atoms O AtomsAykhan DamirliNo ratings yet
- Basic ELECTRICAL ENGINEERING Review BOARD EXAM 2023Document17 pagesBasic ELECTRICAL ENGINEERING Review BOARD EXAM 2023One Piece FanboyNo ratings yet
- PN Juncyion DiodeDocument21 pagesPN Juncyion DiodeSithara JeyarajNo ratings yet
- 03 Chemistry Chemical Bonding To EDITDocument49 pages03 Chemistry Chemical Bonding To EDITmaheshapaNo ratings yet
- Solid State Bonding in SolidsDocument6 pagesSolid State Bonding in SolidsbookregtestNo ratings yet
- 05 Chem Bond - Modul - ChemistryDocument11 pages05 Chem Bond - Modul - Chemistryrudi_z100% (1)
- Chemical Bonding and Molecular Structure: 2.1. Fundamental Concepts of Chemical BondsDocument47 pagesChemical Bonding and Molecular Structure: 2.1. Fundamental Concepts of Chemical BondsNguyễn Quốc HưngNo ratings yet
- Chapter 1, Panda (CHEM)Document4 pagesChapter 1, Panda (CHEM)naifalfarraj3No ratings yet
- Ionic BondingDocument16 pagesIonic BondingOwais Siddiqui IX-M-ANo ratings yet
- FaziraRazak - Chemical BondingDocument71 pagesFaziraRazak - Chemical BondingaieyinHengNo ratings yet
- CHAPTER 5 - Chemical Bond (5.1 - 5.3) 8 JulyDocument25 pagesCHAPTER 5 - Chemical Bond (5.1 - 5.3) 8 JulyNur100% (1)
- Chemical BondingDocument18 pagesChemical BondingAvel Xyphus N. MaravillaNo ratings yet
- Welcome To: Chemical Bonding and Molecular StructureDocument284 pagesWelcome To: Chemical Bonding and Molecular StructureSachin NayakNo ratings yet
- Grade 9 UNIT 1 ScienceDocument3 pagesGrade 9 UNIT 1 ScienceFrancesca Irah MapaNo ratings yet
- Analysis of Concept Chemistry For Senior High School Grade 10 Subject Matter: Chemical BondingDocument4 pagesAnalysis of Concept Chemistry For Senior High School Grade 10 Subject Matter: Chemical BondingSiti Rahma Dani HarahapNo ratings yet
- (CHEM CS) Chapter 2.3 - 2.5 - Chemical BondingDocument16 pages(CHEM CS) Chapter 2.3 - 2.5 - Chemical Bondingfayyaz haqueNo ratings yet
- Solid State PhysicsDocument10 pagesSolid State PhysicsJaymin RayNo ratings yet
- Chemical Bonding - Final PDFDocument47 pagesChemical Bonding - Final PDFKundan ChoudharyNo ratings yet
- ME122 4th LE ExercisesDocument1 pageME122 4th LE ExercisesPaul RodgersNo ratings yet
- Offices Summary GuidelinesDocument6 pagesOffices Summary GuidelinesGeorge StockburgerNo ratings yet
- Catalog HarfitDocument80 pagesCatalog Harfitfajarsanjaya1992No ratings yet
- A Detailed Lesson Plan in Technology and Livelihood Education - Beauty Care (Nail Care)Document4 pagesA Detailed Lesson Plan in Technology and Livelihood Education - Beauty Care (Nail Care)Exequiel Macalisang Ramientos Jr.No ratings yet
- Simpson XT Anchor ICBO CertDocument16 pagesSimpson XT Anchor ICBO CertjarnebergNo ratings yet
- Stevanovic 2016Document7 pagesStevanovic 2016sezgin bayramNo ratings yet
- MMT Validity ReliabilityDocument23 pagesMMT Validity ReliabilitynNo ratings yet
- Canine Zoonosis Its Potential and Association of Soil-Borne Helminthes From Public Parks and Its Gastro-Intestinal Helminthes in Lahore, PakistanDocument5 pagesCanine Zoonosis Its Potential and Association of Soil-Borne Helminthes From Public Parks and Its Gastro-Intestinal Helminthes in Lahore, PakistanhaloninenineNo ratings yet
- Perlindungan Hukum Bagi Profesi Perawat Terhadap Pelaksanaan Praktik KeperawatanDocument5 pagesPerlindungan Hukum Bagi Profesi Perawat Terhadap Pelaksanaan Praktik KeperawatanALWIN WinNo ratings yet
- Assessment of The Ultra High Molecular Weight Polyethylene (UHMWPE) Used in Orthopedic and Spinal DevicesDocument5 pagesAssessment of The Ultra High Molecular Weight Polyethylene (UHMWPE) Used in Orthopedic and Spinal DevicesJoãoNo ratings yet
- Want To Prep But Not Sure Where To Begin?: Sign Up & Download My Urban Survival Plan For FREE!Document20 pagesWant To Prep But Not Sure Where To Begin?: Sign Up & Download My Urban Survival Plan For FREE!Emilio Vicente YepesNo ratings yet
- Department of Labor FLSA Fact SheetDocument6 pagesDepartment of Labor FLSA Fact SheetAme PoseyNo ratings yet
- Practical Report 2Document50 pagesPractical Report 2elviscosmas300No ratings yet
- 93-693 Web ManualDocument13 pages93-693 Web ManualJosé Angel González HernándezNo ratings yet
- Department of Education: Republic of The PhilippinesDocument2 pagesDepartment of Education: Republic of The PhilippinesAirah SantiagoNo ratings yet
- QF-PCR Best Practice GuidelinesDocument11 pagesQF-PCR Best Practice GuidelinesSandeep SharmaNo ratings yet
- Soal Uas Genap 2021Document12 pagesSoal Uas Genap 2021Nur Rokhma SNo ratings yet
- Liver CirrhosisDocument9 pagesLiver CirrhosismedsmracelisNo ratings yet
- Hdi BDDocument22 pagesHdi BDAjker PaychalNo ratings yet
- Infinit 1Document12 pagesInfinit 1Saufiy SarminNo ratings yet
- XYLO Diagnostic Manual ABSDocument96 pagesXYLO Diagnostic Manual ABSData TécnicaNo ratings yet
- Publix Coral Springs Inspection ReportDocument5 pagesPublix Coral Springs Inspection ReportAmanda RojasNo ratings yet
- Stihl FS 45Document36 pagesStihl FS 45swhitton135No ratings yet
- Bitter Kola Nut and Edulis Trees Production To ConsumptionDocument125 pagesBitter Kola Nut and Edulis Trees Production To Consumptionalkhwarizmi1968100% (1)
- 01-34-11 11-10-2016 Brasil - Feasibility Study For The Waste To Energy Plant Vol.1Document255 pages01-34-11 11-10-2016 Brasil - Feasibility Study For The Waste To Energy Plant Vol.1Sekretariat SPSPNo ratings yet
- Groups 070 - 079: Emission Control Systems (EVAP, Sec. AIR, EGR)Document2 pagesGroups 070 - 079: Emission Control Systems (EVAP, Sec. AIR, EGR)Sebastian DamianNo ratings yet
- Peace Corps Azerbaijan Welcome Book - 2012Document83 pagesPeace Corps Azerbaijan Welcome Book - 2012Accessible Journal Media: Peace Corps DocumentsNo ratings yet
- Mangu Kcse Pre-Mock 1 2024 KcseDocument271 pagesMangu Kcse Pre-Mock 1 2024 Kcsephilomenamulee79No ratings yet
Chemical-Bonding Part 01
Chemical-Bonding Part 01
Uploaded by
Sampa SadhukhanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemical-Bonding Part 01
Chemical-Bonding Part 01
Uploaded by
Sampa SadhukhanCopyright:
Available Formats
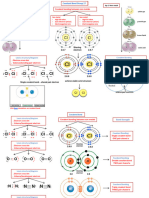
Chemical Bonding
Attractive force that holds ions and atoms together and stabilizes them by overall loss of energy
atoms - - -
atoms
Ionic Bond + + Covalent Bond + +
-
Formed by complete transfer Bonds in which two atoms
sharing
of valence electrons to attain Transfer share a pair of electrons
Of of
stability Electron electrons
- Covalent Attraction
Compounds
- -
+ +
+ +
Nucleus
- + +
- -
Positive Ion Negative Ion Shared Molecule
Coordinate Bond electrons
H
A covalent chemical bond - - - -
between two atoms that is + + + + +
produced when one atom shares H N H - - - -
+ + + + Metallic Bond
a pair of electrons with another
atom lacking such a pair. Also - - - - Bond is formed when metals
called coordinate covalent bond H + + + + share their electrons
Hydrogen Bond
Inter Molecular Hydrogen bonding Hydrogen bond is the chemical bond Intra Molecular Hydrogen bonding
between H-atom and electronegative atom
O
O O
HO N N H
O O
O
HO N
p- nitrophenol o-nitrophenol
O
(Intermolecular hydrogen bonding) Intramolecular hydrogen bonding
Bond Parameters Polarity of Bonds
Bond Length State of atom or molecule having positive and negative charges
in case of magnetic or an electrical poles
Bond Length
A B
Average distance between rA rB
nuclei of two bonded atoms Polar bonds/molecules Non-polar bonds/molecules
in a molecule. Examples- HCl, NH3 Examples- BF3, CCl4
Bond multiplicity
Factors affecting H Cl
F
Size of the atom
Bond Angle .. B
A bond angle is the angle O N
formed between three atoms F F
across at least two bonds. H H H H F
Contribute to the shape of a molecule. Bond Angle H
Bond Enthalpy
Cl
Enthalpy change when one mole of bonds are broken in Bond Order
a substance at 298 K.
The higher its value, the stronger the bond and the more Number of bonds that form
energy required to break it. between two atoms C
H H Cl Cl
Atomic size
Bond order is 1
N N
Factors affecting Electronegativity Cl
Bond order is 3
Extent of overlapping O O
Bond order is 2
You might also like
- Nclex Cheat SheetDocument6 pagesNclex Cheat SheetLeeAnn Marie100% (34)
- 2011 Uaw Ford ContractDocument562 pages2011 Uaw Ford ContractbobologyNo ratings yet
- Donate Electron Accept ElectronDocument2 pagesDonate Electron Accept ElectronPraveen Raj RajamaniNo ratings yet
- Chemical BondingDocument131 pagesChemical BondingAnant VashishtNo ratings yet
- ChemistryDocument20 pagesChemistryFatma SharifNo ratings yet
- Dokumen - Tips Ib Chemistry On VseprDocument14 pagesDokumen - Tips Ib Chemistry On VseprIwona Agata GórnickaNo ratings yet
- Chemical Bonding - Short Notes - Learning Tales 2Document5 pagesChemical Bonding - Short Notes - Learning Tales 2balajibhakte646No ratings yet
- Chemical Bonding Short Notes Raftaar NItesh Devnani PDF CrdownloadDocument5 pagesChemical Bonding Short Notes Raftaar NItesh Devnani PDF Crdownloadaadil0% (1)
- Chemical Bonding Short Notes Nitesh DevnaniDocument5 pagesChemical Bonding Short Notes Nitesh Devnanivrinda11xxNo ratings yet
- Chemical Bonding (Infographic)Document1 pageChemical Bonding (Infographic)pundleturdyyNo ratings yet
- Chemical Bonding One Day One Chapter Nitesh DevnaniDocument41 pagesChemical Bonding One Day One Chapter Nitesh Devnanivrinda11xxNo ratings yet
- Chemical Bonding Reny RosalinaDocument49 pagesChemical Bonding Reny Rosalinawildan ariefNo ratings yet
- Chemical Bonding Reny RosalinaDocument52 pagesChemical Bonding Reny RosalinaViand NugrohoNo ratings yet
- Carbon and Its Compounds - Class - 10Document9 pagesCarbon and Its Compounds - Class - 10Mamta JoshiNo ratings yet
- CAN 1 Ionic Bonding Mat 2Document1 pageCAN 1 Ionic Bonding Mat 2Kev WattsNo ratings yet
- Breakdown in GasesDocument28 pagesBreakdown in GasesGaurav kumarNo ratings yet
- Chemsheet Structure Summary WsDocument1 pageChemsheet Structure Summary WsShiv PatelNo ratings yet
- Structure Types: Monatomic Simple Molecular Giant Covalent Ionic MetallicDocument1 pageStructure Types: Monatomic Simple Molecular Giant Covalent Ionic MetallicZack CurryNo ratings yet
- SARA PEREZ TRUJILLO - Molecules and Bonds 7BDocument4 pagesSARA PEREZ TRUJILLO - Molecules and Bonds 7BApuestas JulanoNo ratings yet
- Electrocoagulation: Program Studi Teknik Pengolahan Limbah - PpnsDocument30 pagesElectrocoagulation: Program Studi Teknik Pengolahan Limbah - PpnsrizkaNo ratings yet
- Enercon Surface Treatment EffectsDocument1 pageEnercon Surface Treatment EffectscavekeNo ratings yet
- Chemical Bonding UssDocument8 pagesChemical Bonding Usstech 2 lifeNo ratings yet
- Biochemistry BasicsDocument22 pagesBiochemistry Basicsjazlamba09No ratings yet
- .Trashed 1702966746 1700327622328Document40 pages.Trashed 1702966746 1700327622328gno667533No ratings yet
- @bohring - Bot 01. Chemical Bonding Syn (1-32)Document32 pages@bohring - Bot 01. Chemical Bonding Syn (1-32)PranavNo ratings yet
- Topic 3: Chemical BondsDocument49 pagesTopic 3: Chemical BondsFlores DavidNo ratings yet
- 1-Kimia Lingkungan 2021Document41 pages1-Kimia Lingkungan 2021Ahmad SyahrulNo ratings yet
- ElectricityDocument20 pagesElectricityJürgen GeermanNo ratings yet
- Ionic BondingDocument2 pagesIonic Bondingdigjhon6No ratings yet
- Chemical Bonding and Molecular StructureDocument1 pageChemical Bonding and Molecular Structuresarthakyedlawar04No ratings yet
- Chemical Bonding and Molecular StructureDocument1 pageChemical Bonding and Molecular StructureRao GootleyNo ratings yet
- Notes On Bonding IDocument38 pagesNotes On Bonding IEdem DumashieNo ratings yet
- Ionic Bonding - FactRecallDocument1 pageIonic Bonding - FactRecallgabby fosterNo ratings yet
- Chapter 8 AssessmentDocument19 pagesChapter 8 AssessmentLeinNo ratings yet
- Notes On Bonding IDocument38 pagesNotes On Bonding INika ReleniNo ratings yet
- 1.3 Revision Guide Bonding AqaDocument8 pages1.3 Revision Guide Bonding AqaRS JNo ratings yet
- Core Bonding - Ionic BondingDocument21 pagesCore Bonding - Ionic BondingMarin PesicNo ratings yet
- Knowledge Organiser: Elements and Compounds Development of The Model of The AtomDocument35 pagesKnowledge Organiser: Elements and Compounds Development of The Model of The AtomOblizinNo ratings yet
- Acids, Bases & Buffers: Ionic BondsDocument4 pagesAcids, Bases & Buffers: Ionic BondsSamantha BolanteNo ratings yet
- Lecture - 05 - Chemical Bonding I Basic ConceptsDocument55 pagesLecture - 05 - Chemical Bonding I Basic ConceptsDuy Do MinhNo ratings yet
- Carbon and Its Compounds - Shobhit NirwanDocument17 pagesCarbon and Its Compounds - Shobhit NirwanBhaskar 8287No ratings yet
- Carbon and Its Compounds CH4Document14 pagesCarbon and Its Compounds CH4SuryaNo ratings yet
- 4A Ionic Bonding Edrolo Study Notes AnnotatedDocument17 pages4A Ionic Bonding Edrolo Study Notes AnnotatedMr FiddleNo ratings yet
- I Graphite As An Electrical Conductor: Si Atoms O AtomsDocument3 pagesI Graphite As An Electrical Conductor: Si Atoms O AtomsAykhan DamirliNo ratings yet
- Basic ELECTRICAL ENGINEERING Review BOARD EXAM 2023Document17 pagesBasic ELECTRICAL ENGINEERING Review BOARD EXAM 2023One Piece FanboyNo ratings yet
- PN Juncyion DiodeDocument21 pagesPN Juncyion DiodeSithara JeyarajNo ratings yet
- 03 Chemistry Chemical Bonding To EDITDocument49 pages03 Chemistry Chemical Bonding To EDITmaheshapaNo ratings yet
- Solid State Bonding in SolidsDocument6 pagesSolid State Bonding in SolidsbookregtestNo ratings yet
- 05 Chem Bond - Modul - ChemistryDocument11 pages05 Chem Bond - Modul - Chemistryrudi_z100% (1)
- Chemical Bonding and Molecular Structure: 2.1. Fundamental Concepts of Chemical BondsDocument47 pagesChemical Bonding and Molecular Structure: 2.1. Fundamental Concepts of Chemical BondsNguyễn Quốc HưngNo ratings yet
- Chapter 1, Panda (CHEM)Document4 pagesChapter 1, Panda (CHEM)naifalfarraj3No ratings yet
- Ionic BondingDocument16 pagesIonic BondingOwais Siddiqui IX-M-ANo ratings yet
- FaziraRazak - Chemical BondingDocument71 pagesFaziraRazak - Chemical BondingaieyinHengNo ratings yet
- CHAPTER 5 - Chemical Bond (5.1 - 5.3) 8 JulyDocument25 pagesCHAPTER 5 - Chemical Bond (5.1 - 5.3) 8 JulyNur100% (1)
- Chemical BondingDocument18 pagesChemical BondingAvel Xyphus N. MaravillaNo ratings yet
- Welcome To: Chemical Bonding and Molecular StructureDocument284 pagesWelcome To: Chemical Bonding and Molecular StructureSachin NayakNo ratings yet
- Grade 9 UNIT 1 ScienceDocument3 pagesGrade 9 UNIT 1 ScienceFrancesca Irah MapaNo ratings yet
- Analysis of Concept Chemistry For Senior High School Grade 10 Subject Matter: Chemical BondingDocument4 pagesAnalysis of Concept Chemistry For Senior High School Grade 10 Subject Matter: Chemical BondingSiti Rahma Dani HarahapNo ratings yet
- (CHEM CS) Chapter 2.3 - 2.5 - Chemical BondingDocument16 pages(CHEM CS) Chapter 2.3 - 2.5 - Chemical Bondingfayyaz haqueNo ratings yet
- Solid State PhysicsDocument10 pagesSolid State PhysicsJaymin RayNo ratings yet
- Chemical Bonding - Final PDFDocument47 pagesChemical Bonding - Final PDFKundan ChoudharyNo ratings yet
- ME122 4th LE ExercisesDocument1 pageME122 4th LE ExercisesPaul RodgersNo ratings yet
- Offices Summary GuidelinesDocument6 pagesOffices Summary GuidelinesGeorge StockburgerNo ratings yet
- Catalog HarfitDocument80 pagesCatalog Harfitfajarsanjaya1992No ratings yet
- A Detailed Lesson Plan in Technology and Livelihood Education - Beauty Care (Nail Care)Document4 pagesA Detailed Lesson Plan in Technology and Livelihood Education - Beauty Care (Nail Care)Exequiel Macalisang Ramientos Jr.No ratings yet
- Simpson XT Anchor ICBO CertDocument16 pagesSimpson XT Anchor ICBO CertjarnebergNo ratings yet
- Stevanovic 2016Document7 pagesStevanovic 2016sezgin bayramNo ratings yet
- MMT Validity ReliabilityDocument23 pagesMMT Validity ReliabilitynNo ratings yet
- Canine Zoonosis Its Potential and Association of Soil-Borne Helminthes From Public Parks and Its Gastro-Intestinal Helminthes in Lahore, PakistanDocument5 pagesCanine Zoonosis Its Potential and Association of Soil-Borne Helminthes From Public Parks and Its Gastro-Intestinal Helminthes in Lahore, PakistanhaloninenineNo ratings yet
- Perlindungan Hukum Bagi Profesi Perawat Terhadap Pelaksanaan Praktik KeperawatanDocument5 pagesPerlindungan Hukum Bagi Profesi Perawat Terhadap Pelaksanaan Praktik KeperawatanALWIN WinNo ratings yet
- Assessment of The Ultra High Molecular Weight Polyethylene (UHMWPE) Used in Orthopedic and Spinal DevicesDocument5 pagesAssessment of The Ultra High Molecular Weight Polyethylene (UHMWPE) Used in Orthopedic and Spinal DevicesJoãoNo ratings yet
- Want To Prep But Not Sure Where To Begin?: Sign Up & Download My Urban Survival Plan For FREE!Document20 pagesWant To Prep But Not Sure Where To Begin?: Sign Up & Download My Urban Survival Plan For FREE!Emilio Vicente YepesNo ratings yet
- Department of Labor FLSA Fact SheetDocument6 pagesDepartment of Labor FLSA Fact SheetAme PoseyNo ratings yet
- Practical Report 2Document50 pagesPractical Report 2elviscosmas300No ratings yet
- 93-693 Web ManualDocument13 pages93-693 Web ManualJosé Angel González HernándezNo ratings yet
- Department of Education: Republic of The PhilippinesDocument2 pagesDepartment of Education: Republic of The PhilippinesAirah SantiagoNo ratings yet
- QF-PCR Best Practice GuidelinesDocument11 pagesQF-PCR Best Practice GuidelinesSandeep SharmaNo ratings yet
- Soal Uas Genap 2021Document12 pagesSoal Uas Genap 2021Nur Rokhma SNo ratings yet
- Liver CirrhosisDocument9 pagesLiver CirrhosismedsmracelisNo ratings yet
- Hdi BDDocument22 pagesHdi BDAjker PaychalNo ratings yet
- Infinit 1Document12 pagesInfinit 1Saufiy SarminNo ratings yet
- XYLO Diagnostic Manual ABSDocument96 pagesXYLO Diagnostic Manual ABSData TécnicaNo ratings yet
- Publix Coral Springs Inspection ReportDocument5 pagesPublix Coral Springs Inspection ReportAmanda RojasNo ratings yet
- Stihl FS 45Document36 pagesStihl FS 45swhitton135No ratings yet
- Bitter Kola Nut and Edulis Trees Production To ConsumptionDocument125 pagesBitter Kola Nut and Edulis Trees Production To Consumptionalkhwarizmi1968100% (1)
- 01-34-11 11-10-2016 Brasil - Feasibility Study For The Waste To Energy Plant Vol.1Document255 pages01-34-11 11-10-2016 Brasil - Feasibility Study For The Waste To Energy Plant Vol.1Sekretariat SPSPNo ratings yet
- Groups 070 - 079: Emission Control Systems (EVAP, Sec. AIR, EGR)Document2 pagesGroups 070 - 079: Emission Control Systems (EVAP, Sec. AIR, EGR)Sebastian DamianNo ratings yet
- Peace Corps Azerbaijan Welcome Book - 2012Document83 pagesPeace Corps Azerbaijan Welcome Book - 2012Accessible Journal Media: Peace Corps DocumentsNo ratings yet
- Mangu Kcse Pre-Mock 1 2024 KcseDocument271 pagesMangu Kcse Pre-Mock 1 2024 Kcsephilomenamulee79No ratings yet