Professional Documents
Culture Documents
Recognizing Vaccine-Induced Immune Thrombotic Thrombocytopenia
Recognizing Vaccine-Induced Immune Thrombotic Thrombocytopenia
Uploaded by
lagopatis4Copyright:
Available Formats
You might also like
- Workbook Answers: Unit 1 RespirationDocument30 pagesWorkbook Answers: Unit 1 RespirationYan En88% (196)
- Coagulopathy of Coronavirus Disease 2019: Review ArticlesDocument7 pagesCoagulopathy of Coronavirus Disease 2019: Review ArticlesBianca IlieNo ratings yet
- Diagnosis and Management of Cerebral Venous Sinus Thrombosis With Vaccine-Induced Immune Thrombotic ThrombocytopeniaDocument5 pagesDiagnosis and Management of Cerebral Venous Sinus Thrombosis With Vaccine-Induced Immune Thrombotic ThrombocytopeniaindanazulfaaNo ratings yet
- Diagnosis and Management of Cerebral Venous Sinus Thrombosis With Vaccine-Induced Immune Thrombotic ThrombocytopeniaDocument10 pagesDiagnosis and Management of Cerebral Venous Sinus Thrombosis With Vaccine-Induced Immune Thrombotic ThrombocytopeniaShin Elcant Del BarçaNo ratings yet
- Lecturio Pericardial Effusion & Cardiac TamponadeDocument1 pageLecturio Pericardial Effusion & Cardiac TamponadeCecil-An DalanonNo ratings yet
- BMJ n1005 FullDocument1 pageBMJ n1005 FullDror BrandNo ratings yet
- Fulminant MeningococcemiaDocument5 pagesFulminant MeningococcemiaRaffaharianggaraNo ratings yet
- What Is Meningitis?Document7 pagesWhat Is Meningitis?laujeroNo ratings yet
- Jurnal RadiologiDocument12 pagesJurnal Radiologiimamlutfi13No ratings yet
- MicrobiologyDocument24 pagesMicrobiologyPRAJWAL PES HDNo ratings yet
- COVID-19 Associated Ischemic Stroke and Hemorrhagic Stroke - Incidence, Potential Pathological Mechanism, and ManagementDocument8 pagesCOVID-19 Associated Ischemic Stroke and Hemorrhagic Stroke - Incidence, Potential Pathological Mechanism, and ManagementWilliam MilesNo ratings yet
- Ischemic Stroke in COVID-19-Positive Patients - An Overview of SARS-CoV-2 and Thrombotic Mechanisms For The NeurointerventionalistDocument6 pagesIschemic Stroke in COVID-19-Positive Patients - An Overview of SARS-CoV-2 and Thrombotic Mechanisms For The Neurointerventionalistarif 2006No ratings yet
- Pathology and Pathogenesis of Infective Endocarditis in Native Heart ValvesDocument8 pagesPathology and Pathogenesis of Infective Endocarditis in Native Heart ValvesIrina TănaseNo ratings yet
- New Perspectives of Infections in Cardiovascular Disease: Ignatius W. FongDocument18 pagesNew Perspectives of Infections in Cardiovascular Disease: Ignatius W. FongCarlos Danilo Noroña CNo ratings yet
- 17-Infective EndocarditisDocument17 pages17-Infective Endocarditisمصطفى محمد جواد كاظمNo ratings yet
- Infective EndocarditisDocument4 pagesInfective EndocarditisXiang Yun TanNo ratings yet
- Brain Abscess: State-Of-The-Art Clinical ArticleDocument17 pagesBrain Abscess: State-Of-The-Art Clinical ArticleIqbal AbdillahNo ratings yet
- Tuning The Thromboinflammatory Response To Venous Flow Interruption by The Ectonucleotidase CD39Document12 pagesTuning The Thromboinflammatory Response To Venous Flow Interruption by The Ectonucleotidase CD39KEERTHI UPPALAPATINo ratings yet
- Brain Abscess: State-Of-The-Art Clinical ArticleDocument17 pagesBrain Abscess: State-Of-The-Art Clinical ArticleAwais KhanNo ratings yet
- 2021 Thrombocytopenia and Intracranial Venous Sinus Thrombosis AfterDocument10 pages2021 Thrombocytopenia and Intracranial Venous Sinus Thrombosis AfterBeatriz VásquezNo ratings yet
- CNS Micro ImpulseDocument44 pagesCNS Micro Impulsedineshvd75No ratings yet
- Coagulop CovidDocument11 pagesCoagulop CovidbacchiocchipaoloNo ratings yet
- Obstructive Shock From Diagnosis To TreatmentDocument11 pagesObstructive Shock From Diagnosis To TreatmentMasjid Jaami' Al Ma'ruf SmdNo ratings yet
- Pathogenesis of Bacterial Meningitis: From Bacteraemia To Neuronal InjuryDocument10 pagesPathogenesis of Bacterial Meningitis: From Bacteraemia To Neuronal InjurymjeanjNo ratings yet
- Nosocomial Infection: 50Th Anniversary ArticlesDocument19 pagesNosocomial Infection: 50Th Anniversary ArticlesAndres RamirezNo ratings yet
- Hypotheses Behind The Very Rare Cases of Thrombosis With Thrombocytopenia Syndrome After SARS-CoV-2 VaccinationDocument10 pagesHypotheses Behind The Very Rare Cases of Thrombosis With Thrombocytopenia Syndrome After SARS-CoV-2 VaccinationAna-Maria AursuleseiNo ratings yet
- Antithrombotic Therapy Forpatients With COVID-19Document19 pagesAntithrombotic Therapy Forpatients With COVID-19dregleavNo ratings yet
- Mec CronicaDocument29 pagesMec CronicaAlfredo Luis Arana MaqueraNo ratings yet
- Disseminated Intravascular CoagulationDocument7 pagesDisseminated Intravascular CoagulationlgebNo ratings yet
- Cshperspectmed BAC A012393Document14 pagesCshperspectmed BAC A012393RaffaharianggaraNo ratings yet
- Cerebral Venous Thrombosis PDFDocument7 pagesCerebral Venous Thrombosis PDFd dNo ratings yet
- Viral MyocarditisDocument12 pagesViral Myocarditisemotic123No ratings yet
- Manejo HicDocument5 pagesManejo HicDianira Chavez RamosNo ratings yet
- Updates On Thrombosis With Thrombocytopenia Syndrome (TTS) : Advisory Committee On Immunization Practices (ACIP)Document39 pagesUpdates On Thrombosis With Thrombocytopenia Syndrome (TTS) : Advisory Committee On Immunization Practices (ACIP)kajelchaNo ratings yet
- Concern About Using Current Covid 19 Vaccines For Mass Vaccination in The Midst of A Pandemic Geert Vanden BosscheDocument21 pagesConcern About Using Current Covid 19 Vaccines For Mass Vaccination in The Midst of A Pandemic Geert Vanden BosscheSlavica Borojevic100% (1)
- Geert Vanden Bossche Concern About Current Covid 19 Mass Vaccination Campaigns Mar 2nd 2021Document14 pagesGeert Vanden Bossche Concern About Current Covid 19 Mass Vaccination Campaigns Mar 2nd 2021Kada Ben youcefNo ratings yet
- Infectious Diseases Pharmacotherapy: Lesson 5 Central Nervous System InfectionDocument63 pagesInfectious Diseases Pharmacotherapy: Lesson 5 Central Nervous System Infectionbest batiNo ratings yet
- Latest Development in PCVDocument9 pagesLatest Development in PCVAndityo SidohutomoNo ratings yet
- Medical Progress: Review ArticleDocument11 pagesMedical Progress: Review ArticleGaby Alejandra Ordonez AndradeNo ratings yet
- Autopsies Prove Covid-19 Is Disseminated Intravascular CoagulationDocument9 pagesAutopsies Prove Covid-19 Is Disseminated Intravascular CoagulationWayne SpringerNo ratings yet
- Thromboembolic Disease in COVID-19 Patients: A Brief Narrative ReviewDocument10 pagesThromboembolic Disease in COVID-19 Patients: A Brief Narrative ReviewSteven PutraNo ratings yet
- 202 FullDocument6 pages202 FullMariana CabralNo ratings yet
- MINOCADocument10 pagesMINOCAmariana virguezNo ratings yet
- COVID 19 Associated Coagulopathy and Inflammatory.2Document10 pagesCOVID 19 Associated Coagulopathy and Inflammatory.2alvNo ratings yet
- Embolic Stroke Circulation2017Document5 pagesEmbolic Stroke Circulation2017Maryani SyahrilNo ratings yet
- Unusual Pattern of Arterial Macrothrombosis Causing Stroke in A Young Adult Recovered From COVID-19Document5 pagesUnusual Pattern of Arterial Macrothrombosis Causing Stroke in A Young Adult Recovered From COVID-19Audrey Beatrice ReyesNo ratings yet
- Jesse Wackerbarth - CNS Pathogens RevisedDocument1 pageJesse Wackerbarth - CNS Pathogens RevisedMicroposterNo ratings yet
- MeningitisDocument2 pagesMeningitisedrian02No ratings yet
- Intracranial Infection - Prof SunartiniDocument12 pagesIntracranial Infection - Prof SunartiniFranciscus BuwanaNo ratings yet
- Covid Is An Endothelial Disease 2020 SeptDocument7 pagesCovid Is An Endothelial Disease 2020 SeptDaniela Mădălina GhețuNo ratings yet
- MAnaagement Koagulapati Dan Thrombolis Pada Pasien CovDocument13 pagesMAnaagement Koagulapati Dan Thrombolis Pada Pasien CovJuwita Rashi LumbantoruanNo ratings yet
- A Radiation-Mimicking Disease of Access - We Initially Believed SARS-CoV-2 Was A Respiratory Virus Is That The Respiratory Tract Is The Initial Point of AccessDocument6 pagesA Radiation-Mimicking Disease of Access - We Initially Believed SARS-CoV-2 Was A Respiratory Virus Is That The Respiratory Tract Is The Initial Point of Accesshansley cookNo ratings yet
- JIR 333887 Common Inflammatory Mechanisms in Covid 19 and ParkinsonDocument33 pagesJIR 333887 Common Inflammatory Mechanisms in Covid 19 and ParkinsonRochiNo ratings yet
- Covid Covid 19 Por Sarscov 2 Adultos y Pediatricos Version Septiembre - Octubre 21Document163 pagesCovid Covid 19 Por Sarscov 2 Adultos y Pediatricos Version Septiembre - Octubre 21ximenadonajiNo ratings yet
- Neonatal Pneumonia PathophysiologyDocument5 pagesNeonatal Pneumonia PathophysiologyRoderick EstrellaNo ratings yet
- Periodontal Disease AND Cardiovascular Disease: Dentaid Medical DepartmentDocument17 pagesPeriodontal Disease AND Cardiovascular Disease: Dentaid Medical DepartmentMunteanu DragosNo ratings yet
- Bcs Article RoleDocument15 pagesBcs Article RoleSowmya ANo ratings yet
- Green and Cream Vintage Aesthetic Group Project Presentation - 20231208 - 000317 - 0000Document22 pagesGreen and Cream Vintage Aesthetic Group Project Presentation - 20231208 - 000317 - 0000onoshaomneyaNo ratings yet
- Assessment of Changes and Complications Caused by Coronavirus Infection in The Lungs Using Computed TomographyDocument6 pagesAssessment of Changes and Complications Caused by Coronavirus Infection in The Lungs Using Computed TomographyCentral Asian StudiesNo ratings yet
- Journey to the Center of a Human Cell.: WE NEED A SECOND OPINION, #1From EverandJourney to the Center of a Human Cell.: WE NEED A SECOND OPINION, #1No ratings yet
- Referencetable 2Document350 pagesReferencetable 2lagopatis4No ratings yet
- 395 925 2 PBDocument22 pages395 925 2 PBlagopatis4No ratings yet
- Kosovo enDocument3 pagesKosovo enlagopatis4No ratings yet
- Lignite Mining Development Strategy: Statem Ent of Principl EDocument8 pagesLignite Mining Development Strategy: Statem Ent of Principl Elagopatis4No ratings yet
- Balkan Watch.11April 05Document1 pageBalkan Watch.11April 05lagopatis4No ratings yet
- COVID-19 Infection SurveyDocument22 pagesCOVID-19 Infection Surveylagopatis4No ratings yet
- Understanding Intimate Partner Violence in The EU: The Role of DataDocument48 pagesUnderstanding Intimate Partner Violence in The EU: The Role of Datalagopatis4No ratings yet
- DLL 4 - Body OrganssDocument4 pagesDLL 4 - Body OrganssAdor IsipNo ratings yet
- Science Grade 7 Second QuarterDocument12 pagesScience Grade 7 Second QuarterAnneKARYLE67% (3)
- IGCSE - BloodDocument7 pagesIGCSE - Bloodmubasherkatbar562No ratings yet
- Blood TypingDocument3 pagesBlood TypingNoriz Ember DominguezNo ratings yet
- Grade 9: ScienceDocument19 pagesGrade 9: ScienceChristian ArcegaNo ratings yet
- Acido TranexamicoDocument8 pagesAcido TranexamicoVianey GarciaVillegasNo ratings yet
- Science Blood TransfusionsDocument10 pagesScience Blood Transfusionsapi-237866019No ratings yet
- Final Lesson Plan For Demo TeachingDocument12 pagesFinal Lesson Plan For Demo Teachingapi-651914206No ratings yet
- Detailed Lesson Plan RealDocument14 pagesDetailed Lesson Plan RealHarold Jay LamangNo ratings yet
- Hema Chapter 21 Part 2Document4 pagesHema Chapter 21 Part 2EMETERIO TUTOR IIINo ratings yet
- ANSCIDocument40 pagesANSCIJessa Lorraine Dela CruzNo ratings yet
- Sains F3 Bab 3.3Document9 pagesSains F3 Bab 3.3HAREYANKA EKAA MADAN MOHAN MoeNo ratings yet
- 1BI0 2H Mark-Scheme 20180822Document25 pages1BI0 2H Mark-Scheme 20180822Xiao ShadowlordNo ratings yet
- Blood Cell PosterDocument1 pageBlood Cell PosternadaNo ratings yet
- First Periodical Test Sci 9Document4 pagesFirst Periodical Test Sci 9Jhaypee SorianoNo ratings yet
- 9B Fit and HealthyDocument24 pages9B Fit and HealthyKassyKas100% (1)
- ROTEM AnalysisDocument42 pagesROTEM AnalysisAnnie ArriNo ratings yet
- Biology C - Lesson 1 - Circulatory SystemDocument46 pagesBiology C - Lesson 1 - Circulatory SystemMuhammad Azrie0% (1)
- PBL Problem 1 Unit IV Week 1Document4 pagesPBL Problem 1 Unit IV Week 1Ibtehal HasanNo ratings yet
- Science Quiz Bee Questions 2015Document3 pagesScience Quiz Bee Questions 2015melba escuetaNo ratings yet
- Circulatory SystemDocument26 pagesCirculatory SystemGlemarie EnriquezNo ratings yet
- CAIELSBIO Y8 c5 tt5Document5 pagesCAIELSBIO Y8 c5 tt5warothkorn29No ratings yet
- Jyoti Singh ReportsDocument5 pagesJyoti Singh ReportsRaghujyotisNo ratings yet
- Step-t19-Ep by Saeed Mdcat TeamDocument18 pagesStep-t19-Ep by Saeed Mdcat TeamMuhammad AnwarNo ratings yet
- Module NCM112Document69 pagesModule NCM112Potato Banana100% (1)
- Diagnostics SampleDocument4 pagesDiagnostics SampleRobin RavagoNo ratings yet
- Hematology 2 TEST QUESTIONSDocument4 pagesHematology 2 TEST QUESTIONSa a r o n b a u t i s t aNo ratings yet
- Nutrients and Waste Transport SystemDocument22 pagesNutrients and Waste Transport SystemItsPotatoQueen MaeNo ratings yet
- MLS3174 Clinical Haematology FEB 18 PDFDocument9 pagesMLS3174 Clinical Haematology FEB 18 PDFVisaNathanNo ratings yet
Recognizing Vaccine-Induced Immune Thrombotic Thrombocytopenia
Recognizing Vaccine-Induced Immune Thrombotic Thrombocytopenia
Uploaded by
lagopatis4Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Recognizing Vaccine-Induced Immune Thrombotic Thrombocytopenia
Recognizing Vaccine-Induced Immune Thrombotic Thrombocytopenia
Uploaded by
lagopatis4Copyright:
Available Formats
ONLINE REVIEW ARTICLE
Recognizing Vaccine-Induced Immune
Thrombotic Thrombocytopenia
Toshiaki Iba, MD1
OBJECTIVES: Vaccine-induced immune thrombotic thrombocytopenia is an Jerrold H. Levy, MD2
unexpected consequence of the coronavirus disease 2019 pandemic era. We
Theodore E. Warkentin, MD3
reviewed the pathogenesis, clinical presentation, diagnosis, and treatment of this
rare side effect.
Downloaded from http://journals.lww.com/ccmjournal by BhDMf5ePHKav1zEoum1tQfN4a+kJLhEZgbsIHo4XMi0hCywCX1AWnYQp/IlQrHD3i3D0OdRyi7TvSFl4Cf3VC4/OAVpDDa8KKGKV0Ymy+78= on 01/02/2022
DATA SOURCES: Online search of published medical literature through PubMed,
Scopus, Web of Science, and Google Scholar using the terms “COVID-19,” “vac-
cine,” “thrombosis” was performed.
STUDY SELECTION: Articles were chosen for inclusion based on their rele-
vance to coronavirus disease 2019, vaccine, and thrombosis.
DATA SYNTHESIS: Vaccine-induced immune thrombotic thrombocytopenia
manifests most often as unusual thromboses (cerebral venous sinus thrombosis,
splanchnic vein thrombosis) but sometimes also “usual” thromboses (arterial
stroke, pulmonary embolism, deep-vein thrombosis), with oftentimes severe throm-
bocytopenia, that becomes clinically evident 5–30 days after adenovirus-vectored
coronavirus disease 2019 vaccine administration. Most patients have dissemi-
nated intravascular coagulation. These features are the result of vaccine-triggered
formation of anti-platelet factor 4 immunoglobulin G that activate platelets, clini-
cally mimicking autoimmune heparin-induced thrombocytopenia. Early recognition
based on thrombosis (sometimes, hemorrhage), thrombocytopenia, and d-dimer
elevation within the day 5–30 postvaccine “window” is important given treatment
with high-dose IV immunoglobulin plus nonheparin anticoagulation.
CONCLUSIONS: Vaccine-induced immune thrombotic thrombocytopenia is a
serious complication of vaccination that is not feasible to anticipate or prevent.
When the patient presents with sustained headache, neurologic symptoms/signs,
abdominal pain, dyspnea, or limb pain/swelling beginning 5–30 days post vacci-
nation, platelet count and d-dimer must be measured, and imaging for thrombosis
performed. Confirmation of vaccine-induced immune thrombotic thrombocyto-
penia diagnosis should be ordered (platelet factor 4/polyanion enzyme-linked
immunosorbent assay; platelet factor 4–enhanced platelet activation testing) as
treatment is initiated (nonheparin anticoagulation, IV immunoglobulin).
KEY WORDS: autoimmune heparin-induced thrombocytopenia; coronavirus Copyright © 2021 The Author(s).
disease 2019; disseminated intravascular coagulation; thrombosis; vaccine Published by Wolters Kluwer Health,
Inc. on behalf of the Society of Critical
O
Care Medicine and Wolters Kluwer
n March 19, 2021, the World Health Organization released a state- Health, Inc. This is an open-access
ment on the risk of thrombotic events that can occur after vaccina- article distributed under the terms of
tion with the AstraZeneca coronavirus disease 2019 (COVID-19) the Creative Commons Attribution-
Non Commercial-No Derivatives
vaccine (ChAdOx1 nCoV-19). According to the statement, “the available data
License 4.0 (CCBY-NC-ND), where
do not suggest any overall increase in clotting conditions such as deep venous it is permissible to download and
thrombosis or pulmonary embolism following administration of COVID-19 share the work provided it is properly
vaccines;” they concluded, “the AZ COVID-19 vaccine continues to have a cited. The work cannot be changed
positive benefit-risk profile, with tremendous potential to prevent infections in any way or used commercially
and reduce deaths across the world.” However, they also stated: “very rare and without permission from the journal.
unique thromboembolic events in combination with thrombocytopenia, such DOI: 10.1097/CCM.0000000000005211
e80 www.ccmjournal.org January 2022 • Volume 50 • Number 1
Online Review Article
as cerebral venous sinus thrombosis (CVST), have also The authors reviewed the 24 relevant references
been reported following vaccination in Europe” (1). identified.
Thereafter, infrequent reports of multiple thrombo-
ses in unusual sites, bleeding, thrombocytopenia, and Epidemiology and Clinical Features
laboratory findings recognized in healthy individuals
According to the European Medicines Agency (EMA),
shortly after receiving the vaccine began to emerge
30 cases of thromboembolic events had been reported
(2). Since the onset of these thromboses was typically
1–2 weeks after the vaccination, and almost all were by March 10, 2021, among the approximately 5 mil-
accompanied by thrombocytopenia, the involvement lion recipients of the AstraZeneca COVID-19 vaccine
of vaccine-induced autoimmune responses resem- in Europe (12). The EMA subsequently stated that
bling heparin-induced thrombocytopenia (HIT) was “The number of thromboembolic events in vaccinated
investigated. On April 13, 2021, the Centers for the people is no higher than the number seen in the general
Disease Control and Prevention and Food and Drug population” (13). Østergaard et al (14) analyzed the
Administration in the United States recommended data from the epidemiologic view and reported that the
pausing the administration of Johnson & Johnson number of thromboembolic events does not seem to
(J&J)/Janssen COVID-19 vaccine (Ad26.COV2.S) be- be increased relative to the expected number estimated
cause of a similar side effect profile in a small number from occurrence rates before the introduction of the
of patients (3, 4). On April 9, 2021, the first two arti- vaccination program. However, the onset of throm-
cles (5, 6) regarding vaccine-induced immune throm- botic events with thrombocytopenia in several healthy
botic thrombocytopenia (VITT) were released, which people shortly after vaccination in unusual sites such as
provided a detailed explanation of this potentially life- cerebral venous sinus and splanchnic veins indicated
threatening side effect of vaccination. A week later, a the possibility of a rare vaccine-induced prothrom-
larger case-series was published (7). The United States botic disorder (15). As a result, Canada, Germany, and
subsequently lifted the pause, and so both vaccines several European countries recommended against the
implicated in VITT—ChAdOx1 nCoV-19 and Ad26. use of the Oxford-AstraZeneca COVID-19 vaccine use
COV2.S—continue to be administered worldwide; in younger people. Following this announcement, the
thus, clinicians need to be able to recognize this rare University of Oxford paused the dosing trial in chil-
but severe prothrombotic complication aware of these dren and teenagers (16). Furthermore, a similar side
adenoviral vector vaccines. In this review, we discuss effect was reported after the administration of J&J/
the pathogenesis and clinical evaluation of suspected Janssen vaccine. As of April 12, 2021, more than 6.8
VITT. million doses have been injected in the United States,
and six CVST cases were seen in combination with
thrombocytopenia. All the cases are female between
LITERATURE SEARCH
the ages of 18 and 48 (3, 4, 8).
We reviewed related articles to the VITT in PubMed, Initial reports indicated certain features of VITT,
Scopus, Web of Science, and Google Scholar. Two including female predominance, relatively young
authors independently searched the articles using fol- age, and unusual sites of thrombosis. In the report by
lowing subject headings: “COVID-19,” “vaccine,” and Greinacher et al (5), 11 cases of VITT were reported,
“thrombosis.” We also checked the related articles, of which nine were women; the median age was 36
press releases, announcements, and home pages of the years (range, 22–49 yr). Schultz et al (6) also re-
medical societies on the websites. The search strategies ported five VITT cases (four female) with ages rang-
were modified for each electronic database using data- ing from 32 to 54 years. At least 13 of the 16 patients
base-specific search terms, field names, and syntax. reported in these two studies had CVST, and four
We identified eight relevant articles published between patients had splanchnic vein thrombosis (indicat-
March 2020 and April 20, 2021 (Table 1). In addition, ing clotting of portal, mesenteric, splenic, or hepatic
relevant literature regarding “heparin-induced throm- veins). However, the predominance of young women
bocytopenia,” “cerebral vein,” “thrombus,” and “antico- may have reflected the population of recipients of this
agulants” were identified in the electronic resources. particular vaccine in Germany and Austria, which
Critical Care Medicine www.ccmjournal.org e81
Iba et al
TABLE 1.
Case-Series and Case Reports of Vaccine-Induced Immune Thrombotic Thrombocytopenia
No. of
Cases
S. Date (Female/ Site of
No. References Published Male) Thrombus Key Findings
1 Muir et al (4) April 14, 2021 1 (1/0) Splanchnic vein The patient showed severe thrombocytopenia (13,000/
mm3) with schistocytes, low fibrinogenemia, and DIC.
2 Greinacher et al (5) April 9, 2021 11 (9/2) CVT: 9, splanchnic Five cases had DIC and one presented fatal
vein: 3, PE: 3, intracranial hemorrhage.
other: 4
3 Schultz et al (6) April 9, 2021 5 (4/1) Cerebral vein: 4, Four cases had major cerebral hemorrhage. Platelet
splanchnic counts increased in all cases despite of the treat-
vein: 1 ment with low-molecular-weight heparin.
4 Scully et al (7) April 16, 2021 23 (14/9) CVT: 13, PE: 4, Secondary cerebral hemorrhage was recognized in
splanchnic vein: some cases after CVT. Two cases had ischemic
2, etc. stroke. Seven (30%) died.
5 Sadoff et al (8) April 16, 2021 1 (0/1) CVT Single case of CVT with thrombocytopenia occurred
in a vaccine recipient during the clinical trial pro-
gram for the Ad26.COV2.S vaccine (of which
〜50,000 received active vaccine).
6 Franchini et al (9) April 12, 2021 1 (0/1) CVT CVT with multiple parenchymal hemorrhage.
7 Mehta et al (10) April 20, 2021 2 (0/2) CVT Both two were males.
8 Thaler et al (11) April 20, 2021 1 (1/0) None Petechiae and hematomas were the only symptoms.
CVT = cerebral venous thrombosis, DIC = disseminated intravascular coagulation, PE = pulmonary embolism.
was mainly healthcare workers (predominantly Early Recognition of VITT
young women). Indeed, in the subsequent report of
VITT from the United Kingdom, where the vaccine VITT presents in a characteristic time period between
was given more broadly in the population, only 14 5 and 30 days post vaccination, usually with signs
of 23 patients (60.9%) were females, and the me- and symptoms indicating thrombosis. The name of
dian age was somewhat higher (46 yr; range, 21–77 the condition—VITT—provides a helpful mnemonic
yr). Although CVST was most common (at least (VITT), as follows: 1) Vaccine, 2) “Interval” (5–30 d),
13/23 patients), and splanchnic vein thrombosis also 3) Thrombosis, and 4) Thrombocytopenia. We rec-
observed (4/23), a more diverse spectrum of throm- ommend in addition coagulation screening (d-dimer,
botic events was evident, including pulmonary em- prothrombin test/international normalized ratio, ac-
bolism (5/23), middle cerebral artery (MCA) stroke tivated partial thromboplastin test, fibrinogen), as the
(2 cases), myocardial infarction (1 case), deep-vein combination of unexpected thrombocytopenia and
thrombosis (3 cases, 1 upper limb and 2 lower limb), elevated d-dimer (often with reduced fibrinogen) in
acute aortic thrombosis (1 case), and bilateral adrenal an otherwise well individual who now has symptoms
hemorrhage (1 case). One patient presented with or signs of thrombosis 5 or more days post vaccina-
“hemorrhagic symptoms only.” Remarkably, the two tion requires further urgent investigation (discussed
patients with MCA stroke were only 21 and 39 years subsequently). These additional coagulation tests are
old. Interestingly, if there is a true female predom- helpful to the diagnosis of VITT and necessary in dis-
inance in VITT, this could mirror that seen in HIT tinguishing VITT from other entities. However, the
(approximately two-fold increase in females) (17). cutoffs of each test remain to be elucidated.
e82 www.ccmjournal.org January 2022 • Volume 50 • Number 1
Online Review Article
A severe and persistent headache or abdominal pain strongly positive (optical density is generally > 2.0)
are the most common symptoms, indicting potential (5–7); however, rapid HIT immunoassays (e.g., latex-
CVST or splanchnic vein thrombosis, but abnormal enhanced immunoassay, chemiluminescence immu-
neurologic symptoms/signs and fever can also occur. noassay) are usually negative (4, 18). Platelet activation
Dyspnea, tachypnea, and tachycardia can indicate pul- assays (performed using a variety of activation end-
monary embolism. Signs of limb ischemia or swelling, points, including aggregation and serotonin release)
chest pain, or even cardiovascular collapse (massive show serum-/plasma-induced heparin-independent
pulmonary embolism, bilateral adrenal hemorrhagic platelet aggregation (5–7, 18). Interestingly, standard
necrosis) are reported. A patient with VITT presenting washed platelet activation assays, usually performed
with sudden unexplained death post vaccination can be using patient serum, are sometimes negative unless
shown to have pathogenic antibodies using a postmortem PF4 is supplemented (5, 18) (Table 2).
blood sample (5). Depending on clinical suspicion, im- The optimal treatment of VITT is not established;
aging studies are required to diagnose thrombosis (MRI however, anticoagulation using nonheparin agents,
venography, CT brain, CT angiography, etc.). including direct thrombin inhibitors (argatroban,
The standard HIT platelet factor 4 (PF4)–dependent bivalirudin), indirect (antithrombin-dependent) Xa
enzyme-linked immunosorbent assays are usually inhibitors (fondaparinux, danaparoid), or direct oral
TABLE 2.
Comparison of Heparin-Induced Thrombocytopenia and Vaccine-Induced Immune
Thrombotic Thrombocytopenia
Vaccine-Induced Immune
Variables HIT Thrombotic Thrombocytopenia
Age Usually older Usually younger?
Sex Slight female predominance Female predominance?
Prevalence 0.2–3% after heparin use 1/20,000 to 1/100,000 post vaccination
Recognition after exposure 5 d to 2 wk 5 d to 4 wk
Site of thrombosis DVT/PE, arterial thrombosis Cerebral venous sinus, splanchnic vein,
DVT/PE, arterial
Bleeding Usually none; if present, usually sec- Possible; usually secondary to
ondary to thrombosis (hemorrhagic thrombosis (e.g., cerebral venous
infarction) or anticoagulation sinus thrombosis)
HIT enzyme-linked immunosorbent assay Anti-PF4/polyanion IgG positive Anti-PF4/polyanionic IgG positive
HIT rapid screening assays (e.g., latex Usually positive Usually negative
immunoassay, chemiluminescence
immunoassay, particle gel immunoassay)
Washed platelet activation assay Usually positive (heparin-dependent ± Often negative unless PF4 is
heparin-independent activation) supplemented
Platelet count Median, ~50,000/mm3 (usual range, Median, ~20,000/mm3 (usual range,
10–150× ×103) 10–100× ×103)
Prothrombin test Normal/mildly prolonged Normal/mildly prolonged
d-dimer Greatly increased Greatly increased
Worsened by heparin administration? Sometimes Uncertain
DVT = deep-vein thrombosis, HIT = heparin-induced thrombocytopenia, IgG = immunoglobulin G, PE = pulmonary embolism, PF4 = platelet
factor 4.
Critical Care Medicine www.ccmjournal.org e83
Iba et al
anticoagulants (DOACs), is currently suggested pend- HIT (16). In the absence of IVIG, the severe platelet-
ing ruling out heparin-dependent increase in platelet activating process can last for several weeks (the nat-
activation or emerging data indicating heparin safety ural history of platelet count recovery in VITT remains
and efficacy. Referral of patient serum/plasmas to ref- unknown). Plasma exchange is a potential option to
erence laboratories is usually required to perform reduce antibody levels. Unless bleeding, platelet trans-
high-quality functional (platelet activation) assays. In fusions and fibrinogen replacement should be consid-
addition, IV immunoglobulin (IVIG, 1.0 g/kg/d for 2 ered relatively contraindicated.
consecutive days) therapy is recommended, as immu- The natural history of VITT is unknown, including
noglobulin inhibits VITT antibody-induced platelet the duration of thrombocytopenia and hypercoagu-
activation and decreases hypercoagulability (18). IVIG lability. Patients usually have severe thrombosis, so
is also known to be effective for treating autoimmune at least 3–6 months of anticoagulation will usually be
required, at which time
thrombocytopenia will
likely have recovered (by
analogy with the usual
natural history of auto-
immune HIT) Also, by
analogy with HIT com-
plicated by thrombosis,
longer term anticoagula-
tion is usually managed
with DOAC therapy after
discharge from hospital.
PATHOPHYSIOLOGY
The most mysterious part
of this new disease cate-
gory is its initial trigger and
subsequent pathogenesis.
Since both AstraZeneca
and J&J/Janssen vaccines
are adenovirus-vectored
vaccines, a vector-specific
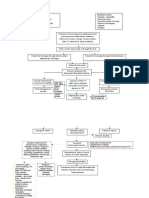
Figure 1. Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia. Adenovirus, a
vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, infects
mechanism is suggested.
dendritic cell/macrophage/monocyte and induces the production of an antispike protein antibody. However, the adenovi-
Since adenovirus is a DNA virus, the vaccine contains viral DNA as well as human proteins. Platelet rus-vectored vaccine has
factor 4 (PF4) released from platelets binds to negatively charged substances such as DNA and a long history of being
heparan sulfate and forms PF4-polyanionic component. After binding, PF4 changes its structure used for making Ebola
and is recognized as an antigen. If high-affinity anti-PF4 platelet-activating antibodies are generated,
and AIDS vaccines, and
these can bind to PF4 (potentially requiring platelet-associated polyanions), inducing platelet
activation and aggregation, as is observed in heparin-induced thrombocytopenia (HIT). Furthermore,
the VITT-mimicking
the antibodies stimulate tissue factor (TF) expression on monocytes and provoke inflammation. thrombosis-related side
These reactions maximize coagulation and inflammation by increasing proinflammatory cytokine effect had not been re-
production, up-regulating TF expression, and releasing TF-bearing extracellular vesicles. As a result ported. The key finding to
of the innate immune system activation, neutrophils eject neutrophil extracellular traps (NETs) that date is that patients have
amplify the coagulation cascade and disrupt antithrombogenicity of the endothelium by damaging
platelet-activating anti-
glycocalyx. The above HIT-mimicking reaction is suspected to be the fundamental mechanism of
vaccine-induced thrombotic thrombocytopenia. Both activated coagulation and platelet aggregation
bodies that target PF4 even
may collaboratively accelerate the clot formation in the unusual sites. Fcγ receptor = receptor for Fc in the absence of heparin
portion of immunoglobulin G, Xa = factor Xa. (5). This phenomenon of
e84 www.ccmjournal.org January 2022 • Volume 50 • Number 1
Online Review Article
heparin-“independent” platelet-activating antibodies 1 Department of Emergency and Disaster Medicine, Juntendo
that target PF4 has been reported with “autoimmune University Graduate School of Medicine, Tokyo, Japan.
2 Department of Anesthesiology, Critical Care, and Surgery,
HIT” (16), which refers to patients who have both
Duke University School of Medicine, Durham, NC.
heparin-dependent and heparin-independent platelet-
3 Department of Pathology and Molecular Medicine, and
activating properties and which explains unusual fea- Department of Medicine, McMaster University, Hamilton,
tures of HIT, including onset after stopping heparin ON, Canada.
(delayed-onset HIT) (19), prolonged thrombocyto- Supported, in part, by a Grant-in-Aid from the Ministry of Health,
penia despite stopping heparin (persisting/refractory Labor and Welfare of Japan and from the Ministry of Education,
Culture, Sports, Science, and Technology of Japan.
HIT) (20), and HIT that occurs only with heparin
Dr. Iba has received a research grant from Japan Blood Products
“flush” exposures (21). In addition, a rare disorder, Organization and JIMRO. Dr. Levy disclosed that he is on the
called “spontaneous HIT,” also features heparin-inde- steering committees for Instrumentation Labs, Merck, and
pendent platelet-activating antibodies that target PF4 Octapharma. Dr. Warkentin reports receiving consulting fees from
Aspen Global, Bayer, CSL Behring, Ergomed, Instrumentation
(22). Some of these patients also present with CVST Laboratory, and Octapharma; he received research support from
(23, 24). Presumably, both in autoimmune HIT as well Instrumentation Laboratory; royalties from Informa (Taylor &
as in VITT, the platelet-activating anti-PF4 antibodies Francis); consulting fees related to medical-legal consulting and
testimony; and disclosed the off-label product use of high-dose
interact with PF4 either in the absence of a polyanion
intravenous immunoglobulin to treat vaccine-induced immune
or in association with platelet-associated polyanions, thrombotic thrombocytopenia.
such as chondroitin sulfate or polyphosphates (16). It is For information regarding this article, E-mail: toshiiba@juntendo.
currently hypothesized that one or more components ac.jp
within the vaccine is somehow triggering a strong anti-
PF4 immune response that helps to explain the broad
similarities between VITT and these autoimmune HIT REFERENCES
disorders. If indeed VITT parallels severe autoimmune 1. WHO Global Advisory Committee on Vaccine Safety (GACVS)
HIT, it is expected that its pathogenesis will include COVID-19 subcommittee. Available at: https://www.who.int/
news/item/19-03-2021-statement-of-the-who-global-advi-
(besides platelet activation) NETs release, monocyte,
sory-committee-on-vaccine-safety-(gacvs)-covid-19-subcom-
and endothelial activation (25). Figure 1 indicates mittee-on-safety-signals-related-to-the-astrazeneca-covid-
potential features of VITT pathogenesis, based on ex- 19-vaccine. Accessed April 17, 2021
isting information as well as expected parallels with se- 2. Pharmacovigilance Risk Assessment Committee of the
European Medicines Agency: Signal assessment report on em-
vere autoimmune HIT.
bolic and thrombotic events (SMQ) with COVID-19 Vaccine
A striking unresolved question is why CVST is so (ChAdOx1-S [recombinant]) – COVID-19 Vaccine AstraZeneca
common in VITT. CVST is rare, accounting for less (Other viral vaccines). Available at: https://www.ema.europa.
than 1% of strokes (26). Non-VITT CVST is reported eu/en/documents/prac-recommendation/signal-assessment-
report-embolic-thrombotic-events-smq-covid-19-vaccine-
in the relatively younger patients, with 70% younger chadox1-s-recombinant-covid_en.pdf. Accessed April 11, 2021
than 50 years old and more common in females (27). 3. Schuchat A, Marks P: Available at: https://www.cdc.gov/media/
Further studies of VITT pathogenesis may help in a releases/2021/s0413-JJ-vaccine.html. Accessed April 17, 2021
better understanding of CVST occurrence. 4. Muir KL, Kallam A, Koepsell SA, et al: Thrombotic thrombocy-
topenia after Ad26.COV2.S vaccination. N Engl J Med 2021;
384:1964–1965
SUMMARY 5. Greinacher A, Thiele T, Warkentin TE, et al: Thrombotic throm-
bocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J
VITT is a serious complication of vaccination which Med 2021; 384:2092–2101
unfortunately cannot be anticipated or prevented. 6. Schultz NH, Sørvoll IH, Michelsen AE, et al: Thrombosis and
Clinicians need to have a high index of suspicion when thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N
patients develop any of a number of symptoms or signs Engl J Med 2021; 384:2124–2130
(headache, neurologic, abdominal, chest, limb) within 7. Scully M, Singh D, Lown R, et al: Pathologic antibodies to
platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl
the day 5–30 window post vaccination. In this situa- J Med 2021; 384:2202–2211
tion, check the platelet count and d-dimer. If thrombo- 8. Sadoff J, Davis K, Douoguih M: Thrombotic thrombocytopenia
cytopenia is recognized, and especially if the d-dimer after Ad26.COV2.S vaccination-response from the manufac-
is elevated, urgent further investigation is mandatory. turer. N Engl J Med 2021; 384:1965–1966
Critical Care Medicine www.ccmjournal.org e85
Iba et al
9. Franchini M, Testa S, Pezzo M, et al: Cerebral venous throm- thrombocytopenia. N Engl J Med 2021 Jun 9. [online
bosis and thrombocytopenia post-COVID-19 vaccination. ahead of print]
Thromb Res 2021; 202:182–183 19. Warkentin TE, Kelton JG: Delayed-onset heparin-induced
10. Mehta PR, Apap Mangion S, Benger M, et al: Cerebral venous thrombocytopenia and thrombosis. Ann Intern Med 2001; 135:
sinus thrombosis and thrombocytopenia after COVID-19 vac- 502–506
cination - A report of two UK cases. Brain Behav Immun 2021; 20. Warkentin TE, Pai M, Linkins LA: Direct oral anticoagulants for
95:514–517 treatment of HIT: Update of hamilton experience and literature
11. Thaler J, Ay C, Gleixner KV, et al: Successful treatment of review. Blood 2017; 130:1104–1113
vaccine-induced prothrombotic immune thrombocytopenia 21. Mian H, Warkentin TE, Sheppard JI, et al: Autoimmune HIT
(VIPIT). J Thromb Haemost 2021; 19:1819-1822 due to apheresis catheter heparin flushes for stem cell har-
12. Wise J: Covid-19: European countries suspend use of Oxford- vesting before autotransplantation for myeloma. Blood 2017;
AstraZeneca vaccine after reports of blood clots. BMJ 2021; 130:1679–1682
372:n699 22. Warkentin TE, Makris M, Jay RM, et al: A spontaneous pro-
13. Pharmacovigilance Risk Assessment Committee of the
thrombotic disorder resembling heparin-induced thrombocy-
European Medicines Agency: COVID-19 Vaccine AstraZeneca: topenia. Am J Med 2008; 121:632–636
PRAC investigating cases of thromboembolic events - vac- 23. Hwang SR, Wang Y, Weil EL, et al: Cerebral venous sinus
cine’s benefits currently still outweigh risks - Update. Available thrombosis associated with spontaneous heparin-induced
at: https://www.ema.europa.eu/en/news/covid-19-vaccine- thrombocytopenia syndrome after total knee arthroplasty.
astrazeneca-prac-investigating-cases-thromboembolic- Platelets 2020 Oct 1. [online ahead of print]
events-vaccines-benefits. Accessed April 17, 2021
24. Moores G, Warkentin TE, Farooqi MAM, et al: Spontaneous
14. Østergaard SD, Schmidt M, Horváth-Puhó E, et al:
heparin-induced thrombocytopenia syndrome presenting as
Thromboembolism and the Oxford-AstraZeneca COVID- cerebral venous sinus thrombosis. Neurology: Clinical Practice
19 vaccine: Side-effect or coincidence? Lancet 2021; 2015; 26:602-607
397:1441–1443
25. Chong BH: Evolving concepts of pathogenesis of heparin-
15. Vogel G, Kupferschmidt K: Side effect worry grows for
induced thrombocytopenia: Diagnostic and therapeutic impli-
AstraZeneca vaccine. Science 2021; 372:14–15 cations. Int J Lab Hematol 2020; 42(Suppl 1):25–32
16. Greinacher A, Selleng K, Warkentin TE: Autoimmune hepa- 26. Maali L, Khan S, Qeadan F, et al: Cerebral venous thrombosis:
rin-induced thrombocytopenia. J Thromb Haemost 2017; Continental disparities. Neurol Sci 2017; 38:1963–1968
15:2099–2114
27. Alet M, Ciardi C, Alemán A, et al; Argentinian Stroke
17. Warkentin TE, Sheppard JA, Sigouin CS, et al: Gender imbal- and Cerebrovascular Diseases Study Group - Argentine
ance and risk factor interactions in heparin-induced thrombo- Neurological Society: Cerebral venous thrombosis in
cytopenia. Blood 2006; 108:2937–2941 Argentina: Clinical presentation, predisposing factors, out-
18. Bourguignon A, Arnold DM, Warkentin TE, et al: Adjunct comes and literature review. J Stroke Cerebrovasc Dis 2020;
immune globulin for vaccine-induced thrombotic 29:105145
e86 www.ccmjournal.org January 2022 • Volume 50 • Number 1
You might also like
- Workbook Answers: Unit 1 RespirationDocument30 pagesWorkbook Answers: Unit 1 RespirationYan En88% (196)
- Coagulopathy of Coronavirus Disease 2019: Review ArticlesDocument7 pagesCoagulopathy of Coronavirus Disease 2019: Review ArticlesBianca IlieNo ratings yet
- Diagnosis and Management of Cerebral Venous Sinus Thrombosis With Vaccine-Induced Immune Thrombotic ThrombocytopeniaDocument5 pagesDiagnosis and Management of Cerebral Venous Sinus Thrombosis With Vaccine-Induced Immune Thrombotic ThrombocytopeniaindanazulfaaNo ratings yet
- Diagnosis and Management of Cerebral Venous Sinus Thrombosis With Vaccine-Induced Immune Thrombotic ThrombocytopeniaDocument10 pagesDiagnosis and Management of Cerebral Venous Sinus Thrombosis With Vaccine-Induced Immune Thrombotic ThrombocytopeniaShin Elcant Del BarçaNo ratings yet
- Lecturio Pericardial Effusion & Cardiac TamponadeDocument1 pageLecturio Pericardial Effusion & Cardiac TamponadeCecil-An DalanonNo ratings yet
- BMJ n1005 FullDocument1 pageBMJ n1005 FullDror BrandNo ratings yet
- Fulminant MeningococcemiaDocument5 pagesFulminant MeningococcemiaRaffaharianggaraNo ratings yet
- What Is Meningitis?Document7 pagesWhat Is Meningitis?laujeroNo ratings yet
- Jurnal RadiologiDocument12 pagesJurnal Radiologiimamlutfi13No ratings yet
- MicrobiologyDocument24 pagesMicrobiologyPRAJWAL PES HDNo ratings yet
- COVID-19 Associated Ischemic Stroke and Hemorrhagic Stroke - Incidence, Potential Pathological Mechanism, and ManagementDocument8 pagesCOVID-19 Associated Ischemic Stroke and Hemorrhagic Stroke - Incidence, Potential Pathological Mechanism, and ManagementWilliam MilesNo ratings yet
- Ischemic Stroke in COVID-19-Positive Patients - An Overview of SARS-CoV-2 and Thrombotic Mechanisms For The NeurointerventionalistDocument6 pagesIschemic Stroke in COVID-19-Positive Patients - An Overview of SARS-CoV-2 and Thrombotic Mechanisms For The Neurointerventionalistarif 2006No ratings yet
- Pathology and Pathogenesis of Infective Endocarditis in Native Heart ValvesDocument8 pagesPathology and Pathogenesis of Infective Endocarditis in Native Heart ValvesIrina TănaseNo ratings yet
- New Perspectives of Infections in Cardiovascular Disease: Ignatius W. FongDocument18 pagesNew Perspectives of Infections in Cardiovascular Disease: Ignatius W. FongCarlos Danilo Noroña CNo ratings yet
- 17-Infective EndocarditisDocument17 pages17-Infective Endocarditisمصطفى محمد جواد كاظمNo ratings yet
- Infective EndocarditisDocument4 pagesInfective EndocarditisXiang Yun TanNo ratings yet
- Brain Abscess: State-Of-The-Art Clinical ArticleDocument17 pagesBrain Abscess: State-Of-The-Art Clinical ArticleIqbal AbdillahNo ratings yet
- Tuning The Thromboinflammatory Response To Venous Flow Interruption by The Ectonucleotidase CD39Document12 pagesTuning The Thromboinflammatory Response To Venous Flow Interruption by The Ectonucleotidase CD39KEERTHI UPPALAPATINo ratings yet
- Brain Abscess: State-Of-The-Art Clinical ArticleDocument17 pagesBrain Abscess: State-Of-The-Art Clinical ArticleAwais KhanNo ratings yet
- 2021 Thrombocytopenia and Intracranial Venous Sinus Thrombosis AfterDocument10 pages2021 Thrombocytopenia and Intracranial Venous Sinus Thrombosis AfterBeatriz VásquezNo ratings yet
- CNS Micro ImpulseDocument44 pagesCNS Micro Impulsedineshvd75No ratings yet
- Coagulop CovidDocument11 pagesCoagulop CovidbacchiocchipaoloNo ratings yet
- Obstructive Shock From Diagnosis To TreatmentDocument11 pagesObstructive Shock From Diagnosis To TreatmentMasjid Jaami' Al Ma'ruf SmdNo ratings yet
- Pathogenesis of Bacterial Meningitis: From Bacteraemia To Neuronal InjuryDocument10 pagesPathogenesis of Bacterial Meningitis: From Bacteraemia To Neuronal InjurymjeanjNo ratings yet
- Nosocomial Infection: 50Th Anniversary ArticlesDocument19 pagesNosocomial Infection: 50Th Anniversary ArticlesAndres RamirezNo ratings yet
- Hypotheses Behind The Very Rare Cases of Thrombosis With Thrombocytopenia Syndrome After SARS-CoV-2 VaccinationDocument10 pagesHypotheses Behind The Very Rare Cases of Thrombosis With Thrombocytopenia Syndrome After SARS-CoV-2 VaccinationAna-Maria AursuleseiNo ratings yet
- Antithrombotic Therapy Forpatients With COVID-19Document19 pagesAntithrombotic Therapy Forpatients With COVID-19dregleavNo ratings yet
- Mec CronicaDocument29 pagesMec CronicaAlfredo Luis Arana MaqueraNo ratings yet
- Disseminated Intravascular CoagulationDocument7 pagesDisseminated Intravascular CoagulationlgebNo ratings yet
- Cshperspectmed BAC A012393Document14 pagesCshperspectmed BAC A012393RaffaharianggaraNo ratings yet
- Cerebral Venous Thrombosis PDFDocument7 pagesCerebral Venous Thrombosis PDFd dNo ratings yet
- Viral MyocarditisDocument12 pagesViral Myocarditisemotic123No ratings yet
- Manejo HicDocument5 pagesManejo HicDianira Chavez RamosNo ratings yet
- Updates On Thrombosis With Thrombocytopenia Syndrome (TTS) : Advisory Committee On Immunization Practices (ACIP)Document39 pagesUpdates On Thrombosis With Thrombocytopenia Syndrome (TTS) : Advisory Committee On Immunization Practices (ACIP)kajelchaNo ratings yet
- Concern About Using Current Covid 19 Vaccines For Mass Vaccination in The Midst of A Pandemic Geert Vanden BosscheDocument21 pagesConcern About Using Current Covid 19 Vaccines For Mass Vaccination in The Midst of A Pandemic Geert Vanden BosscheSlavica Borojevic100% (1)
- Geert Vanden Bossche Concern About Current Covid 19 Mass Vaccination Campaigns Mar 2nd 2021Document14 pagesGeert Vanden Bossche Concern About Current Covid 19 Mass Vaccination Campaigns Mar 2nd 2021Kada Ben youcefNo ratings yet
- Infectious Diseases Pharmacotherapy: Lesson 5 Central Nervous System InfectionDocument63 pagesInfectious Diseases Pharmacotherapy: Lesson 5 Central Nervous System Infectionbest batiNo ratings yet
- Latest Development in PCVDocument9 pagesLatest Development in PCVAndityo SidohutomoNo ratings yet
- Medical Progress: Review ArticleDocument11 pagesMedical Progress: Review ArticleGaby Alejandra Ordonez AndradeNo ratings yet
- Autopsies Prove Covid-19 Is Disseminated Intravascular CoagulationDocument9 pagesAutopsies Prove Covid-19 Is Disseminated Intravascular CoagulationWayne SpringerNo ratings yet
- Thromboembolic Disease in COVID-19 Patients: A Brief Narrative ReviewDocument10 pagesThromboembolic Disease in COVID-19 Patients: A Brief Narrative ReviewSteven PutraNo ratings yet
- 202 FullDocument6 pages202 FullMariana CabralNo ratings yet
- MINOCADocument10 pagesMINOCAmariana virguezNo ratings yet
- COVID 19 Associated Coagulopathy and Inflammatory.2Document10 pagesCOVID 19 Associated Coagulopathy and Inflammatory.2alvNo ratings yet
- Embolic Stroke Circulation2017Document5 pagesEmbolic Stroke Circulation2017Maryani SyahrilNo ratings yet
- Unusual Pattern of Arterial Macrothrombosis Causing Stroke in A Young Adult Recovered From COVID-19Document5 pagesUnusual Pattern of Arterial Macrothrombosis Causing Stroke in A Young Adult Recovered From COVID-19Audrey Beatrice ReyesNo ratings yet
- Jesse Wackerbarth - CNS Pathogens RevisedDocument1 pageJesse Wackerbarth - CNS Pathogens RevisedMicroposterNo ratings yet
- MeningitisDocument2 pagesMeningitisedrian02No ratings yet
- Intracranial Infection - Prof SunartiniDocument12 pagesIntracranial Infection - Prof SunartiniFranciscus BuwanaNo ratings yet
- Covid Is An Endothelial Disease 2020 SeptDocument7 pagesCovid Is An Endothelial Disease 2020 SeptDaniela Mădălina GhețuNo ratings yet
- MAnaagement Koagulapati Dan Thrombolis Pada Pasien CovDocument13 pagesMAnaagement Koagulapati Dan Thrombolis Pada Pasien CovJuwita Rashi LumbantoruanNo ratings yet
- A Radiation-Mimicking Disease of Access - We Initially Believed SARS-CoV-2 Was A Respiratory Virus Is That The Respiratory Tract Is The Initial Point of AccessDocument6 pagesA Radiation-Mimicking Disease of Access - We Initially Believed SARS-CoV-2 Was A Respiratory Virus Is That The Respiratory Tract Is The Initial Point of Accesshansley cookNo ratings yet
- JIR 333887 Common Inflammatory Mechanisms in Covid 19 and ParkinsonDocument33 pagesJIR 333887 Common Inflammatory Mechanisms in Covid 19 and ParkinsonRochiNo ratings yet
- Covid Covid 19 Por Sarscov 2 Adultos y Pediatricos Version Septiembre - Octubre 21Document163 pagesCovid Covid 19 Por Sarscov 2 Adultos y Pediatricos Version Septiembre - Octubre 21ximenadonajiNo ratings yet
- Neonatal Pneumonia PathophysiologyDocument5 pagesNeonatal Pneumonia PathophysiologyRoderick EstrellaNo ratings yet
- Periodontal Disease AND Cardiovascular Disease: Dentaid Medical DepartmentDocument17 pagesPeriodontal Disease AND Cardiovascular Disease: Dentaid Medical DepartmentMunteanu DragosNo ratings yet
- Bcs Article RoleDocument15 pagesBcs Article RoleSowmya ANo ratings yet
- Green and Cream Vintage Aesthetic Group Project Presentation - 20231208 - 000317 - 0000Document22 pagesGreen and Cream Vintage Aesthetic Group Project Presentation - 20231208 - 000317 - 0000onoshaomneyaNo ratings yet
- Assessment of Changes and Complications Caused by Coronavirus Infection in The Lungs Using Computed TomographyDocument6 pagesAssessment of Changes and Complications Caused by Coronavirus Infection in The Lungs Using Computed TomographyCentral Asian StudiesNo ratings yet
- Journey to the Center of a Human Cell.: WE NEED A SECOND OPINION, #1From EverandJourney to the Center of a Human Cell.: WE NEED A SECOND OPINION, #1No ratings yet
- Referencetable 2Document350 pagesReferencetable 2lagopatis4No ratings yet
- 395 925 2 PBDocument22 pages395 925 2 PBlagopatis4No ratings yet
- Kosovo enDocument3 pagesKosovo enlagopatis4No ratings yet
- Lignite Mining Development Strategy: Statem Ent of Principl EDocument8 pagesLignite Mining Development Strategy: Statem Ent of Principl Elagopatis4No ratings yet
- Balkan Watch.11April 05Document1 pageBalkan Watch.11April 05lagopatis4No ratings yet
- COVID-19 Infection SurveyDocument22 pagesCOVID-19 Infection Surveylagopatis4No ratings yet
- Understanding Intimate Partner Violence in The EU: The Role of DataDocument48 pagesUnderstanding Intimate Partner Violence in The EU: The Role of Datalagopatis4No ratings yet
- DLL 4 - Body OrganssDocument4 pagesDLL 4 - Body OrganssAdor IsipNo ratings yet
- Science Grade 7 Second QuarterDocument12 pagesScience Grade 7 Second QuarterAnneKARYLE67% (3)
- IGCSE - BloodDocument7 pagesIGCSE - Bloodmubasherkatbar562No ratings yet
- Blood TypingDocument3 pagesBlood TypingNoriz Ember DominguezNo ratings yet
- Grade 9: ScienceDocument19 pagesGrade 9: ScienceChristian ArcegaNo ratings yet
- Acido TranexamicoDocument8 pagesAcido TranexamicoVianey GarciaVillegasNo ratings yet
- Science Blood TransfusionsDocument10 pagesScience Blood Transfusionsapi-237866019No ratings yet
- Final Lesson Plan For Demo TeachingDocument12 pagesFinal Lesson Plan For Demo Teachingapi-651914206No ratings yet
- Detailed Lesson Plan RealDocument14 pagesDetailed Lesson Plan RealHarold Jay LamangNo ratings yet
- Hema Chapter 21 Part 2Document4 pagesHema Chapter 21 Part 2EMETERIO TUTOR IIINo ratings yet
- ANSCIDocument40 pagesANSCIJessa Lorraine Dela CruzNo ratings yet
- Sains F3 Bab 3.3Document9 pagesSains F3 Bab 3.3HAREYANKA EKAA MADAN MOHAN MoeNo ratings yet
- 1BI0 2H Mark-Scheme 20180822Document25 pages1BI0 2H Mark-Scheme 20180822Xiao ShadowlordNo ratings yet
- Blood Cell PosterDocument1 pageBlood Cell PosternadaNo ratings yet
- First Periodical Test Sci 9Document4 pagesFirst Periodical Test Sci 9Jhaypee SorianoNo ratings yet
- 9B Fit and HealthyDocument24 pages9B Fit and HealthyKassyKas100% (1)
- ROTEM AnalysisDocument42 pagesROTEM AnalysisAnnie ArriNo ratings yet
- Biology C - Lesson 1 - Circulatory SystemDocument46 pagesBiology C - Lesson 1 - Circulatory SystemMuhammad Azrie0% (1)
- PBL Problem 1 Unit IV Week 1Document4 pagesPBL Problem 1 Unit IV Week 1Ibtehal HasanNo ratings yet
- Science Quiz Bee Questions 2015Document3 pagesScience Quiz Bee Questions 2015melba escuetaNo ratings yet
- Circulatory SystemDocument26 pagesCirculatory SystemGlemarie EnriquezNo ratings yet
- CAIELSBIO Y8 c5 tt5Document5 pagesCAIELSBIO Y8 c5 tt5warothkorn29No ratings yet
- Jyoti Singh ReportsDocument5 pagesJyoti Singh ReportsRaghujyotisNo ratings yet
- Step-t19-Ep by Saeed Mdcat TeamDocument18 pagesStep-t19-Ep by Saeed Mdcat TeamMuhammad AnwarNo ratings yet
- Module NCM112Document69 pagesModule NCM112Potato Banana100% (1)
- Diagnostics SampleDocument4 pagesDiagnostics SampleRobin RavagoNo ratings yet
- Hematology 2 TEST QUESTIONSDocument4 pagesHematology 2 TEST QUESTIONSa a r o n b a u t i s t aNo ratings yet
- Nutrients and Waste Transport SystemDocument22 pagesNutrients and Waste Transport SystemItsPotatoQueen MaeNo ratings yet
- MLS3174 Clinical Haematology FEB 18 PDFDocument9 pagesMLS3174 Clinical Haematology FEB 18 PDFVisaNathanNo ratings yet