Professional Documents
Culture Documents
Dr. Uttam Kumar Kanp Dr. Uttam Kumar Kanp: Auxin
Dr. Uttam Kumar Kanp Dr. Uttam Kumar Kanp: Auxin
Uploaded by
SasmitaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dr. Uttam Kumar Kanp Dr. Uttam Kumar Kanp: Auxin
Dr. Uttam Kumar Kanp Dr. Uttam Kumar Kanp: Auxin
Uploaded by
SasmitaCopyright:
Available Formats
AUXIN
Dr. Uttam Kumar Kanp
Contents: 1. Discovery, 2. Chemical nature
nature, 3. Bioassay, 4. Physical role,
role 5. Uses
of Auxins.
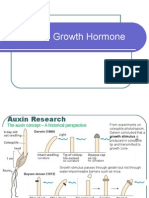
1. Discovery of Auxins:
Charles Darwin and his son Francis Darwin (1880) found tthat
hat the sensation of unilateral
illumination was picked up by the coleoptile tip of Canary Grass (Phalaris canarensis).
A decapitated coleoptile did not receive the sensation. Cole
Coleoptile
optile tip covered by an opaque
tin foil cap also could not perceive the stimulus of light. The sensat
sensation
ion picked up by the coleoptiles
tip is transmitted to the sub-apical
apical part which it bends in relation to the direction of light.
Boysen-Jensen (1910-1913)
1913) showed that the sensation of phototropism picked up by
coleoptile tip could be transmitted to sub-apical region through a block of gelatine but not
through a mica plate. Paal (1919) replaced the previously exposed excised tip eccentrically over
the stump of coleoptile. He observed greater growth on that side even in dark. Went (1928)
collected the growth stimulating substance in agar jelly.
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5: PLANT GROWTH
REGULATORS - AUXIN
discovered that the hormone travelled basipetally, i.e., from tip or apex towards the base.
Agar block containing the chemical caused bending of a decapitated coleoptile according to its
concentration. The growth promoting substance was named by him as auxin (Gk. auxein- to
grow). Kogl and Haagen-Smith
Smith (1931) isolated three chemicals from human urine.
They were named as auxin a, auxin b, and hetero-auxin. Kogl (1934) found that hetero
auxin is the real plant auxin and is chemically indole 3-acetic acid or IAA. It is also present in
urine of human beings suffering from pellagra, a disease caused by deficiency of niacin (=
nicotinic acid).
2. Chemical Nature: Indole 3-Acetic Acid is the universal natural auxin. It was discovered
by Kogl (1934). Related chemicals are indole 3-acetaldehyde, indole 3-acetonitrile,
acetonitrile, indole 3-
butyric acid (IBA), phenyl acetic acid and 4-chloro indole acetic acid. All of them have auxin
like activity.
Auxin is synthesised in shoot apices, leaf primordia and developing seeds from amino acid
tryptophan. A tryptophan independent pathway has also been discovered recently. Auxin
passes from shoot tip to the region of elongation. Auxin movement is polar. It is basipetal in stem
but acropetal in the root. Auxin helps in the elongation of both roots and shoots. However, the
optimum for the two is quite different
different. It is 10 ppm for stem and 0.0001 ppm for the root. In
higher concentration auxin inhibits growth.
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5: PLANT GROWTH
REGULATORS - AUXIN
The raw material which is used in synthesis of auxin is called auxin precursor. It is
tryptophan for IAA. Certain compounds inhibit action of auxin. They are called anti-auxins,
anti e.g., p-
chlorophenoxy isobutyric acid (PCIB). TIBA (2, 3, 5 triiodobenzoic acid also acts as anti-
auxin by blocking the transport of auxin. Active form of auxin is free auxin or auxin which can be
extracted easily. Auxin which cannot be extracted easily except with the help of organic solvents
is called bound auxin, e.g., IAA--aspartic acid, IBA-alanine, IAA-myoinositol,
myoinositol, IAA-glucan,
IAA IAA-
glycoprotein. Bound auxin is believed to be hormonally inactive (Hangarter and Good, 1981),
being meant for storage and protection against degradation.
Synthetic Auxins:
Many synthetic auxins are also being manufactured. The important ones are 2: 4 D (2:
4-di chlorophenoxy acetic acid), 2 : 4 : 5-T (2 : 4 : 5-tri-chlorophenoxy acetic acid), IBA (indole
3- butyric acid), NAA (naphthalene acetic acid). MCPA (2
(2-methyl 4- chloro-phenoxyacetic
phenoxyacetic acid),
Dicamba (2-methoxy 3-, 6-di-chlorobenzoic
chlorobenzoic acid). IBA is both natural and synthetic. Synthetic
auxins move in all directions inside plants.
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5: PLANT GROWTH
REGULATORS - AUXIN
3. Bioassay of Auxins:
It is testing of a biological activity like growth response of a substance by employing a
living material like plant or plant part. Auxin bioassay is quantitative test as it measures
concentration of auxin to produce the effect and the amount of effect.
1. Avena Curvature Test :
The test is based upon exper
experiments of Went (1928). 10° curvature is produced by
auxin concentration of 150 µg/litre at 25° C and 90% relative humidity. The test can measure
auxin upto 300 pg/litre.
Auxin from a shoot tip or any other plant organ is allowed to diffuse in a standard size agar
block (generally 2 x 2 x 1 mm). Auxin can also be dissolved directly in agar. 15-30 mm long
oat coleoptile grown in dark is held vertically over water. 1 mm tip of coleoptile is removed
without injuring the primary leaf.
After 3 hours a second decapitation is carried out for a distance of 4 mm. Primary leaf is
now pulled loose and agar block supported against it at the tip of decapitated coleoptile. After 90-
110 minutes, the coleoptile is found to have bent. The curvature is measured. It can also be
photographed and the curvature known from shadow graph.
2. Root Growth Inhibition:
Sterilized seeds of Cress are allowed to germinate on moist filter paper. As the roots reach a
length of 1 cm or so, root lengths are measured. 50% of the seedlings are placed in a test solution
while the remaining are allowed to grow over moist paper.
Lengths of the roots are measured after 48 hours. It is seen that the seedlings placed in test solution
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5: PLANT GROWTH
REGULATORS - AUXIN
show very little root growth while root growth is normal in control seedlings.
4. Physiological role of Auxins:
1. Respiration: Auxins stimulate
imulate respiration most probably by increasing availability of
respiratory substrate.
2. Metabolism: Application of auxin has been found to enhance metabolism due to mobilization
of plant resources.
3. Solutes: Auxins increase storage of solutes inside the cells.
4. Cell Enlargement : It is the most fundamental activity of auxins. Cell enlargement is caused by
solubilisation of carbohydrates, loosening of wall micro
micro-fibrils, synthesis of more wall materials, increased
membrane permeability and respiration.
5. Cambial Activity: Degree of cambial activity is directly proportional to auxin concentration.
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5: PLANT GROWTH
REGULATORS - AUXIN
Auxin also controls xylem differentiation.
6. Cell Division: Auxin is known to promote division in the cells of vascular cambium.
7. Tissue Culture: In tissue culture, the development of callus or mass of undifferentiated cells
is promoted by auxin. Differentiation of callus occurs in the presence of both auxin and cytokinin.
8. Root Formation: Auxin promotes root initiation at concentration which is inhibitory for
growth of intact root.
9. Apical Dominance (Fig. ): Apical dominance is the phenomenon by which presence of apical
bud does not allow the nearby lateral buds to grow. When the apical bud is removed, the lateral buds sprout.
This produces dense bushy growth. The phenomenon is widely used in tea plucking and hedge making.
Apical bud inhibits the growth of lateral buds by releasing auxins. It is confirmed by painting the cut end
of decapitated shoot by a paste of auxin. The lateral buds remain inhibited, as if the apical bud is present.
10. Inhibition of Abscission: Auxin delays abscission of young leaves and fruits. Its effect is
through non-formation
formation of abscission zone below a leaf or fruit. Abscission zone cuts off nutrients and water
supply. However, auxin
xin promotes the abscission of mature or older leaves and fruits.
11. Tropic Movements: Differential distribution of in
in-dole 3-acetic
acetic acid produces tropical plant
responses like phototropism and geotropism.
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5: PLANT GROWTH
REGULATORS - AUXIN
12. Sex: Auxins have a feminizing effect on some plants.
13. Seedless Fruits: The carpels producing seedless or parthenocarpic fruits have a higher internal
production of auxin that supports the development of fruits, e.g., Banana.
14. Ethylene: Higher concentration of IAA induces synthesis of ethylene.
15. Membrane Potential: It produces a negative potential on the cell membrane.
5. Uses of Auxins:
1. Rooting: Auxins stimulate root formation on the stem cutting, e.g., IBA, IBA-alanine, NAA.
2. Parthenocarpy: Application of auxins (e.g., IAA, IBA) and conjugate auxins (e.g., IBA-
alanine) to un-pollinated
pollinated pistils make them develop into seedless fruits or parthenocarps which carry a
better market price than the normal fruits having seeds.
3. Weedicides (= Herbicides): They are chemicals which kill weeds growing in the fields.
Application of 2: 4-D
D and 2:4: 55-T
T removes broad leaved weeds in cereal crops and lawns
because they do not affect mature monocotyledons while Dalapon (2-2 di-chloropropionic
chloropropionic acid) kills
grasses in broad leaved crops. Weedicides should be used very carefully and only occasionally
as they have wide spectrum and long lasting action. Thus weedicides or defoliants used in Vietnam
have exterminated the wild relatives of Citrus.
4. Flowering: NAA and 2, 4-D
D are often employed for inducing flowering in Litchi and Pineapple.
5. Storage: Methyl ester of NAA prevents the sprouting of Potato tubers kept in storage.
6. Pre-Harvest Fruit Drop:
In low concentration 2, 4-D
D is useful in preventing pre
pre-harvest
harvest fruit drop of Orange and Apple.
NAA is similarly useful for checking fruit drop of Tomato.
7. Vegetable Crops: Chlorophenoxy propionic acid enhances the quality of vegetable crops by
preventing flower formation.
8. Fruits: Auxins enhance sweetening of fruits, e.g., IBA.
9. Prevention of Lodging: Naphthalene acetamide (NAAM) prevents lodging or falling of crop
plants during windy season.
10. Dwarf Shoots: In Apple, flowers and fruits are formed on dwarf shoots. Application of
naphthalene acetic acid increases the number of dwarf shoots as well as the number of fruits.
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5: PLANT GROWTH
REGULATORS - AUXIN
REFERENCES:
1. Plant Physiology by S.N. Pandey and B. K. Sinha. Forth edition.
2. Applied Plant Physiology by Arup Kumar Mitra (2014).
3. Fundamentals of Plant Physiology by Dr. V. K. Jain, 2017. (S. Chand).
4. Studies in Botany (Vol. 2); D. Mitra, J. Guha and S. K. Chowdhuri. Moulik Library (2006).
5. https://www.biologydiscussion.com/plant
https://www.biologydiscussion.com/plant-physiology-2/plant-hormones/auxins
mones/auxins-history
bioassay-function-and-uses/44757
uses/44757.
This information, including the figures, are collected from the above references and will be used
solely for academic purpose.
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5: PLANT GROWTH
REGULATORS - AUXIN
GIBBERELLINS
Dr. Uttam Kumar Kanp
Content: 1. Discovery 2. Chemical nature 3. Bioassay 4. Physiological roles 5. Uses
of Gibberellins.
1. Discovery of Gibberellins:
The effect of gibberellins had been known in Japan since early 1800 where certain rice plants
were found to suffer from bakane or bakanae (foolish seedling) disease. Such rice plants were thin,
pale green, spindle shaped, longer by 50% than the healthy plants, and were sterile. The disease was
found by Hori (1918) and Kurosawa (1926) to be caused by a fungus, Gibberella fujikori.
fujikori The fungus
is the perfect stage of Fusarium moniliforme
moniliforme.
Kurosawa also found that the sterile filtrate of the fungus also caus
caused
ed appearance of disease
symptoms in uninfected rice seedlings. The active substance was separated and named gibberellin by
Yabuta (1935). Yabuta (1938) also prepared crystalline form of gibberellin (it actually consisted of
six gibberellins).
2. Chemical Nature:
Japanese work came to light only after World War II. Gibberellic acid or GA3 was isolated in
pure form by Brian et al in 1955. Cross (1961) worked out the structure of gibberellic acid, GA3
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5:
5: PLANT GROWTH
REGULATORS - GIBBERELLIN
It is chemically C19H22O6. GA3 is one of the most intensively studied gibberellin. A mixture of
GA4 and GA7 is used commercially. Until now 125 different gibberellins have been identified. Many
of them occur naturally in plants and fungi. Gibberella fujikori has as many as 15 gibberellins.
A single plant also possesses a number of gibberellins. This is in contrast to auxin, where a
single natural hormone occurs. Gibberellins are synthesised in the apical shoot buds (young
leaves), root tips and developing seeds. The precursors for their synthesis is mevalonic acid (derived
from acetyl coenzyme A). Gibberellin transport occurs through sim
simple
ple diffusion as well as through
conducting channels.
3. Bioassay of Gibberellins:
A. Dwarf Pea:
Seeds of dwarf pea are allowed to germinate till the formation of coleoptile. GA solution is
applied to some seedlings. Others are kept as control. After 5 days, epicotyl length is measured. GA
stimulates epicotyl growth with a concentration as low as 1 Nano gram.
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5:
5: PLANT GROWTH
REGULATORS - GIBBERELLIN
B. Barley Endosperm:
Endosperms are detached from embryos, sterilized and allowed to remain in 1 ml of test
solution for 1-22 days. There is a build-up of reducing sugars. The content of reducing sugar is
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5:
5: PLANT GROWTH
REGULATORS - GIBBERELLIN
proportional to gibberellin concentration. Reducing sugars are not formed in control experiment
where endosperms are kept in plain water.
4. Physiological roles of Gibberellins:
1. Stem and Leaf Growth:
Gibberellins help in cell growth of stem, leaves and other aerial parts. Therefore, they increase the
size of stem, leaves, flowers and fruits. Gibberellins, however, do not seem to play any such part in
case of roots.
2. Dwarf Shoots:
Besides general increase in stem length, gibberellins specifically induce intermodal growth in some
genetically dwarf varieties of plants like Pea and Maize. It appears that dwarfness of such varieties is
due to internal deficiency of gibberellins.
3. Bolting:
Gibberellins induce sub-apical meristem to develop faster. This causes elongation of reduced stem or
bolting in case of rosette plants (e.g., Henbane, Cabbage, Fig. 15.25) and root crops [e.g., Radish). A
weekly doze of O.J mg gibberellic acid made cabbage plants to grow taller than 3.5 m. Normally
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5:
5: PLANT GROWTH
REGULATORS - GIBBERELLIN
bolting occurs at the onset of reproductive
oductive phase. It is favoured in nature by either cold nights or long
days.
4. Dormancy:
Gibberellins overcome the natural dormancy of buds, tubers, seeds, etc. and allow them to grow. In
this function they are antagonistic to abscisic acid (ABA).
5. Seed Germination:
During seed germination, especially of cereals, gibberellins stimulate he production of
the
some messenger RNAs and then hydrolytic enzymes like amylases, lipases,
ribonucleases and proteases. The enzymes solubilize the reserve food of the seed.
The same is transferred to embryo axis for its growth.
6. Fruit Development:
Along with auxin, gibberellins control fruit growth and development. They can be replaced by
induce parthenocarpy or development of seedless fruits from unfertilized pistils,
especially in case of pomes (e.g., Apple, Pear).
7. Flowering:
They promote flowering in long day plants during non
non-inductive periods.
8. Vernalization:
Vernalization or low temperature requirement of some plants can gibberellins.
9. Sex Expression:
Gibberellins promote the formation of male flowers on genetically female plants of
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5:
5: PLANT GROWTH
REGULATORS - GIBBERELLIN
Cannabis. They can also replace female flowers with male flowers on monoecious plants of cucurbits.
10. Curvatures:
In Sunflower, phototropic and geotropic responses of shoot tips are due to redistribution of
gibberellins.
5. Uses of Gibberellins:
1. Fruit Growth:
Application of gibberellins increases the number and size of several fruits, e.g., Grape,
Gr Tomato. The
hormone creates more room by increasing the size of stalks so that fruits can grow in size. Size and
shape of Apple fruits is enhanced by application of GA4 and GA7 mixture.
2. Parthenocarpy:
Seedless pomaceous fruits can be produced by appli
application of gibberellins to un-pollinated
pollinated flowers.
3. Malt:
Gibberellins (e.g., GA3) increase the yield of malt from barley grains.
4. Overcoming Dormancy:
Gibberellins can be employed for breaking seed and bud dormancy. They induce germination of
positively photoblastic seeds of Tobacco and Lettuce in complete darkness.
5. Delayed Ripening:
GA7 delays senescence so that fruit can be left on the tree for longer period It extends period of
marketing. Ripening of Citrus fruits can be delayed with the help of gibber
gibberellins.
ellins. This is useful in
storing the fruits.
6. Flowering:
Gibberellins can be used in inducing offseason flowering in many long day plants as well as plants
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5:
5: PLANT GROWTH
REGULATORS - GIBBERELLIN
requiring vernalisation.
7. Sugarcane:
Spraying of sugarcane crop with gibberellins increases length of stem and yield of sugarcane to as
much as 20 tonnes/acre.
8. Early Maturity:
Juvenile conifers sprayed with mixture of GA4 and GA7 reach maturity quite early resulting in early
seed production.
REFERENCES:
1. Plant Physiology by S.N. Pandey and B. K. Sinha. Forth edition.
2. Applied Plant Physiology by Arup Kumar Mitra (2014).
3. Fundamentals of Plant Physiology by Dr. V. K. Jain, 2017. (S. Chand).
4. Studies in Botany (Vol. 2);
); D. Mitra, J. Guha and S. K. Chowdhuri. Moulik Library (2006).
(
5. https://www.biologydiscussion.com/plant
//www.biologydiscussion.com/plant-physiology-2/plant-hormones/gibberellins
gibberellins
This information, including the figures, are collected from the above references and will be
used solely for academic purpose.
BOTANY: SEM – V, PAPER-C12T:
C12T: PLANT PHYSIOLOGY, UNIT
UNIT-5:
5: PLANT GROWTH
REGULATORS - GIBBERELLIN
COMPILED AND CIRCULATED BY PROF. SANJAY KUMAR
DATTA, DEPT. OF BOTANY, NARAJOLE RAJ COLLEGE
TRANSPIRATION
Definition :
Transpiration is the process of water movement through a plant and its evaporation from
aerial parts, such as leaves, stems and flowers. Water is necessary for plants but only a
small amount of water taken up by the roots is used for growth and metabolism.
Types of Transpiration:
Depending on the organ that performs transpiration, the different types are:
• Stomatal transpiration: It is the evaporation of water through stomata. Stomata are
specialized pores in the leaves. They account for around 80 to 90% of the total water loss
from the plants.
• Cuticular transpiration: Cuticle is an impermeable covering present on the leaves and
stem. It causes around 20% of transpiration in plants. Cuticular transpiration is lesser in
xerophytes because they have thicker cuticles.
• Lenticular Transpiration: It is the evaporation of water through lenticels. Lenticels are
the tiny openings present on the woody bark.
Factors affecting transpiration
The rate of transpiration is affected by several factors. These include:
• Temperature
• humidity
BOTANY : SEM-V, PAPER-C12T : PLANT PHYSIOLOGY, UNIT-1: PLANT WATER
RELATIONS
COMPILED AND CIRCULATED BY PROF. SANJAY KUMAR
DATTA, DEPT. OF BOTANY, NARAJOLE RAJ COLLEGE
• air movement
• light intensity
Change in factor that
increases transpiration
Factor Explanation
rate
Increases molecular movement so that more water
molecules evaporate from cell surfaces. The rate of
Temperature Increase
diffusion of water molecules from the leaf is
increased.
Reduces the concentration of water molecules outside
Humidity Decrease
the leaf. Diffusion of water from the leaf increases.
Removes water vapour from leaf surfaces. More water
Air movement Increase
diffuses from the leaf.
Increases the rate of photosynthesis. Stomata open so
Light intensity Increase
that water diffuses out of the leaf.
BOTANY : SEM-V, PAPER-C12T : PLANT PHYSIOLOGY, UNIT-1: PLANT WATER
RELATIONS
COMPILED AND CIRCULATED BY PROF. SANJAY KUMAR
DATTA, DEPT. OF BOTANY, NARAJOLE RAJ COLLEGE
Opening and closing of stomata:
The opening and closing of stomata depend on the turgor pressure, caused by the osmotic flow
of water in the guard cells. When the guard cells are turgid, they expand resulting in
the opening of stomata. When the guard cells lose water, they become flaccid leading
to stomatal closure.
Starch - sugar interconversion hypothesis of stomatal opening and closing:
During day time: According to this theory, the CO2 released in respiration is utilized in the process
of photosynthesis, which makes the medium of the guard cell alkaline. Due to this high pH, the starch
produced in the night is converted into sugar in the presence of enzyme phosphorylase. Sugar is
soluble in water and consequently increases to the OP of the guard cells. Therefore, the cells become
turgid. In this state, the thin outer wall of guard cell stretches outward and opens the stomata.
During the night: During the night, the CO2 produced in respiration is not utilized and diffuses into
the cytoplasm of guard cells. It makes the medium of the guard cells acidic (low pH). At this low pH,
the sugar made during daytime is also converted into starch. Starch being insoluble in water reduces
the osmotic pressure of the guard cell. Consequently, water moves from guard cells to the attached
subsidiary cells. It makes the guard cells flaccid and therefore, stomata close.
BOTANY : SEM-V, PAPER-C12T : PLANT PHYSIOLOGY, UNIT-1: PLANT WATER
RELATIONS
COMPILED AND CCIRCULATED
IRCULATED BY PROF. SANJAY KUMAR
DATTA, DEPT. OF BOTANY, NARAJOLE RAJ COLLEGE
Stomatal movement based on K+ ion-transport:
Malate or K+ ion pump hypothesis was proposed by Levitt. According to this theory, the change that
takes place in the turgor pressure of the guard cells that open and close the stomata causes the
absorption and loss of K+ ions by guard cells.
Opening of stomata in light:
Starch in guard cells is metabolised into phosphoenol pyruvate (PEP) and later converted into malic
acid by the enzyme PEP carboxylase. Malic acid dissociates into H+ and malate ions in the guard
cells. These H+ ions are transported to the epidermal cells and K+ ions moves from thee epidermal
cells to the guard cells through the hydrogen - potassium ion exchange pump in the plasma
membrane. It is an active process and requires ATP. In the guard cells, K+ ions are balanced by
malate ions and a small amount of Cl ions are also absorbed to neutralize a small percentage
BOTANY : SEM-V, PAPER-C
C12T : PLANT PHYSIOLOGY, UNIT-1: PLANT WATER
RELATIONS
COMPILED AND CIRCULATED BY PROF. SANJAY KUMAR
DATTA, DEPT. OF BOTANY, NARAJOLE RAJ COLLEGE
of K+ ions. Increased K+ ions and anion concentration in the guard cells increases their osmotic
concentration and results in the water uptake by the guard cells. This osmosis process increases the
turgor pressure of the guard cells and so the stoma opens.
Closing of Stomata in Dark:
During night, CO2 is not utilized in photosynthesis, hence its concentration increases in the sub-
stomatal cavity. Organic acids are converted into starch. THe abscisic acid (ABA) hormone functions
in the presence ofCO2 and inhibits the uptake of K+ ions by changing the diffusion and permeability
of the guard cells. The K+ ions are transported back to the epidermal cells from the guard cells. Due
to this, the osmotic concentration of the guard cells decreases and results in the movement of water out
of the guard cells by exosmosis. The guard cells becomes flaccid and so the stoma closes.
BOTANY : SEM-V, PAPER-C12T : PLANT PHYSIOLOGY, UNIT-1: PLANT WATER
RELATIONS
COMPILED AND CIRCULATED BY PROF. SANJAY KUMAR
DATTA, DEPT. OF BOTANY, NARAJOLE RAJ COLLEGE
Significance of Transpiration:
• Plants waste much of their energy in absorbing large quantities of water and most
of which is ultimately lost through transpiration.
• Transpiration is a unique feature in the plant system and referred as necessary evil
as it is advantageous to plant under certain circumstances and harmful in some
other situations.
• Transpiration is necessary
1. Role in the movement of water
Water plays an important role in the upward movement of water i.e. Ascent of sap in
plants.
But, it does not mean that the translocation of water will be stopped without it.
2. Role in the absorption and translocation of mineral salts
• Absorption of water and mineral salts are entirely independent process.
• Therefore transpiration has nothing to do with the absorption of mineral salts.
• However, once mineral salts have been absorbed by the plants, their further
translocation and distribution may be facilitated by transpiration through
translocation of water in the xylem elements.
3. Role of regulation of temperature
Some of the light energy absorbed by the leaves is utilized in photosynthesis, rest is
converted into heat energy which raise the leaf temperature.
• Transpiration plays an important role in controlling the temperature of the plants.
BOTANY : SEM-V, PAPER-C12T : PLANT PHYSIOLOGY, UNIT-1: PLANT WATER
RELATIONS
COMPILED AND CIRCULATED BY PROF. SANJAY KUMAR
DATTA, DEPT. OF BOTANY, NARAJOLE RAJ COLLEGE
• Rapid evaporation of water from the aerial parts of the plant through
transpiration brings down the temperature and thus prevents them from excessive
heating.
• Transpiration is one of the chief ways for the dissipation of excess energy, which
the plant receives from the sun.
• Shull (1930) estimated that approximately 0.8 cal of energy is received upon each
square cm of leaf surface per minute, of which about 10% is reflected and 25% is
transmitted.
• The remaining 65% (0.52 cal) will increase the temperature of the leaves very
rapidly.
• If the weight of the leaf tissue is 0.02g/cm2 with the specific heat of 0.879, then
the rise in temperature would be at 32ºC per minute.
• With this rate of increase in temperature, the plants will be killed in less than two
minutes, if there is no dissipation of energy.
• Transpiration plays a significant role here.
• It helps in dissipating this excess energy which will otherwise raise the
temperature.
4. Role on growth and development:
• Winneberger (1958) has observed that the buds of hardy pear cease to grow under
conditions of high humidity and that under the same conditions growth of the
sunflower plant is reduced to about half of the normal.
BOTANY : SEM-V, PAPER-C12T : PLANT PHYSIOLOGY, UNIT-1: PLANT WATER
RELATIONS
COMPILED AND CIRCULATED BY PROF. SANJAY KUMAR
DATTA, DEPT. OF BOTANY, NARAJOLE RAJ COLLEGE
• So it is clear that transpiration necessary factor in the normal growth of these two
plants.
• Most important point is that cell growth depends on absorption of water which is
passively absorbed by the roots of plants due to transpiration pull.
• Plants showing high rate of transpiration exhibit adequate development of
mechanical tissues. Transpiration also shows that plants showing high rate
of transpiration exhibit extensive root system.
5. Involves in improvement in the quality of fruits:
• Increased sugar and mineral contents of fruits follows high rate of transpiration.
6. Transpiration help in hardening process:
• Transpiration induces hardening which imparts resistance of plant to drought.
7. Transpiration help in removal of excess water:
• It has been held that plants absorb far more amount of water than is actually used
by the plant by the plant. Transpiration removes excess of water.
PROBABLE QUESTIONS :
1. Define Transpiration.
2. Mention the types of Transpiration.
3. What are the major factors which controlling Transpiration, explain in brief ?
4. Mention the role of Stomata in controlling Transpiration.
5. What are the significance of Transpiration?
BOTANY : SEM-V, PAPER-C12T : PLANT PHYSIOLOGY, UNIT-1: PLANT WATER
RELATIONS
COMPILED AND CIRCULATED BY PROF. SANJAY KUMAR
DATTA, DEPT. OF BOTANY, NARAJOLE RAJ COLLEGE
REFERENCES:
1. Studies in Botany ( Vol.III) : D Mitra, J Guha and S K Chowdhury. Published by Moulik
Library (2006).
2. Plant physiology, A.K.Ghosh, New Central (2006)
3. Biology (Moderns ABC) Dr. B.B. Arora and A.K. Sabharwal (2016)
BOTANY : SEM-V, PAPER-C12T : PLANT PHYSIOLOGY, UNIT-1: PLANT WATER
RELATIONS
You might also like
- The Chinese Yam or Light Root Dioscorea batatas: Fundamental research into the possible beneficial health effects of this plant as a nutrientFrom EverandThe Chinese Yam or Light Root Dioscorea batatas: Fundamental research into the possible beneficial health effects of this plant as a nutrientNo ratings yet
- Encyclopedia of Biological Chemistry - Vol - 1Document895 pagesEncyclopedia of Biological Chemistry - Vol - 1aishbiyaNo ratings yet
- Plant HormonesDocument34 pagesPlant HormonesSimamkeleNo ratings yet
- AuxinDocument9 pagesAuxinVINDHYA SHANKERNo ratings yet
- Auxin As A Natural PGR (Phytoharmone)Document5 pagesAuxin As A Natural PGR (Phytoharmone)Saad Ahmed SiddiquiNo ratings yet
- AUXINSDocument28 pagesAUXINSdewifirNo ratings yet
- Growth PhysiologyDocument12 pagesGrowth PhysiologyEntirety 4u100% (1)
- 633652229777383750Document45 pages633652229777383750Elakkuvan SomasundaramNo ratings yet
- Plant Hormone and Growth and DevelopmentDocument36 pagesPlant Hormone and Growth and DevelopmentdeeNo ratings yet
- Plant HormonesDocument6 pagesPlant HormonesAsad Afridi100% (1)
- Pesticides 2 & PGRDocument15 pagesPesticides 2 & PGRmehakfaiz.07No ratings yet
- Apical Dominance, Abscission and Biological Rhythm: Mindanao State University General Santos CityDocument11 pagesApical Dominance, Abscission and Biological Rhythm: Mindanao State University General Santos CityJohn Arist LCNo ratings yet
- AUXINDocument23 pagesAUXINJose S. Cabato IIINo ratings yet
- Biology TermsDocument120 pagesBiology TermsNiamat UllahNo ratings yet
- Plant Harmons PDFDocument15 pagesPlant Harmons PDFRahul GauravNo ratings yet
- AuxinDocument9 pagesAuxinsextansNo ratings yet
- Chapter 8 - Plant HormonesDocument49 pagesChapter 8 - Plant Hormonessayidah nafisah100% (2)
- Regulating Growth and Development The Plant HormonesDocument30 pagesRegulating Growth and Development The Plant HormonesMoath EnnabNo ratings yet
- Crop - Lecture - Growth HormonesDocument9 pagesCrop - Lecture - Growth HormonesCynel DelaNo ratings yet
- Axunis BDocument49 pagesAxunis BAbebe fikaduNo ratings yet
- AuxinsDocument16 pagesAuxinsICPH 2021No ratings yet
- General Biology 2Document6 pagesGeneral Biology 2Adrienne CabanigNo ratings yet
- Plant Growth Hormones: TiwariDocument19 pagesPlant Growth Hormones: TiwariVinayak SabnisNo ratings yet
- Plant HormonesDocument6 pagesPlant HormonesThư PhạmNo ratings yet
- Auxin: The First Discovered Plant Growth Hormone: Ayushi Mandloi 2019H1290104P M.E. BiotechnologyDocument33 pagesAuxin: The First Discovered Plant Growth Hormone: Ayushi Mandloi 2019H1290104P M.E. BiotechnologyAyushi MandloiNo ratings yet
- Note PhytohormonesDocument47 pagesNote PhytohormonesAyuni Mokhtar100% (1)
- TTPB7THESTORYOFAUXIN ShortDocument65 pagesTTPB7THESTORYOFAUXIN ShortDiksha GahlotNo ratings yet
- PLANT HORMONE - ModuleDocument24 pagesPLANT HORMONE - ModuleMarinel Almencion MagyanoNo ratings yet
- Plant Growth RegulatorsDocument55 pagesPlant Growth RegulatorsXimena Libano0% (1)
- Auxin: Greek Auxein, Meaning "To Increase" or "To Grow."Document31 pagesAuxin: Greek Auxein, Meaning "To Increase" or "To Grow."gouthami shivaswamyNo ratings yet
- Auxin PresentationDocument66 pagesAuxin PresentationAman BishtNo ratings yet
- Chapter 4 PLANTDocument3 pagesChapter 4 PLANTSedsed QuematonNo ratings yet
- Plant Physiology Lec. 7 Growth RegulatorsDocument4 pagesPlant Physiology Lec. 7 Growth RegulatorsMuhamad MuhamadNo ratings yet
- Plant HormonesDocument38 pagesPlant Hormonesbaha hafizNo ratings yet
- CRP 101 Lecture No 26Document16 pagesCRP 101 Lecture No 26AnandKuttiyanNo ratings yet
- AuksinDocument8 pagesAuksinhabib arief prakosoNo ratings yet
- Lab 7 - Internal and External Plant Growth Regulators: Xperiment AND Pical OminanceDocument10 pagesLab 7 - Internal and External Plant Growth Regulators: Xperiment AND Pical OminanceJeemCarloFagelaPula0% (1)
- Assignment: Submitted To: Dr. JS DEOL Submitted By: Anish Jalota (L-2015-A-1-BTFT) 1 YearDocument19 pagesAssignment: Submitted To: Dr. JS DEOL Submitted By: Anish Jalota (L-2015-A-1-BTFT) 1 YearAnish JalotaNo ratings yet
- Effect Auksin Dan Sitokinin PDFDocument22 pagesEffect Auksin Dan Sitokinin PDFAnisaNo ratings yet
- Nutrition - Food SupplyingsystemDocument29 pagesNutrition - Food SupplyingsystemRavi KiranNo ratings yet
- AuksiiinnDocument30 pagesAuksiiinnIrfan SuliansyahNo ratings yet
- Botanical Society of AmericaDocument10 pagesBotanical Society of AmericaArinal Haq Izzawati NurrahmaNo ratings yet
- 02 Plant Hormones (P2)Document33 pages02 Plant Hormones (P2)psharifah97No ratings yet
- Phytohormones: Presented By:-ShiyasDocument39 pagesPhytohormones: Presented By:-ShiyaserikabeltranNo ratings yet
- Cytokinins-Discovery, Biosynthesis and Physiological Role: Unit 5.1Document6 pagesCytokinins-Discovery, Biosynthesis and Physiological Role: Unit 5.1RimeiaNo ratings yet
- Auxin Biosynthesis and Its Role in Plant DevelopmentDocument4 pagesAuxin Biosynthesis and Its Role in Plant DevelopmentAnastasia SylviankaNo ratings yet
- Phytohormone - Types and FunctionsDocument23 pagesPhytohormone - Types and FunctionsminiNo ratings yet
- The Ups and Downs of AuxinDocument17 pagesThe Ups and Downs of AuxinAshrita PradhanNo ratings yet
- ExposicionDocument23 pagesExposicionisabelaNo ratings yet
- CytokininsDocument5 pagesCytokininskwNo ratings yet
- Regulation Ethylene Evolution Abscission: of and Leaf by Auxin'Document7 pagesRegulation Ethylene Evolution Abscission: of and Leaf by Auxin'BelmaNo ratings yet
- Ultrastructural Alterations in Lepocinclis Acus (Euglenophyta) Induced by Medium With High Organic Matter ContentDocument8 pagesUltrastructural Alterations in Lepocinclis Acus (Euglenophyta) Induced by Medium With High Organic Matter ContentSxmx Xho OutsiderNo ratings yet
- CHEMICAL COORDINATION IN PLANTS - NotesDocument6 pagesCHEMICAL COORDINATION IN PLANTS - NotesMinal Shahbaz saeedNo ratings yet
- L J. C, M E. M C, M J. C, C X. H, L E. C. LDocument12 pagesL J. C, M E. M C, M J. C, C X. H, L E. C. LMarijanaNo ratings yet
- PhotosynthesisDocument15 pagesPhotosynthesisSultana KhanNo ratings yet
- AP 10th Class Biology Study MaterialDocument212 pagesAP 10th Class Biology Study MaterialPreeti ThakurNo ratings yet
- AuxinDocument18 pagesAuxinMadhusmita BalNo ratings yet
- Biology - XI - Photosynthesis in Higher Plants - IntroductionDocument47 pagesBiology - XI - Photosynthesis in Higher Plants - IntroductionSDO BSNL NALAGARHNo ratings yet
- 2019 Maruri Lopez Journey SADocument11 pages2019 Maruri Lopez Journey SAPriscila MezzomoNo ratings yet
- Plant Growth RegulatorsDocument5 pagesPlant Growth RegulatorsYousef H YousefNo ratings yet
- Plant Growth Regulators (2014)Document34 pagesPlant Growth Regulators (2014)matthewNo ratings yet
- Variations in Pro Line Accumulation and Relative Water Content UnderDocument8 pagesVariations in Pro Line Accumulation and Relative Water Content Underyustina_183741565No ratings yet
- Chapter 11 - Transport in Vascular PlantDocument35 pagesChapter 11 - Transport in Vascular PlantMASYITAH MOHD PADZILNo ratings yet
- Second Lecture Exam ReviewerDocument7 pagesSecond Lecture Exam ReviewerKen RubioNo ratings yet
- LE GRADE 9 SCIENCE, Q1 W6-NewDocument16 pagesLE GRADE 9 SCIENCE, Q1 W6-NewESMERALDA GAMILNo ratings yet
- Photosynthesis and Respiration (Hopkins W.G., 2006)Document177 pagesPhotosynthesis and Respiration (Hopkins W.G., 2006)Houssein Ya Mazloum100% (3)
- Transpiration Notes Class 10Document4 pagesTranspiration Notes Class 10proodootNo ratings yet
- Acclimatization of Micropropagated Silvan Blackberry PDFDocument107 pagesAcclimatization of Micropropagated Silvan Blackberry PDFBrij Mohan Singh100% (1)
- Guard Cell - WikipediaDocument42 pagesGuard Cell - WikipediaBashiir NuurNo ratings yet
- Gas Exchange in PlantsDocument2 pagesGas Exchange in PlantsWhat do you mean mhNo ratings yet
- Leaf Epidermal Peel - LabPreparation PDFDocument2 pagesLeaf Epidermal Peel - LabPreparation PDFRoxy BrownNo ratings yet
- PHOTOBIOLOGYDocument49 pagesPHOTOBIOLOGYserimawarNo ratings yet
- Water StressDocument24 pagesWater StressTikTok ZappingNo ratings yet
- Trial Sem II 2018 (SMK Sungai Merah, Sibu)Document8 pagesTrial Sem II 2018 (SMK Sungai Merah, Sibu)Wong SeptemberNo ratings yet
- Gas Exchange in PlantsDocument6 pagesGas Exchange in PlantsMaria Theresa HerreroNo ratings yet
- TranspirationDocument19 pagesTranspirationproodootNo ratings yet
- Chap 2 F5 BiologyDocument37 pagesChap 2 F5 Biologyg-16355677No ratings yet
- Plant Responses To Abiotic StressDocument303 pagesPlant Responses To Abiotic StresskamalNo ratings yet
- Chap 1-3 F5 BIOLOGYDocument12 pagesChap 1-3 F5 BIOLOGYg-16355677No ratings yet
- Lesson Explainer Transpiration NagwaDocument2 pagesLesson Explainer Transpiration NagwaTeng Xuen ChanNo ratings yet
- Gaseous ExchangeDocument74 pagesGaseous ExchangeMartina BugejaNo ratings yet
- UPCAT-Reviewer-Session-3Document51 pagesUPCAT-Reviewer-Session-3이성은No ratings yet
- Full PHD Thesis Crop Physiology Shahid Iqbal (2006-Ag-1903)Document124 pagesFull PHD Thesis Crop Physiology Shahid Iqbal (2006-Ag-1903)King KhanNo ratings yet
- IGCSE Revision Material - Part BDocument82 pagesIGCSE Revision Material - Part Braghav.gupta.unofficialNo ratings yet
- 14 Homeostasis 2021-225 A Level Biology 9700 Notes by Mr. ADEEL AHMADDocument18 pages14 Homeostasis 2021-225 A Level Biology 9700 Notes by Mr. ADEEL AHMADADEEL AHMADNo ratings yet
- Plant PhysiologyDocument19 pagesPlant PhysiologyLester Eslava Orpilla100% (2)
- Dse Bio Ch10 & Cross Topic AnsDocument11 pagesDse Bio Ch10 & Cross Topic AnsyungcheukyinryanNo ratings yet
- Cells Under The Microscope PDFDocument5 pagesCells Under The Microscope PDFAra LimNo ratings yet
- Chapter Transpiration Worksheet SolutionDocument24 pagesChapter Transpiration Worksheet SolutionZawad7No ratings yet
- Dr. Uttam Kumar Kanp Dr. Uttam Kumar Kanp: AuxinDocument24 pagesDr. Uttam Kumar Kanp Dr. Uttam Kumar Kanp: AuxinSasmitaNo ratings yet