Professional Documents
Culture Documents
Dojcansky, J., Heinrich, J. Saturated Vapour Pressure of Acetonitrile, Chem. Zvesti, 28, 2, 157-159, (1974)
Dojcansky, J., Heinrich, J. Saturated Vapour Pressure of Acetonitrile, Chem. Zvesti, 28, 2, 157-159, (1974)
Uploaded by
Andrew RolandOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dojcansky, J., Heinrich, J. Saturated Vapour Pressure of Acetonitrile, Chem. Zvesti, 28, 2, 157-159, (1974)
Dojcansky, J., Heinrich, J. Saturated Vapour Pressure of Acetonitrile, Chem. Zvesti, 28, 2, 157-159, (1974)
Uploaded by
Andrew RolandCopyright:
Available Formats
Saturated vapour pressure of acetonitrile
J . DOJCANSKY and J . H E I N R I C H
Department of Chemical Engineering, Slovak Technical University,
880 37 Bratislava
Received 12 September 1973
The saturated vapour pressures of acetonitrile have been measured over
the range from 50 to 950 torr. The obtained data have been evaluated statis
tically by using the Antoine equation. The found constants A = 7.27748,
В = 1424.47, С = 242.202 reproduce the values of pressure in torr with
a standard deviation, of 1.41.
In studying the properties of some acetonitrile solutions it was necessary to know
the data on the vapour pressure of this solvent. Since there were great discrepancies
in the data available in literature [1, 2, 4] the temperature dependence of vapour pressure
was determined experimentally.
Experimental
Acetonitrile obtained by rectifying a chemical grade product of Laborchemie Apolda
VEB did not contain any impurities according to the chromatographic analysis perfor
med on an Hewlett — Packard apparatus. The vapour pressure was measured simul
taneously by the static method according to Surový [6] and in an ebulliometer accord-
ing to Swietoslawski. The values of pressure were read on the scale of a mercury mano-
meter by means of a cathetometer accurate to ±0.2 torr; the temperature was measured
with a mercury thermometer accurate to ±0.05 K.
Results
The obtained data were correlated by the Antoine equation
log P = A — (1)
С + t
(where pressure P is given in torr and temperature t in °C) with the statistical weight
[8.9]
u C + t
'= \( У (0-2Y2~ /——V (0.05)2 1 \ (2)
[\2.303P/ \G + t/ J
The system of nonlinear equations ensuing from the least squares method was solved
by the Newton method. The following values of the constants in eqn (1) were calculated
A = 7.27748, В = 1424.472, G = 242.202. (3)
(Чн-ш. zvesti 28 {'!) 157-159 (1974) i e-y
J. DOJCAXSKÝ, J. HEINRICH
T h e e x p e r i m e n t a l d a t a P E x (ebulliometric d a t a a r e d e n o t e d b y e) a r e c o n f r o n t e d
w i t h t h e v a l u e s Pc c a l c u l a t e d according t o (1) a n d (2) in T a b l e 1, t h e s t a n d a r d d e v i a t i o n
being
{4)
-ÍTZ-3—-1-41-
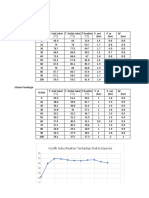
Table 1
T e m p e r a t u r e d e p e n d e n c e of t h e v a p o u r p r e s s u r e of a c e t o n i t r i l e
t pĽx pc pEx _ J
[°C] [torr] [torr]
15.1 55.2 55.1 0.1
20.1 70.6 70.3 0.3
25.2 89.4 89.2 0.2
30.7 114.4 114.2 0.2
35.0 137.2 137.6 -0.4
39.95 168.5 e 169.4 -0.9
40.0 169.7 169.7 0.0
44.9 207.3 207.0 0.3
50.1 253.5 253.6 -0.1
54.9 303.9 304.0 -0.1
60.0 367.7 366.3 1.4
64.4 428.9 428.0 0.9
64.95 435.8 e 436.3 -0.5
70.0 517.3 518.6 -1.3
73.05 575.4 e 574.1 1.3
75.1 610.4 614.0 -3.6
77.2 659.7 e 657.2 2.5
81.1 745.8 e 743.8 2.0
85.2 842.8 844.6 -1.8
88.2 924.0 925.0 -1.0
89.2 935.9 953.1 0.8
Discussion
T h e values of t h e v a p o u r pressure of a c e t o n i t r i l e o b t a i n e d b y us a r e n e a r t o t h e d a t a
of Brown [1]. T h e d a t a of Heim [4] d e v i a t e from o u r s especially a t lower t e m p e r a t u r e s .
T h e d a t a of Dreisbach [2] a r e m e r e l y a n e s t i m a t e a c c o r d i n g t o t h e boiling p o i n t b a s e d
on t h e Cox d i a g r a m . The}'' c a n n o t be c o m p a r e d w i t h t h e o t h e r s in t h e whole i n v e s t i g a t e d
p r e s s u r e r a n g e a n d c a n n o t be r e c o m m e n d e d .
T h e c i t e d original d a t a m a y b e f o u n d in t h e form of t h e c o n s t a n t s of t h e A n t o i n e
e q u a t i o n . Hála et al. [3] give t h e c o n s t a n t s b a s e d on t h e d a t a of Brown [1] while Wichterle
a n d Linek [8] use t h e c o n s t a n t s given b y Heim [4]. On t h e o t h e r h a n d , Weissberger [7]
t a k e s over t h e c o n s t a n t s of D r e i s b a c h . H e i m ' s d a t a h a v e b e e n processed g r a p h i c a l l y
in t a b l e s b y Stull [5].
Chcm. zvesti 28 (2) 157-150 (1974)
158
VAPOUR PRESSURE OF ACETONITRILE
References
1. Brown, I., Aust. J. Chem. 7, 269 (1954).
2. Dreisbach, R. R, and Martin, R. A., Ind. Eng. Chem. 41, 2875 (1949).
3. Hála, E., Wichterle, L, Polák, J., and Boublík, Т., Vapour —Liquid Equilibrium
Data, p. 23. Pergamem Press, Oxford, 1968.
4. Heim, D., Bull. Soc. Chim. Beiges 42, 467 (1933).
5. Stull, D. R., Ind. Eng. Chem. 39, 517 (1947).
6. Surový, J., Habilitation Thesis, p. 18. Slovak Technical University, Bratislava, 1969.
7. Weissberger, A. and Proskauer, E. S., Organic Solvents, p. 224. Wiley, New York,
1955.
8. Wichterle, I. and Linek, J., Antoine Vapor Pressure Constants of Pure Compounds,
p. 14. Academia, Prague, 1971.
9. Willingham, С. В., Taylor, W. J., Fignocco, J . M., and Rossini, F. D., J. Res. Nat.
Bur. Stand. 35, 219 (1954).
Translated by R. Doiuan.sk ý
Chem. zvesti 28 (2) 157-150 (1974)
159
You might also like
- UW Chem 152 Lab 1Document7 pagesUW Chem 152 Lab 1Jimmy Dang91% (22)
- Mech 343 Lab 1 ReportDocument18 pagesMech 343 Lab 1 ReportYousef Meguid0% (1)
- GPA 2140 - Liquefied Petroleum Gas Specifications and Test MethodsDocument56 pagesGPA 2140 - Liquefied Petroleum Gas Specifications and Test Methodsclementer92% (12)
- Chem 152 Lab Report 1Document7 pagesChem 152 Lab Report 1Sabica NasarNo ratings yet
- CE 370 HW 5 - Poisson's Distribution-SolutionsDocument9 pagesCE 370 HW 5 - Poisson's Distribution-SolutionsfaisalasgharNo ratings yet
- PH102 LabDocument10 pagesPH102 LabPeggy CockerNo ratings yet
- Fluid Dynamics: Impinging Jet Experiment ReportDocument16 pagesFluid Dynamics: Impinging Jet Experiment ReportAmar BayasgalanNo ratings yet
- Chem152 Kinetics2 Report PC Gradescope 011420 PDFDocument6 pagesChem152 Kinetics2 Report PC Gradescope 011420 PDFNam NguyenNo ratings yet
- Cet2 PDFDocument28 pagesCet2 PDFfebriNo ratings yet
- EJERCICIOS EN CLASE EvaporacionDocument1 pageEJERCICIOS EN CLASE EvaporacionMiguel Andres Espitia LopezNo ratings yet
- Sensor CalibrationDocument11 pagesSensor Calibrationupeksha erandiNo ratings yet
- Lab Report 4 MM322Document6 pagesLab Report 4 MM322JnrNo ratings yet
- Marcet Boiler ReportDocument20 pagesMarcet Boiler Reportalwaysbethere100% (1)
- Tarea 2 Variograma de 2DDocument6 pagesTarea 2 Variograma de 2DMiguelito Vargas SolisNo ratings yet
- Instituto Politécnico Nacional: Escuela Superior de Ingeniería Química E Industrias ExtractivasDocument10 pagesInstituto Politécnico Nacional: Escuela Superior de Ingeniería Química E Industrias ExtractivasArmando ValladaresNo ratings yet
- Supporting Information: Mccollom 10.1073/pnas.1611843113Document3 pagesSupporting Information: Mccollom 10.1073/pnas.1611843113MuhalamNasyrahNo ratings yet
- T2 EosDocument17 pagesT2 EosKush ShahNo ratings yet
- Interactive TD 280: Compensation of Oxygen MeasurementsDocument17 pagesInteractive TD 280: Compensation of Oxygen MeasurementsHariNo ratings yet
- Physics Experiment On Stefan's Law.Document10 pagesPhysics Experiment On Stefan's Law.Ayush SrivastavaNo ratings yet
- H 0,2m H 0,4m H 0,5m H (M) T(S) H (M) T(S) H (M) T(S) : Tabla 1Document3 pagesH 0,2m H 0,4m H 0,5m H (M) T(S) H (M) T(S) H (M) T(S) : Tabla 1Cindy Juranny Duque VanegasNo ratings yet
- Vibration Lab 4-Mass Spring System B Lab 4Document19 pagesVibration Lab 4-Mass Spring System B Lab 4Fajobi AbeebNo ratings yet
- CL351: Chemical Engineering Lab-II Semester 1, 2014-2015 IIT GandhinagarDocument7 pagesCL351: Chemical Engineering Lab-II Semester 1, 2014-2015 IIT GandhinagarPradeep DiwakarNo ratings yet
- Full Name - Student Id Group..................................................... Teacher's Confirmation PointDocument3 pagesFull Name - Student Id Group..................................................... Teacher's Confirmation PointLe Tung QuanNo ratings yet
- Experiment 3 Kinematics and Kinerics of MachinesDocument8 pagesExperiment 3 Kinematics and Kinerics of Machinesuzair jahanzebNo ratings yet
- Lab7 2Document4 pagesLab7 2Paweekan HansungnoenNo ratings yet
- Experiment 1 Moment of Inertia and Angular MomentumDocument3 pagesExperiment 1 Moment of Inertia and Angular MomentumRikachuNo ratings yet
- CE374L HW 6 Solution Pump Test 2019 PDFDocument9 pagesCE374L HW 6 Solution Pump Test 2019 PDFHerlou GarpaoNo ratings yet
- ME2142E Feedback and Control Lab - Frequency ResponseDocument9 pagesME2142E Feedback and Control Lab - Frequency ResponseLinShaodun100% (4)
- 1997 Gioncu & Petcu - Available Rotation Capacity of Wide-Flange Beams and Beam-Columns Part 2. Experimental and Numerical TestsDocument26 pages1997 Gioncu & Petcu - Available Rotation Capacity of Wide-Flange Beams and Beam-Columns Part 2. Experimental and Numerical TestsAKNo ratings yet
- Heat TransferDocument11 pagesHeat Transfercracking khalifNo ratings yet
- Zub Indeterminate Truss DegDocument13 pagesZub Indeterminate Truss DegAnonymous jmo0bcjAsNo ratings yet
- Dpipe: Calculation Sample and Recommended Sequence For Entering Input DataDocument14 pagesDpipe: Calculation Sample and Recommended Sequence For Entering Input DataAbhinav OjhaNo ratings yet
- RSM Case StudyDocument12 pagesRSM Case StudyVbaluyoNo ratings yet
- Aa + BB +CC+DD RR A+ (B/a) B + (C/a) C + (D/a) D (R/a) R R - KC C C C N N (1-X) N N - (B/a) (N - N) N N - (C/a) (N - N) N N - (D/a) (N - N) C N /VDocument7 pagesAa + BB +CC+DD RR A+ (B/a) B + (C/a) C + (D/a) D (R/a) R R - KC C C C N N (1-X) N N - (B/a) (N - N) N N - (C/a) (N - N) N N - (D/a) (N - N) C N /VChemical EngineeringNo ratings yet
- UNIT 6 Dah BeresDocument10 pagesUNIT 6 Dah BeresrahmadNo ratings yet
- MCEN2001 Lab Report 1Document8 pagesMCEN2001 Lab Report 1AntonyNo ratings yet
- Mass Moment of Inertia Lab ReportDocument9 pagesMass Moment of Inertia Lab ReportOmair Sadiq100% (1)
- Supporting Information For: Characterization of Crude Oil by Real-Component SurrogatesDocument15 pagesSupporting Information For: Characterization of Crude Oil by Real-Component SurrogatesHarmanNo ratings yet
- PerhitunganDocument5 pagesPerhitunganEvelineFauziahNo ratings yet
- AppendixDocument17 pagesAppendixtinoNo ratings yet
- 11711515ppt2019 190914160308Document28 pages11711515ppt2019 190914160308JeTengine EaterNo ratings yet
- Ep211 THE FUNDAMENTAL PRESSURE-TEMPERATURE RELATIONSHIP OF SATURATED STEAM IN EQUILIBRIUMDocument9 pagesEp211 THE FUNDAMENTAL PRESSURE-TEMPERATURE RELATIONSHIP OF SATURATED STEAM IN EQUILIBRIUMMoontarij JahanNo ratings yet
- Theory:: Figure: Verification of Voltages and Currents in Different Branches in A Ladder NetworkDocument4 pagesTheory:: Figure: Verification of Voltages and Currents in Different Branches in A Ladder NetworkAhmed Nesar Tahsin ChoudhuryNo ratings yet
- Data Hasil Mohamad FadliDocument7 pagesData Hasil Mohamad FadliMuhammad IkramullahNo ratings yet
- Nhiet Vi SaiDocument8 pagesNhiet Vi SaiVinh PhamNo ratings yet
- Analysis of DataDocument6 pagesAnalysis of DataJustinNo ratings yet
- Reported Data Corrected Data For 760 MMHG and Vol. Loss Fraction # P, MM HG Cutt (F) V% Sum V% SP - GR@ 60/60 (F) Corrected T (F) Corrected V%Document3 pagesReported Data Corrected Data For 760 MMHG and Vol. Loss Fraction # P, MM HG Cutt (F) V% Sum V% SP - GR@ 60/60 (F) Corrected T (F) Corrected V%Siraj AL sharifNo ratings yet
- Marcet Boiler Lab ReportDocument4 pagesMarcet Boiler Lab ReportJohnConor95% (41)
- Laboratory A: Steady State Heat Conduction in A CylinderDocument14 pagesLaboratory A: Steady State Heat Conduction in A CylindercfellowNo ratings yet
- Lab 1Document7 pagesLab 1Kashif hussainNo ratings yet
- Gas Absorption Lab ReportDocument12 pagesGas Absorption Lab ReportGracylla RoseNo ratings yet
- Small Scale Dosimetry: Beta Emitters (SS-) : Manuel Bardiès, INSERM UMR 892, Nantes, France Manuel - Bardies@Document39 pagesSmall Scale Dosimetry: Beta Emitters (SS-) : Manuel Bardiès, INSERM UMR 892, Nantes, France Manuel - Bardies@Madalina-Elena CostacheNo ratings yet
- Fundamentals of Fluid MechanicsDocument1 pageFundamentals of Fluid MechanicsJuan Pablo Giraldo FrancoNo ratings yet
- Laprak STRDocument9 pagesLaprak STRAliya SyNo ratings yet
- Experiment 12 - Unsteady State Heat Transfer (GR5)Document8 pagesExperiment 12 - Unsteady State Heat Transfer (GR5)Shreya Pagaria (B20CH042)No ratings yet
- Thermo II Lab 1 Report PDFDocument13 pagesThermo II Lab 1 Report PDFPeter LauNo ratings yet
- Group 1 - ALEJANO - DEZOLLER - GRATIS - BATCH REACTORDocument8 pagesGroup 1 - ALEJANO - DEZOLLER - GRATIS - BATCH REACTORJohn Frix AlejanoNo ratings yet
- Kinetics of OxidationDocument9 pagesKinetics of OxidationTanyaTouchéNo ratings yet
- CL351: Chemical Engineering Lab-II Semester 1, 2014-2015 IIT GandhinagarDocument6 pagesCL351: Chemical Engineering Lab-II Semester 1, 2014-2015 IIT GandhinagarPradeep DiwakarNo ratings yet
- Study of An EvaporatorDocument10 pagesStudy of An EvaporatorMdSiShovonNo ratings yet
- H2S en AguaDocument3 pagesH2S en AguaBrayan UribeNo ratings yet
- Principles and Applications of Thermal AnalysisFrom EverandPrinciples and Applications of Thermal AnalysisPaul GabbottRating: 4 out of 5 stars4/5 (1)
- Vapour Pressure of Water - Marcet Boiler: Page 1/2 11/2014Document2 pagesVapour Pressure of Water - Marcet Boiler: Page 1/2 11/2014Muhammad UsmanNo ratings yet
- Determination of Vapor Density and Molecular Weight of Acetone Using Victor Meyer MethodDocument7 pagesDetermination of Vapor Density and Molecular Weight of Acetone Using Victor Meyer MethodKyle Delos Santos100% (5)
- Q3 G11 Physical Science Module 6Document20 pagesQ3 G11 Physical Science Module 6Lebz Ricaram100% (2)
- Topic 3 - Absorption Refrigeration CyclesDocument45 pagesTopic 3 - Absorption Refrigeration CyclesOk SokNo ratings yet
- Ansi C29.1Document17 pagesAnsi C29.1kevin brown0% (1)
- Phases and SolutionsDocument105 pagesPhases and SolutionsMolina ThirumalNo ratings yet
- Sherwood 1939Document7 pagesSherwood 1939Ahmed AliNo ratings yet
- Storage Vacuum CollapseDocument7 pagesStorage Vacuum CollapsebehnamhfNo ratings yet
- Chemistry Sahodaya PaperDocument10 pagesChemistry Sahodaya PaperflippodynamicsNo ratings yet
- ThermodynamicsDocument16 pagesThermodynamicsaneeda shabirNo ratings yet
- Fluid Mechanics PDFDocument196 pagesFluid Mechanics PDFMayordz JonixNo ratings yet
- Properties of Pure SubstancesDocument48 pagesProperties of Pure SubstancesJack BalsNo ratings yet
- States of MatterDocument17 pagesStates of MatterThaarvena RetinaNo ratings yet
- Exercise - 1: Basic Objective Questions: Concentration TermsDocument14 pagesExercise - 1: Basic Objective Questions: Concentration TermsJohn WickNo ratings yet
- Physical Characteristics of Boiling WaterDocument2 pagesPhysical Characteristics of Boiling WaterCatalin CataNo ratings yet
- Thermochemistry of Selected Fission Product CompoundsDocument11 pagesThermochemistry of Selected Fission Product Compoundsnarayana bhaktaNo ratings yet
- Phase Equilibrium: Physical Chemistry For STPMDocument60 pagesPhase Equilibrium: Physical Chemistry For STPMDavidson ChanNo ratings yet
- Mee2161 Fluid Mechanics - 2021Document99 pagesMee2161 Fluid Mechanics - 2021dany rwagatareNo ratings yet
- Improvement in Patel Teja Eqn of StatesDocument10 pagesImprovement in Patel Teja Eqn of StatesSumukh VermaNo ratings yet
- MECE0423 Lecture 01 Thermodynamics 02Document28 pagesMECE0423 Lecture 01 Thermodynamics 02DANIEL JOSEF SANCHEZNo ratings yet
- R 410a Si PDFDocument20 pagesR 410a Si PDFMarin Munteanu0% (1)
- Nylon 6 Avey PDFDocument296 pagesNylon 6 Avey PDFMohamed Sayed AbdoNo ratings yet
- Outlines of A New System of Thermodynamic Chemistry G. N. Lewis 1907Document36 pagesOutlines of A New System of Thermodynamic Chemistry G. N. Lewis 1907Victor VazquezNo ratings yet
- Warm Humid Climate FinalDocument26 pagesWarm Humid Climate FinalLakshmiRaviChanduKolusuNo ratings yet
- Advanced Pharmaceutical SolidsDocument534 pagesAdvanced Pharmaceutical SolidssevensonyNo ratings yet
- Chemical Engineering GATE 1999Document13 pagesChemical Engineering GATE 1999Anonymous 8pCXXsNo ratings yet
- Universidad Del Atlántico Edgardo Benitez, Maria Martinez Thermodynamics Chemistry II Exercise No.1Document4 pagesUniversidad Del Atlántico Edgardo Benitez, Maria Martinez Thermodynamics Chemistry II Exercise No.1Alen SuarezNo ratings yet