Professional Documents
Culture Documents
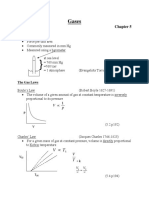
Equilibrium Constant of Pressure
Equilibrium Constant of Pressure
Uploaded by
Mic DropOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Equilibrium Constant of Pressure
Equilibrium Constant of Pressure
Uploaded by
Mic DropCopyright:
Available Formats
Kp
=
pressure of products
pressure of reactants
Equilibrium constant of pressure – Kp
Pressure of a gas: Number of collisions between the particles and the walls of the container that it’s
in.
The Partial pressure of a gas is if it were alone in a container. E.g.
N2
+ ¿ O2
= 100, 000 Pa = partial pressures PN 2
PO 2
=80,000 Pa =20,000 Pa
Total pressure = PN + PO
2 2
Mole Fraction = number of moles of gas A
total number of moles of gas in mixture
Sum of all the mole fraction always equals 1.
How to calculate partial pressure?
If a mixture had 15 moles of nitrogen and 10 moles of oxygen, then the mole fraction of each
nitrogen and oxygen is
Nitrogen = 15 ÷ (15 + 10) = 0.6 – mole fraction
Oxygen = 10 ÷ (15 + 10) = 0.4 – mole fraction
If the mixture had a total pressure of 130 Pa, then to work out the partial pressure you,
PA
¿
Total pressure x mole fraction = 130 Pa x 0.6 = PN
=
2
for Nitrogen
¿ 78 Pa
130 Pa x 0.4 = PO
=
2
for Oxygen
52 Pa
Question: 5 mol of CO is reacted with 7 mol of H2 . At equilibrium, the pressure of the mixture is 400
Pa and the yield of CH
OH
3is 2 mol. Calculate the partial pressure of carbon monoxide in this
equilibrium mixture.
CO (g) + 2 H2 (g) ↔ C
H3 (g)
OH
First how many moles of gas there are in each equilibrium.
C
CO 2 H2 H3
OH
Initial moles 5 7 0
Change in moles -2 -4 +2
Equilibrium moles 3 3 2
Change in moles for CO = ¿ = 2 Equilibrium moles = 5-2 = 3
1
1
Change in moles for H2 = 2 × 2 = 4 Equilibrium moles = 7-4 = 3
1
3+3+2=8
CO 3 moles of CO = proportion of CO = 3 = mole fraction = 0.375 PCO = 0.375 × 400 = 150 Pa
8
You might also like
- Chapter 12-13 Uygulama PDFDocument45 pagesChapter 12-13 Uygulama PDFEylül Nur ÇakırNo ratings yet
- The Ultimate Reality - Vol.1Document368 pagesThe Ultimate Reality - Vol.1anakin6895% (19)
- 1.10 Partial Pressures and KP: Mole FractionDocument3 pages1.10 Partial Pressures and KP: Mole Fractionbazel mukuzeNo ratings yet
- Chemical Equilibria - KDocument9 pagesChemical Equilibria - KBeatriz MatthiasNo ratings yet
- 4.5 Equilibria PDFDocument6 pages4.5 Equilibria PDFNyak PereraNo ratings yet
- Chap 05BDocument13 pagesChap 05BAbhishek DasNo ratings yet
- BSG 104 Gas LawsDocument35 pagesBSG 104 Gas LawsCJ DRBNo ratings yet
- Investigative Activity of Ideal Gas LawDocument7 pagesInvestigative Activity of Ideal Gas LawShaina AdralesNo ratings yet
- Unit 4.3Document2 pagesUnit 4.3Azeem iftikharNo ratings yet
- Example 1Document8 pagesExample 1jgolloberNo ratings yet
- Kesetimbangan KimiaDocument51 pagesKesetimbangan KimiaNaufal ThoriqNo ratings yet
- An Ideal Gas Mixture Consists of 2kmol of N2 and 6 Kmol of CO2. The Mass Fraction of CO2 IsDocument9 pagesAn Ideal Gas Mixture Consists of 2kmol of N2 and 6 Kmol of CO2. The Mass Fraction of CO2 IsLance Andrew LagmanNo ratings yet
- 5.0 States of MatterDocument106 pages5.0 States of MatterTasya KassimNo ratings yet
- Lecture3 - Gas Laws2Document19 pagesLecture3 - Gas Laws2lytonchirwa882No ratings yet
- General Chemistry 1: NRT P Atm Mol KDocument2 pagesGeneral Chemistry 1: NRT P Atm Mol KViannix GameplayNo ratings yet
- Reaksi GasDocument19 pagesReaksi Gaszainal mustaqimNo ratings yet
- ChapterII - GasesDocument40 pagesChapterII - Gasesjumanahelmy12No ratings yet
- Gaseous StateDocument23 pagesGaseous StateSiddhartha KumarNo ratings yet
- Bab 1 SolutionsDocument37 pagesBab 1 SolutionsNgọc HuyềnNo ratings yet
- Physical Chemistry Chapter 2 ProblemsDocument37 pagesPhysical Chemistry Chapter 2 ProblemsS. GreenNo ratings yet
- GasesDocument12 pagesGasesghs26w5s2tNo ratings yet
- Jawaban Pemisahan PAK AMIRDocument93 pagesJawaban Pemisahan PAK AMIRRifai Partogi ManaluNo ratings yet
- Characteristics of Chemical EquilibriumDocument43 pagesCharacteristics of Chemical Equilibriumpimpin1No ratings yet
- CH 5Document50 pagesCH 5Paul ArcillaNo ratings yet
- Chapter 5 GasesDocument8 pagesChapter 5 GasessamNo ratings yet
- Gases: Course Name: Chemistry 101 Course CodeDocument28 pagesGases: Course Name: Chemistry 101 Course CodeHeartcheNo ratings yet
- Gas Absorption PDFDocument93 pagesGas Absorption PDFIngeniería Industrias Alimentarias Itsm100% (1)
- Gas Absorption PDFDocument93 pagesGas Absorption PDFAnonymous JDg7HCVWNo ratings yet
- UntitledDocument93 pagesUntitledSiphelele MalembeNo ratings yet
- Gas LawDocument14 pagesGas LawRoszelan Majid100% (1)
- Chemical EquilibriumDocument27 pagesChemical EquilibriumMashnoor HossainNo ratings yet
- Study Guide Gas LawsDocument3 pagesStudy Guide Gas LawsAdamNo ratings yet
- Kimia Fisika 1Document7 pagesKimia Fisika 1Agung Pribadi WicaksonoNo ratings yet
- CH 301 CH5 AnswersDocument4 pagesCH 301 CH5 AnswersArnav ChhabraNo ratings yet
- Assignment-3 Chem-Eng SolutionDocument4 pagesAssignment-3 Chem-Eng SolutionDuy Do MinhNo ratings yet
- Chang Chap 5Document49 pagesChang Chap 5MR no oneNo ratings yet
- Reading and Writing SkillsDocument41 pagesReading and Writing SkillsFrancynn ManliguezNo ratings yet
- CH 15 Gas Law (Studebt Notes) - 071112Document21 pagesCH 15 Gas Law (Studebt Notes) - 071112celine.yxtehNo ratings yet
- 1.2+pre Lecture+NotesDocument28 pages1.2+pre Lecture+NotesJonathan TranNo ratings yet
- 4 (G) o 4 4 (G) 2 (G) 2 (G)Document13 pages4 (G) o 4 4 (G) 2 (G) 2 (G)Annissa RiskyNo ratings yet
- Kimia Teknik TS Ke-5 (07102013)Document28 pagesKimia Teknik TS Ke-5 (07102013)Radja NurNo ratings yet
- Catatan Before MidDocument6 pagesCatatan Before MidDidin MuhidinNo ratings yet
- AP CH 5 GasesDocument37 pagesAP CH 5 GasesyourstrulyrahulNo ratings yet
- Lecture 08 GasesDocument42 pagesLecture 08 GasesDuy Do MinhNo ratings yet
- Chang Chap 5 JKDocument40 pagesChang Chap 5 JKAmal Abu KhalilNo ratings yet
- Topic 4 States of MatterDocument43 pagesTopic 4 States of MatterJowyn SeetNo ratings yet
- Chapter 5 GasesDocument42 pagesChapter 5 GasesPerlita MorongNo ratings yet
- C10 F13 3Document0 pagesC10 F13 3Rohit BandaNo ratings yet
- Chapter 5 Gases PDFDocument49 pagesChapter 5 Gases PDFAbou WalidNo ratings yet
- Tugas Teknik Reaksi Kimia Lanjut EXAMPLE 10.2 & PROBLEM 10.3Document13 pagesTugas Teknik Reaksi Kimia Lanjut EXAMPLE 10.2 & PROBLEM 10.3Andre Fahriz Perdana HarahapNo ratings yet
- Topic 17 1 - Equilibrium LawDocument14 pagesTopic 17 1 - Equilibrium LawMichelle EnkhsaikhanNo ratings yet
- 6 - ch5 Aa 0Document49 pages6 - ch5 Aa 0Edlyn RamirezNo ratings yet
- Module 11 - The Gas PhaseDocument15 pagesModule 11 - The Gas PhaseAna Maria Millan RinconNo ratings yet
- Change The % Into Grams (23% Na - 23g Na) : Grams of An Element Total MolesDocument2 pagesChange The % Into Grams (23% Na - 23g Na) : Grams of An Element Total MolesAliance Mary AdorioNo ratings yet
- Genchem FormulasDocument2 pagesGenchem FormulasAliance Mary AdorioNo ratings yet
- Chapter 4 States of Matter 2021Document24 pagesChapter 4 States of Matter 2021suh mey chongNo ratings yet
- Solution & Colligative Properties_Faculty copyDocument8 pagesSolution & Colligative Properties_Faculty copyanshwitripathi59No ratings yet
- Environmental Science and Engineering: Assignment No. 8: Solid Waste ManagementDocument3 pagesEnvironmental Science and Engineering: Assignment No. 8: Solid Waste ManagementLucienne Iriana0% (1)
- Gas StoichiometryDocument17 pagesGas StoichiometryJamless ChimChimNo ratings yet
- General Chemistry 11th Edition Ebbing Test Bank 1Document54 pagesGeneral Chemistry 11th Edition Ebbing Test Bank 1kathleen100% (49)
- From Abdul Aziz YS To EveryoneDocument3 pagesFrom Abdul Aziz YS To EveryoneAdinda LarasatiNo ratings yet
- The Impacts of Environmental Pollutants On Microalgae and CyanobacteriaDocument124 pagesThe Impacts of Environmental Pollutants On Microalgae and CyanobacteriaDeepakNo ratings yet
- Planet Earth, Explained: Our Dance Around The SunDocument5 pagesPlanet Earth, Explained: Our Dance Around The SunAristeo EbioNo ratings yet
- Understanding Geography Form 3&4Document263 pagesUnderstanding Geography Form 3&4JOHN100% (2)
- Chapter19 PDFDocument30 pagesChapter19 PDFrestoffical100% (1)
- Climatology 4Document8 pagesClimatology 4Ritu RaniNo ratings yet
- Elements of ClimateDocument2 pagesElements of Climatevidh VRNo ratings yet
- A Review of Gravity Three - Phase SeparatorsDocument12 pagesA Review of Gravity Three - Phase SeparatorsMelis AllakNo ratings yet
- Tank FixturesDocument21 pagesTank FixturesASSSSSSSSSSSSNo ratings yet
- Unit 1 Introduction To Environmental StudiesDocument9 pagesUnit 1 Introduction To Environmental Studiesankit kaleNo ratings yet
- Directions. Read The Essay and Answer The Following QuestionsDocument9 pagesDirections. Read The Essay and Answer The Following QuestionsJohn Rey LayderosNo ratings yet
- Do-It-Yourself Climate Prediction: Sylvia Knight, Atmospheric, Oceanic and Planetary Physics, University of OxfordDocument47 pagesDo-It-Yourself Climate Prediction: Sylvia Knight, Atmospheric, Oceanic and Planetary Physics, University of OxfordMax PowerNo ratings yet
- Lưu ý: - Thí sinh làm bài trên tờ giấy thi, ghi theo đúng thứ tự câu từ 1 đến 100Document6 pagesLưu ý: - Thí sinh làm bài trên tờ giấy thi, ghi theo đúng thứ tự câu từ 1 đến 100Ngọc VươngNo ratings yet
- Advanced Reservoir EngineeringDocument75 pagesAdvanced Reservoir Engineeringginozky50% (2)
- fc13 CJT KardashevDocument37 pagesfc13 CJT KardashevleapZeeg SurrealistNo ratings yet
- Lec19 WEATHER AND CLIMATE, MICRO-CLIMATEDocument7 pagesLec19 WEATHER AND CLIMATE, MICRO-CLIMATEYashasvi ThakurNo ratings yet
- Escritos de Los Audios de InglesDocument14 pagesEscritos de Los Audios de InglesRenato RequenaNo ratings yet
- Wind Power MeteorologyDocument46 pagesWind Power MeteorologyNemanja KomatinovicNo ratings yet
- Chapter-2-Underground Environment - Mine VentilationDocument23 pagesChapter-2-Underground Environment - Mine VentilationSahil SokhalNo ratings yet
- Reuse The Carbon Dioxide From Industries As FuelDocument11 pagesReuse The Carbon Dioxide From Industries As FuelkaviobitoNo ratings yet
- CcycleDocument31 pagesCcycleSUNILNo ratings yet
- Thermal Properties of Matter - 3Document16 pagesThermal Properties of Matter - 3Nik AshrafNo ratings yet
- Natural Resources NewDocument9 pagesNatural Resources NewHari mv013No ratings yet
- List of RefrigerantsDocument13 pagesList of RefrigerantsAshique Muhammed T MNo ratings yet
- Chemestry Ponderal LawsDocument2 pagesChemestry Ponderal LawsMarinö Chavez100% (1)
- Causes of Global Warming Lesson For Middle School by SlidesgoDocument56 pagesCauses of Global Warming Lesson For Middle School by Slidesgonguyenngochienthao2007No ratings yet
- Chapter-3 PPTDocument109 pagesChapter-3 PPTnunuNo ratings yet
- Technology Selection For Liquefied Natural Gas (LNG) On Baseload PlantsDocument15 pagesTechnology Selection For Liquefied Natural Gas (LNG) On Baseload Plantsbkonly4uNo ratings yet