Professional Documents
Culture Documents
Virtual Lab Report: NSCI115L Aviation Chemistry & Physics Experiment # 6: Ideal Gas Law: Apply To Save A Life
Virtual Lab Report: NSCI115L Aviation Chemistry & Physics Experiment # 6: Ideal Gas Law: Apply To Save A Life
Uploaded by
Benedict CarandangCopyright:
Available Formats
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5824)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- PATTS SCHEDULE FORM - Carandang, Benedict J. 200Document1 pagePATTS SCHEDULE FORM - Carandang, Benedict J. 200Benedict CarandangNo ratings yet
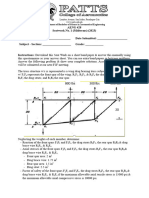
- AENG 428 - Simple Trusses Sample Problem Assignment V2Document3 pagesAENG 428 - Simple Trusses Sample Problem Assignment V2Benedict CarandangNo ratings yet
- AENG 428 SW1 MidDocument1 pageAENG 428 SW1 MidBenedict CarandangNo ratings yet
- Airport Parts 1B (GROUP 1)Document33 pagesAirport Parts 1B (GROUP 1)Benedict CarandangNo ratings yet
- AENG 428 A1 MidDocument1 pageAENG 428 A1 MidBenedict CarandangNo ratings yet
- Nsci 125Document1 pageNsci 125Benedict CarandangNo ratings yet
- Battery Ignition System - Page 3Document1 pageBattery Ignition System - Page 3Benedict CarandangNo ratings yet
- Benedict Carandang - 09 - 13 - Assignment No. 2Document5 pagesBenedict Carandang - 09 - 13 - Assignment No. 2Benedict CarandangNo ratings yet
- 1B-G2 Amte-426 CarandangDocument4 pages1B-G2 Amte-426 CarandangBenedict CarandangNo ratings yet
- 000Document1 page000Benedict CarandangNo ratings yet
- Prelim ExamDocument1 pagePrelim ExamBenedict CarandangNo ratings yet
- 325 - PW SW 1Document5 pages325 - PW SW 1Benedict CarandangNo ratings yet
- 1B Amte426 CarandangDocument18 pages1B Amte426 CarandangBenedict CarandangNo ratings yet
- Part 3 Answer With SolutionsDocument1 pagePart 3 Answer With SolutionsBenedict CarandangNo ratings yet
- 1B G2 Amte 426 CarandangDocument7 pages1B G2 Amte 426 CarandangBenedict CarandangNo ratings yet
- Magneto Ignition System - Page 2Document1 pageMagneto Ignition System - Page 2Benedict CarandangNo ratings yet
- 236 Quiz 1Document1 page236 Quiz 1Benedict CarandangNo ratings yet
- HARDWARE2Document102 pagesHARDWARE2Benedict CarandangNo ratings yet
- 1B G2 Amte 426 CarandangDocument5 pages1B G2 Amte 426 CarandangBenedict CarandangNo ratings yet
- Activity #4Document1 pageActivity #4Benedict CarandangNo ratings yet
- Air Turbine StarterDocument1 pageAir Turbine StarterBenedict CarandangNo ratings yet
- RECIPROCATING ENGINEDocument54 pagesRECIPROCATING ENGINEBenedict CarandangNo ratings yet
- TH REE Laws in Physic S: Add A SubheadingDocument1 pageTH REE Laws in Physic S: Add A SubheadingBenedict CarandangNo ratings yet
- Civil Aviation Authority of The Philippines Aircraft Accident Investigation and Inquiry Board Aircraft Accident ReportDocument2 pagesCivil Aviation Authority of The Philippines Aircraft Accident Investigation and Inquiry Board Aircraft Accident ReportBenedict CarandangNo ratings yet
- Studying Geodesics in The ClassroomDocument13 pagesStudying Geodesics in The ClassroomRafael AlmeidaNo ratings yet
- Main ISM Ch07Document14 pagesMain ISM Ch07Shoja Sammy RahimianNo ratings yet
- HArvard PysicsDocument3 pagesHArvard PysicsAndrei Joshua TorresNo ratings yet
- Engineering Thermodynamics - Open Systems ExercisesDocument1 pageEngineering Thermodynamics - Open Systems Exercisesloli XxxxNo ratings yet
- Heat Transfer: SKMM3443 Section 04Document20 pagesHeat Transfer: SKMM3443 Section 04Izzat AshraffNo ratings yet
- 15-Book 1 CH 15 IntegrationDocument21 pages15-Book 1 CH 15 IntegrationBen ChanNo ratings yet
- Thermodynamics of Polymer Blends PDFDocument5 pagesThermodynamics of Polymer Blends PDFpedroNo ratings yet
- Special Relativity and Minkowski SpacesDocument13 pagesSpecial Relativity and Minkowski Spacesgraviton6No ratings yet
- Updated Slides of Compressible FlowsDocument39 pagesUpdated Slides of Compressible FlowsFurqan YousafzaiNo ratings yet
- HMT Unit 3 MCQsDocument10 pagesHMT Unit 3 MCQsSatish kumar patleNo ratings yet
- Lewis 2007Document13 pagesLewis 2007david decena ortegaNo ratings yet
- EntropyDocument39 pagesEntropyRohit Singh LatherNo ratings yet
- Exp 1 Calorimetry IntroDocument3 pagesExp 1 Calorimetry IntroMarco FranciscoNo ratings yet
- Spontaneously Not Vice Versa: Second-Law - PPT Modified 10/9/02Document37 pagesSpontaneously Not Vice Versa: Second-Law - PPT Modified 10/9/02T Hari PrasadNo ratings yet
- Wed 7 MargineDocument27 pagesWed 7 Margineshubham patelNo ratings yet
- Revision Part2 PDFDocument23 pagesRevision Part2 PDFrawadNo ratings yet
- Schroedinger vs. N-SDocument14 pagesSchroedinger vs. N-Sdr_s_m_afzali8662No ratings yet
- Peng-Robinson Equation of State For A Pure FluidDocument2 pagesPeng-Robinson Equation of State For A Pure FluidANDRES CAMILO LEYTON ALVAREZNo ratings yet
- AD II Unit I & II 2 Marks Q&ADocument6 pagesAD II Unit I & II 2 Marks Q&AthandialNo ratings yet
- Transformation of Graph (New)Document89 pagesTransformation of Graph (New)18A Kashish PatelNo ratings yet
- Problems and Solutions: Physical ChemistryDocument179 pagesProblems and Solutions: Physical ChemistryRialeeNo ratings yet
- Nonlinear System Analysis: Practice ProblemsDocument7 pagesNonlinear System Analysis: Practice ProblemsRama Krushna PradhanNo ratings yet
- SCES3083 Topic7 ThermodynamicsDocument100 pagesSCES3083 Topic7 Thermodynamics胡佳玲100% (1)
- Outline of PhysicsDocument56 pagesOutline of Physicsaditya00012No ratings yet
- Transport Phenomena Transport CoefficientsDocument3 pagesTransport Phenomena Transport CoefficientsDubistWhiteNo ratings yet
- Mémoire Final Menasra AminaDocument105 pagesMémoire Final Menasra Aminaأمينة مناصرةNo ratings yet
- Nonlinear Dynamics of A Duffing-Like Negative StifDocument13 pagesNonlinear Dynamics of A Duffing-Like Negative StifPietro TestaNo ratings yet
- Thermodynamic Processes: Analysis of Thermodynamic Processes by Applying 1 & 2 Law of ThermodynamicsDocument10 pagesThermodynamic Processes: Analysis of Thermodynamic Processes by Applying 1 & 2 Law of Thermodynamicsmohdmehrajanwar1860No ratings yet
- (Exam) CAD-CAM-ThiCuoiKi (trắc nghiệm) - English (send)Document2 pages(Exam) CAD-CAM-ThiCuoiKi (trắc nghiệm) - English (send)Trường Nguyễn HuyNo ratings yet
- 14 RadiationDocument27 pages14 RadiationZhu Chen ChuanNo ratings yet
Virtual Lab Report: NSCI115L Aviation Chemistry & Physics Experiment # 6: Ideal Gas Law: Apply To Save A Life
Virtual Lab Report: NSCI115L Aviation Chemistry & Physics Experiment # 6: Ideal Gas Law: Apply To Save A Life
Uploaded by
Benedict CarandangOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Virtual Lab Report: NSCI115L Aviation Chemistry & Physics Experiment # 6: Ideal Gas Law: Apply To Save A Life
Virtual Lab Report: NSCI115L Aviation Chemistry & Physics Experiment # 6: Ideal Gas Law: Apply To Save A Life
Uploaded by
Benedict CarandangCopyright:
Available Formats
Virtual Lab Report
NSCI115L Aviation Chemistry & Physics
Experiment # 6: Ideal Gas Law: Apply to
save a life
Geronimo, John Earl
Flores, Ponce Aries
Estrella, Paul Andrey
Gabote, John Israel
Gauiran, Decie Marl Hendrix
Natural Sciences Department
PATTS College of Aeronautics
I. Introduction
The goal of the experiment aims to understand the concept of Ideal Gas
Law. Furthermore, the specific goals and objectives of the experiment are:
● To observe how Ideal Gas molecules behave according to the Ideal
Gas Law.
● To understand the relationship between pressure, volume and
temperature in gases using gas thermometry.
● To define the physical concept of temperature and absolute zero.
● To be able to learn and create our own temperature scale using three
reference points.
Copyright Labster ApS 2021
All Rights Reserved
II. Theory
Ideal gas is a theoretical gas composed of many randomly moving point
particles that are not subject to interparticle interactions. The ideal gas
concept is useful because it obeys the ideal gas law, a simplified equation of
state, and is amenable to analysis under statistical mechanics.
Ideal gas law, also called the general gas equation, is the equation of state of
a hypothetical ideal gas. It is a good approximation of the behavior of many
gases under many conditions, although it has several limitations.
Kinetic theory of gases is a simple, historically significant classical model of
the thermodynamic behavior of gases, with which many principal concepts of
thermodynamics were established.
Gas thermometry is a method of measuring temperatures with gas as the
thermometric fluid. Gas thermometry is the primary source of information
about a fundamental physical parameter, temperature, over the range from
about 3 to 900 K (-454 to 1160°F).
Absolute zero is the lowest limit of the thermodynamic temperature scale, a
state at which the enthalpy and entropy of a cooled ideal gas reach their
minimum value, taken as zero kelvins.
Pressure is the force applied perpendicular to the surface of an object per unit
area over which that force is distributed. Gauge pressure is the pressure
relative to the ambient pressure. Various units are used to express pressure.
Copyright Labster ApS 2021
All Rights Reserved
III. Methodology
This experiment is all about Ideal Gas law, which helps to define the
pressure at three different temperatures for a random amount of gas
molecules contained in a tank.
Activity 1:
In this experiment, the use of the three different reservoirs which contain
a water's boiling point will start once it turns on the variac and it will help to
determine the temperature scale. When a gas is being compressed into
smaller volumes, molecular collisions increase, raising the temperature and
pressure of the gas . It helps to measure the changes in heat that are
associated with the possible chemical reaction However, it also helps to
observe the absolute zero of the gas within the dipper.
Copyright Labster ApS 2021
All Rights Reserved
Activity 2:
Having a concept of P-T graph, it will help to demonstrate the result of
the temperature dependency of the pressure of a constant gas volume which
is shown in the graph. The result must be observed as the reaction occurs
when the heat will be generated by combusting the heat up the oxygen
vessels and to the surrounding water. No matter what amount of a gas , the
absolute zero temperature remains the same.
Copyright Labster ApS 2021
All Rights Reserved
IV. Results and Discussion
We are transporting a lung to the hospital for transplant surgery, and it
needs to arrive in one piece. We must arrive on time to avoid causing
damage to the organ; unfortunately, there has been an accident, and all
traffic ahead of us has come to a halt. We'll need to wait for a while.
We need to preserve the organ during this traffic accident so it will not
be damaged. According to the organ transplant guidance, the lungs must be
transported at 4 ℃ and its nearly 40 ℃ outside. We need to maintain the
right amount of temperature but the driver destroys our only thermometer
and we don’t know when to add cool packs to maintain the temperature.
We can use the Ideal Gas Law during our training and use a manometer and
a pressurized vessel to make our own temperature sensing device.
The main goal for today’s experiment is to create our own temperature
scale using three reference points. The three reservoirs will provide us with
these temperature reference points, starting from the lowest to the highest;
the boiling nitrogen temperature, the ice water temperature and the boiling
water temperature.
We will use the gas thermometry to construct a temperature scale and
determine the absolute zero temperature. This experiment demonstrates the
temperature dependency of the pressure of a constant gas volume. Generally,
gases used in gas thermometry are Ideal gases
Figure 1 :For a constant volume and amount of air, the pressure and temperature are
directly proportional, provided the temperature is in kelvin. (Measurements cannot be
made at lower temperatures because of the condensation of the gas.) When this line is
extrapolated to lower pressures, it reaches a pressure of 0 at –273 °C, which is 0 on the
kelvin scale and the lowest possible temperature, called absolute zero.
Copyright Labster ApS 2021
All Rights Reserved
Figure 2: Determine the pressure required to keep the temperature at 4℃
Assuming that the pressure inside the dipper is atmospheric, that means
that the inclined line representing the pressure change is the one presented
below. If we want to maintain the temperature at 4 ℃ we will need to have
approximately 750 torr to keep the temperature constant.
Figure 3: Explains how to think if you are in a situation when you have to keep
a temperature constant using a gas thermometer
Copyright Labster ApS 2021
All Rights Reserved
We can say that the amount of molecules confined in a gas tank, the
pressure reading on the manometer can define the temperature according to
the Ideal Gas Law. We just need to maintain the pressure in the vessel at 750
Torr to maintain the temperature we need.
V. Conclusions and Applications
With the help of this experiment/simulation,The first experiment observed
how the ideal gas molecules behave according to it. DIscussed also the
relationship between pressure,volume and temperature in gas.If you add too
many molecules then everything inside the container might turn into solid or
liquid. And the second and last experiment is creating your own temperature
scale using three different points.The three reservoirs will provide these
temperature reference points,starting from lowest to the highest.The boiling
nitrogen temperature , the ice water temperature and lastly is the boiling water.
Copyright Labster ApS 2021
All Rights Reserved
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5824)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- PATTS SCHEDULE FORM - Carandang, Benedict J. 200Document1 pagePATTS SCHEDULE FORM - Carandang, Benedict J. 200Benedict CarandangNo ratings yet
- AENG 428 - Simple Trusses Sample Problem Assignment V2Document3 pagesAENG 428 - Simple Trusses Sample Problem Assignment V2Benedict CarandangNo ratings yet
- AENG 428 SW1 MidDocument1 pageAENG 428 SW1 MidBenedict CarandangNo ratings yet
- Airport Parts 1B (GROUP 1)Document33 pagesAirport Parts 1B (GROUP 1)Benedict CarandangNo ratings yet
- AENG 428 A1 MidDocument1 pageAENG 428 A1 MidBenedict CarandangNo ratings yet
- Nsci 125Document1 pageNsci 125Benedict CarandangNo ratings yet
- Battery Ignition System - Page 3Document1 pageBattery Ignition System - Page 3Benedict CarandangNo ratings yet
- Benedict Carandang - 09 - 13 - Assignment No. 2Document5 pagesBenedict Carandang - 09 - 13 - Assignment No. 2Benedict CarandangNo ratings yet
- 1B-G2 Amte-426 CarandangDocument4 pages1B-G2 Amte-426 CarandangBenedict CarandangNo ratings yet
- 000Document1 page000Benedict CarandangNo ratings yet
- Prelim ExamDocument1 pagePrelim ExamBenedict CarandangNo ratings yet
- 325 - PW SW 1Document5 pages325 - PW SW 1Benedict CarandangNo ratings yet
- 1B Amte426 CarandangDocument18 pages1B Amte426 CarandangBenedict CarandangNo ratings yet
- Part 3 Answer With SolutionsDocument1 pagePart 3 Answer With SolutionsBenedict CarandangNo ratings yet
- 1B G2 Amte 426 CarandangDocument7 pages1B G2 Amte 426 CarandangBenedict CarandangNo ratings yet
- Magneto Ignition System - Page 2Document1 pageMagneto Ignition System - Page 2Benedict CarandangNo ratings yet
- 236 Quiz 1Document1 page236 Quiz 1Benedict CarandangNo ratings yet
- HARDWARE2Document102 pagesHARDWARE2Benedict CarandangNo ratings yet
- 1B G2 Amte 426 CarandangDocument5 pages1B G2 Amte 426 CarandangBenedict CarandangNo ratings yet
- Activity #4Document1 pageActivity #4Benedict CarandangNo ratings yet
- Air Turbine StarterDocument1 pageAir Turbine StarterBenedict CarandangNo ratings yet
- RECIPROCATING ENGINEDocument54 pagesRECIPROCATING ENGINEBenedict CarandangNo ratings yet
- TH REE Laws in Physic S: Add A SubheadingDocument1 pageTH REE Laws in Physic S: Add A SubheadingBenedict CarandangNo ratings yet
- Civil Aviation Authority of The Philippines Aircraft Accident Investigation and Inquiry Board Aircraft Accident ReportDocument2 pagesCivil Aviation Authority of The Philippines Aircraft Accident Investigation and Inquiry Board Aircraft Accident ReportBenedict CarandangNo ratings yet
- Studying Geodesics in The ClassroomDocument13 pagesStudying Geodesics in The ClassroomRafael AlmeidaNo ratings yet
- Main ISM Ch07Document14 pagesMain ISM Ch07Shoja Sammy RahimianNo ratings yet
- HArvard PysicsDocument3 pagesHArvard PysicsAndrei Joshua TorresNo ratings yet
- Engineering Thermodynamics - Open Systems ExercisesDocument1 pageEngineering Thermodynamics - Open Systems Exercisesloli XxxxNo ratings yet
- Heat Transfer: SKMM3443 Section 04Document20 pagesHeat Transfer: SKMM3443 Section 04Izzat AshraffNo ratings yet
- 15-Book 1 CH 15 IntegrationDocument21 pages15-Book 1 CH 15 IntegrationBen ChanNo ratings yet
- Thermodynamics of Polymer Blends PDFDocument5 pagesThermodynamics of Polymer Blends PDFpedroNo ratings yet
- Special Relativity and Minkowski SpacesDocument13 pagesSpecial Relativity and Minkowski Spacesgraviton6No ratings yet
- Updated Slides of Compressible FlowsDocument39 pagesUpdated Slides of Compressible FlowsFurqan YousafzaiNo ratings yet
- HMT Unit 3 MCQsDocument10 pagesHMT Unit 3 MCQsSatish kumar patleNo ratings yet
- Lewis 2007Document13 pagesLewis 2007david decena ortegaNo ratings yet
- EntropyDocument39 pagesEntropyRohit Singh LatherNo ratings yet
- Exp 1 Calorimetry IntroDocument3 pagesExp 1 Calorimetry IntroMarco FranciscoNo ratings yet
- Spontaneously Not Vice Versa: Second-Law - PPT Modified 10/9/02Document37 pagesSpontaneously Not Vice Versa: Second-Law - PPT Modified 10/9/02T Hari PrasadNo ratings yet
- Wed 7 MargineDocument27 pagesWed 7 Margineshubham patelNo ratings yet
- Revision Part2 PDFDocument23 pagesRevision Part2 PDFrawadNo ratings yet
- Schroedinger vs. N-SDocument14 pagesSchroedinger vs. N-Sdr_s_m_afzali8662No ratings yet
- Peng-Robinson Equation of State For A Pure FluidDocument2 pagesPeng-Robinson Equation of State For A Pure FluidANDRES CAMILO LEYTON ALVAREZNo ratings yet
- AD II Unit I & II 2 Marks Q&ADocument6 pagesAD II Unit I & II 2 Marks Q&AthandialNo ratings yet
- Transformation of Graph (New)Document89 pagesTransformation of Graph (New)18A Kashish PatelNo ratings yet
- Problems and Solutions: Physical ChemistryDocument179 pagesProblems and Solutions: Physical ChemistryRialeeNo ratings yet
- Nonlinear System Analysis: Practice ProblemsDocument7 pagesNonlinear System Analysis: Practice ProblemsRama Krushna PradhanNo ratings yet
- SCES3083 Topic7 ThermodynamicsDocument100 pagesSCES3083 Topic7 Thermodynamics胡佳玲100% (1)
- Outline of PhysicsDocument56 pagesOutline of Physicsaditya00012No ratings yet
- Transport Phenomena Transport CoefficientsDocument3 pagesTransport Phenomena Transport CoefficientsDubistWhiteNo ratings yet
- Mémoire Final Menasra AminaDocument105 pagesMémoire Final Menasra Aminaأمينة مناصرةNo ratings yet
- Nonlinear Dynamics of A Duffing-Like Negative StifDocument13 pagesNonlinear Dynamics of A Duffing-Like Negative StifPietro TestaNo ratings yet
- Thermodynamic Processes: Analysis of Thermodynamic Processes by Applying 1 & 2 Law of ThermodynamicsDocument10 pagesThermodynamic Processes: Analysis of Thermodynamic Processes by Applying 1 & 2 Law of Thermodynamicsmohdmehrajanwar1860No ratings yet
- (Exam) CAD-CAM-ThiCuoiKi (trắc nghiệm) - English (send)Document2 pages(Exam) CAD-CAM-ThiCuoiKi (trắc nghiệm) - English (send)Trường Nguyễn HuyNo ratings yet
- 14 RadiationDocument27 pages14 RadiationZhu Chen ChuanNo ratings yet