Professional Documents
Culture Documents
Experiment 6: Solubility Diagram of Two Partially Miscible Liquids Determination of Critical Solution Temperature of Water-Phenol System
Experiment 6: Solubility Diagram of Two Partially Miscible Liquids Determination of Critical Solution Temperature of Water-Phenol System
Uploaded by
John NdambukiCopyright:
Available Formats
You might also like
- Experiment 10: Iodine Clock ReactionDocument11 pagesExperiment 10: Iodine Clock ReactionJohn NdambukiNo ratings yet
- Bio 140 Lab BeetsDocument9 pagesBio 140 Lab Beetsflawson0078970100% (2)
- HWK 5Document1 pageHWK 5Kelly SmithNo ratings yet
- Pcc-Yltyvqza-Police Clearance Certificate p0Document2 pagesPcc-Yltyvqza-Police Clearance Certificate p0John Ndambuki100% (1)
- Temperature Effect On Heart Rate of DaphniaDocument4 pagesTemperature Effect On Heart Rate of DaphniaAparnaDasNo ratings yet
- Lab 5 Phenol-Water SystemDocument5 pagesLab 5 Phenol-Water SystemPaulraj Mosae SelvakumarNo ratings yet
- PhychemDocument10 pagesPhychemMinette PacisNo ratings yet
- Solid-Liquid Phase DiagramDocument4 pagesSolid-Liquid Phase DiagramMichael Go67% (9)
- Deluge System General PDFDocument2 pagesDeluge System General PDFNajam24No ratings yet
- Problems and Solutions in Organic Chemistry: PressDocument21 pagesProblems and Solutions in Organic Chemistry: PressSandipan Saha100% (1)
- Phase Diagram (Two Phase For Water-Phenol)Document8 pagesPhase Diagram (Two Phase For Water-Phenol)Sham J. HamaNo ratings yet
- Water PhenolDocument18 pagesWater PhenolSayd KamalNo ratings yet
- Lab 2Document5 pagesLab 2tariqwaece100% (1)
- Industrial Process Control: Control of Distillation TowersDocument20 pagesIndustrial Process Control: Control of Distillation TowersAmeya DalviNo ratings yet
- Acid Base Titration ExperimentDocument2 pagesAcid Base Titration ExperimentDark_KiroNo ratings yet
- Separo Quiz No. 2Document1 pageSeparo Quiz No. 2Bench GuecoNo ratings yet
- Leaching 7Document37 pagesLeaching 7tesfayregs gebretsadikNo ratings yet
- The Philippine Women'S University Biochemistry Laboratory Experiment/sDocument4 pagesThe Philippine Women'S University Biochemistry Laboratory Experiment/sskyler andradaNo ratings yet
- Isolation of Invertase Formal ReportDocument3 pagesIsolation of Invertase Formal ReportGabbySantosNo ratings yet
- 1.3 Newton's Law of Viscosity and RheologyDocument51 pages1.3 Newton's Law of Viscosity and RheologyfamiliarityNo ratings yet
- 07 Chapter 10 (Compiled)Document86 pages07 Chapter 10 (Compiled)Sofea IzyanNo ratings yet
- GlycerolDocument10 pagesGlycerolAshwani KumarNo ratings yet
- Membrane ProcessesDocument49 pagesMembrane ProcessesNur AmaninaNo ratings yet
- Organic Lab - Distillation PDFDocument2 pagesOrganic Lab - Distillation PDFDaryayBaharNo ratings yet
- Department of Chemistry: Determination of Critical Solution Temperature of Phenol-Water SystemDocument11 pagesDepartment of Chemistry: Determination of Critical Solution Temperature of Phenol-Water Systemzh2607903No ratings yet
- Physical Pharmacy: - Two Component System Containing Liquid Phases - ExpDocument9 pagesPhysical Pharmacy: - Two Component System Containing Liquid Phases - ExpcrtgyhujikNo ratings yet
- Experiment 2 Mutual Solubility of Binary System Phenol-Water A. PurposeDocument11 pagesExperiment 2 Mutual Solubility of Binary System Phenol-Water A. PurposeLinda MartianiNo ratings yet
- Experiment AIM: To Determine The Critical Solution Temperature (CST) and Composition of The SolutionDocument2 pagesExperiment AIM: To Determine The Critical Solution Temperature (CST) and Composition of The SolutionRoshan KumarNo ratings yet
- Practical Physical Chemistry (II) Laboratory ManualDocument25 pagesPractical Physical Chemistry (II) Laboratory Manualabdu30esNo ratings yet
- Water PhenolDocument12 pagesWater PhenolChemistry DepartmentNo ratings yet
- Phychem Lab Notes Ex 5Document3 pagesPhychem Lab Notes Ex 5Skye DiazNo ratings yet
- Determination of Critical Solution Temperature - ThermolabDocument2 pagesDetermination of Critical Solution Temperature - ThermolabindumathijayakaranNo ratings yet
- Title Phase Diagram Mutual Solubility - HTMLDocument12 pagesTitle Phase Diagram Mutual Solubility - HTMLAnoif Naputo AidnamNo ratings yet
- Solubility CurveDocument2 pagesSolubility Curvemohammed mahmoudNo ratings yet
- Laporan Resmi Kesetimbangan Fasa 2 KomponenDocument18 pagesLaporan Resmi Kesetimbangan Fasa 2 KomponenFika Fajariyah ArifinNo ratings yet
- Partially Miscible LiquidsDocument16 pagesPartially Miscible LiquidsYuppie Raj86% (7)
- Experiment 3 Lab ReportDocument10 pagesExperiment 3 Lab ReportVanessa Denise AguilarNo ratings yet
- Phase Diagram of Binary System PDFDocument20 pagesPhase Diagram of Binary System PDFGrgtNo ratings yet
- Applications of Critical Solution TemperatureDocument5 pagesApplications of Critical Solution TemperatureParveen88% (8)
- Basra University College of Science and Technology Pharmacy DepartmentDocument9 pagesBasra University College of Science and Technology Pharmacy DepartmentcrtgyhujikNo ratings yet
- Basra University College of Science and Technology Pharmacy DepartmentDocument9 pagesBasra University College of Science and Technology Pharmacy DepartmentcrtgyhujikNo ratings yet
- Unit 11 PDFDocument15 pagesUnit 11 PDFS M Arshad Rashadi100% (1)
- Physical Chemistry II (TKK-1237) : Review ReviewDocument7 pagesPhysical Chemistry II (TKK-1237) : Review ReviewUlvatus Sa' DiyahNo ratings yet
- Ans To Question + ConclusionDocument5 pagesAns To Question + ConclusionSpearMintNo ratings yet
- Two Component System Containing Liquid PhasesDocument6 pagesTwo Component System Containing Liquid Phasesعلاوي البرشلونيNo ratings yet
- Appendix 2 Lab Report Example 1Document5 pagesAppendix 2 Lab Report Example 1qihashiba523No ratings yet
- Appendix 02. Lab Report Example 1Document5 pagesAppendix 02. Lab Report Example 1Linh LinhNo ratings yet
- APC - Chapter 5 - Part 3 SP23Document11 pagesAPC - Chapter 5 - Part 3 SP23iB13eNo ratings yet
- Chapter 3 Lab ReportDocument5 pagesChapter 3 Lab ReportJabin Sta. TeresaNo ratings yet
- I. Ternary SystemsDocument33 pagesI. Ternary SystemsCatriona BlackNo ratings yet
- Adnan Akhtar Adnan Akhtar: Volumetric Properties of Pure FluidsDocument16 pagesAdnan Akhtar Adnan Akhtar: Volumetric Properties of Pure FluidsAdnan AKhtarNo ratings yet
- Experiment No:1 Determination of Critical Solution Temperature of Phenol-Water SystemDocument1 pageExperiment No:1 Determination of Critical Solution Temperature of Phenol-Water SystemDelin Shaji John100% (1)
- PhenolDocument7 pagesPhenolAkhmad RidhaniNo ratings yet
- Determination of The Differential Heat of SolutionDocument3 pagesDetermination of The Differential Heat of SolutionLoveFreequencyNo ratings yet
- Bin Liq MixDocument6 pagesBin Liq MixUche S AguNo ratings yet
- Volumetric Properties of Pure FluidsDocument40 pagesVolumetric Properties of Pure FluidsAleem Ahmed100% (1)
- Norkus Paper Rev0 FinalDocument18 pagesNorkus Paper Rev0 Finaljlcheefei9258No ratings yet
- Ex.1.2 PhamHongAnh 20201367Document5 pagesEx.1.2 PhamHongAnh 20201367Hồng Anh PhạmNo ratings yet
- Unit-2 Solutions 2 CST and Nernst Distribution LawDocument21 pagesUnit-2 Solutions 2 CST and Nernst Distribution LawSANKARA RAO NEIGAPULANo ratings yet
- Experiment 5: Boiling Point and Melting Point DeterminationDocument7 pagesExperiment 5: Boiling Point and Melting Point Determinationscsa31619No ratings yet
- State of Matter Lec 5Document40 pagesState of Matter Lec 5johnsmithprayNo ratings yet
- Equilibrium-Chapter-7: Pressure Etc Do Not Show Any Change With The Passage of TimeDocument12 pagesEquilibrium-Chapter-7: Pressure Etc Do Not Show Any Change With The Passage of TimeAman SinghNo ratings yet
- Binary Vapour Liquid EquiibrumDocument6 pagesBinary Vapour Liquid Equiibrumnishant shindeNo ratings yet
- Phase Diagram of Binary Mixture and Freezing Point DepressionDocument6 pagesPhase Diagram of Binary Mixture and Freezing Point DepressionAli RostamiNo ratings yet
- Pillars of Society MatrixDocument5 pagesPillars of Society MatrixJohn NdambukiNo ratings yet
- Quality Criteria Checklist For Review PapersDocument3 pagesQuality Criteria Checklist For Review PapersJohn NdambukiNo ratings yet
- Part 3Document2 pagesPart 3John NdambukiNo ratings yet
- Order 4184547 - Anesthetic Care For A Patient JOURNAL ARTICLEDocument11 pagesOrder 4184547 - Anesthetic Care For A Patient JOURNAL ARTICLEJohn NdambukiNo ratings yet
- Order 4184489-How To Succeed at A Job InterviewDocument5 pagesOrder 4184489-How To Succeed at A Job InterviewJohn NdambukiNo ratings yet
- Order 4161202 - Medical EthicsDocument5 pagesOrder 4161202 - Medical EthicsJohn NdambukiNo ratings yet
- Course Report: Grade Number of Pages DetailsDocument17 pagesCourse Report: Grade Number of Pages DetailsJohn Ndambuki100% (1)
- Stablcal Msds 2.16.10Document59 pagesStablcal Msds 2.16.10Cristian VegaNo ratings yet
- Polyrib Catalogue PDF NEW PDFDocument16 pagesPolyrib Catalogue PDF NEW PDFsekarNo ratings yet
- Membrane Cleaning and Support ChemicalsDocument5 pagesMembrane Cleaning and Support ChemicalsMohsin AliNo ratings yet
- Welding Procedure Specification: Company Dodsal Pte Ltd. Approved by KBRT Signature Name DateDocument1 pageWelding Procedure Specification: Company Dodsal Pte Ltd. Approved by KBRT Signature Name DateS GoudaNo ratings yet
- Detection of Coating Defects in Pipelines Using In-Line Inspection ToolsDocument3 pagesDetection of Coating Defects in Pipelines Using In-Line Inspection ToolsmohdrameNo ratings yet
- The Uni-Bell PVC Pipe Association's Guide To Understanding The AWWA C900-16 StandardDocument20 pagesThe Uni-Bell PVC Pipe Association's Guide To Understanding The AWWA C900-16 StandardUnibellNo ratings yet
- Chemical Kinetics: Lecture Notes Edited by John Reif From PPT Lectures byDocument128 pagesChemical Kinetics: Lecture Notes Edited by John Reif From PPT Lectures byFaith Escote100% (1)
- Astm e 140 (2005)Document21 pagesAstm e 140 (2005)henry_zambranoNo ratings yet
- ALUMINIUM LOUVERS CLADDING (O)Document14 pagesALUMINIUM LOUVERS CLADDING (O)Sunitha P. SunithaNo ratings yet
- 1.SolidState KCET PYQsDocument1 page1.SolidState KCET PYQsgangi reddy100% (1)
- Single Aisle Technical Training Manual Maintenance Course - T1 (V2500-A5/Me) OxygenDocument60 pagesSingle Aisle Technical Training Manual Maintenance Course - T1 (V2500-A5/Me) OxygenEvanildoLealNo ratings yet
- New J. Chem., 2012, 36, 2292-2301Document10 pagesNew J. Chem., 2012, 36, 2292-2301yokeshNo ratings yet
- Beacon Monitor Installation and Operations Manual: Tech-205Document10 pagesBeacon Monitor Installation and Operations Manual: Tech-205MerkoNo ratings yet
- Natural Gas Hydrocarbon Contamination in AmineDocument8 pagesNatural Gas Hydrocarbon Contamination in AmineRicardo BecNo ratings yet
- Bertrand Et Al. 2021 - Estimation of Pore Dimensions in Lipid Membranes Induced by Peptides and Other Biomolecules A ReviewDocument18 pagesBertrand Et Al. 2021 - Estimation of Pore Dimensions in Lipid Membranes Induced by Peptides and Other Biomolecules A ReviewAmadorRevillaNo ratings yet
- Newco Cast Steel Valves Tech DataDocument86 pagesNewco Cast Steel Valves Tech Dataeduardo goveaNo ratings yet
- Sewage Treatment PlantDocument56 pagesSewage Treatment Plantjin shodanNo ratings yet
- Che Cost Index Jan 2015Document2 pagesChe Cost Index Jan 2015JulianNo ratings yet
- Apple and Tomato Peels An AlternativeDocument18 pagesApple and Tomato Peels An AlternativeJahara MontesNo ratings yet
- Oxidation of Heterocyclic CompoundsDocument25 pagesOxidation of Heterocyclic CompoundsNam NguyenNo ratings yet
- Casting Inspection PDFDocument8 pagesCasting Inspection PDFamol koteNo ratings yet
- TT 21Document37 pagesTT 21Sahilpreet 5inghNo ratings yet
- Mellowing (6 2019)Document10 pagesMellowing (6 2019)majodvvNo ratings yet
- PG 329-357 PDFDocument29 pagesPG 329-357 PDFfilke100% (1)
- Metric Tube - ParkerDocument6 pagesMetric Tube - ParkerJenny Cecilia Ureña ZuriNo ratings yet
- Toxic Plants in Traditional Indian Systems of Medicine: Thomas M.Walter, Gopi G.RadhaDocument8 pagesToxic Plants in Traditional Indian Systems of Medicine: Thomas M.Walter, Gopi G.Radharajesh_rajesh_rajeshNo ratings yet
- MP Board Class 12 Chemistry Previous Year Paper 2018Document4 pagesMP Board Class 12 Chemistry Previous Year Paper 2018Varun PatidarNo ratings yet
- Msds Msds Soda Caustica LiquidaDocument5 pagesMsds Msds Soda Caustica LiquidaMorelis VelasquezNo ratings yet
Experiment 6: Solubility Diagram of Two Partially Miscible Liquids Determination of Critical Solution Temperature of Water-Phenol System
Experiment 6: Solubility Diagram of Two Partially Miscible Liquids Determination of Critical Solution Temperature of Water-Phenol System
Uploaded by
John NdambukiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Experiment 6: Solubility Diagram of Two Partially Miscible Liquids Determination of Critical Solution Temperature of Water-Phenol System
Experiment 6: Solubility Diagram of Two Partially Miscible Liquids Determination of Critical Solution Temperature of Water-Phenol System
Uploaded by
John NdambukiCopyright:
Available Formats
Experiment 6: Solubility Diagram of two Partially Miscible Liquids
Determination of critical solution temperature of water-phenol system.
Abstract
When a small quantity of phenol is mixed with water and shaken, the phenol undergoes
dissolution, resulting in a single layer. When larger phenol quantities are added, there are two
layers of liquids formed. The lower layer is made of small amounts of water dissolved in
phenol and the upper layer consists of phenol dissolved in water. Two partially miscible
liquids may become completely miscible at a higher temperature since solubility increases
with temperature generally. This miscibility temperature is different for different
compositions of the mixture. The highest miscibility temperature is called the critical solution
temperature or CST. Above this temperature, all compositions of this mixture are completely
miscible.
Introduction
To determine the conditions existing in a system of two liquids that are partially miscible, the
physical properties of the solutions on both sides of the solubility curve were measures. This
was done over the whole range of concentrations at a series of temperatures up to and above
the critical solution temperature. The water-phenol system was used in the experiment, and
the densities of the solutions of phenol in water and water in phenol were determined every 10
degrees between 20 degrees and 70 degrees. The densities of the conjugate solutions were
measures frequently near the critical solution temperature.
Procedure:
1. In a clean dry test tube (phenol tube) weigh accurately about 1 g phenol.
2. From a burette, add 0.5 ml of distilled water. Cover the tube with a cork stopper carrying a
thermometer and a stirrer, and then place in a beaker containing water to serve as bath.
3. Heat (or cool) gradually while the mixture is constantly stirred until the two layers
disappear forming one homogeneous layer. The two temperatures (T1 and T2), at which
this occurs on passing from a lower (T1) to a higher (T2) temperature and the reverse, are
recorded. These two temperatures should be nearly the same, and their mean gives the
miscibility temperature of the mixture used.
4. To the same mixture add the necessary volumes of water (0.5, 1, 1.5,…) and heat
gradually, then determine the miscibility temperature of the new mixture as described
above.
Calculations:
1. Record the volume of water used for each composition.
2. Plot miscibility temperature against percentage water or phenol for the various mixtures.
3. From the curve obtained find out the critical solution temperature and the corresponding
composition.
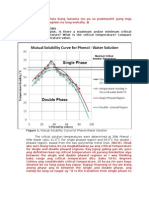
Results and Discussion
For a binary mixture of two partially miscible liquids e-g phenol and water the ‘certain
condition’ heterogeneous phase is formed under it studied by continuous adding of water in
phenol until a saturated solution of water in phenol obtained. Thus continuous adding of water
in phenol results saturated solution of phenol in water after a certain time. So two phases
form. On increase in volume of water first phase ( i.e water in phenol) decreases and second
phase ( i.e phenol in water) increases. Here component 2, phase 2, so degrees of freedom
(F)=2-2+2=2 i.e Bivariant . In this experiment if pressure remain constant then, F=2-2+1=1 i.e
univarient system. With increase in temperature mutual solubility increases . At a certain
temperature the mutual solubility curve attain maximum, this temperature is called critical
solution temperature (CST)or consolute temperature (CT) and corresponding composition is
called consolute composition . Under a constant pressure above the CST the homogeneous
phase is obtained. For phenol water system the critical solution temperature is 65.9℃ and
consolute composition is 34% The expected mutual solubility curve is as follows
Phase 1 is heterogeneous phase consisting of binary mixture of phenol and water. Phase 2 is
homogeneous phase. The composition can be calculated by drawing tie line in the curve
‘between A and A’. In the point A and A’, no of phase two, component 2 so degrees of
freedom F=C-P+1 = 2-2+1= 1 i.e univarient so this expressed as line. In the phase 2, no of
phase (p) = 1 component 2, so F=2-1+1=2 i.e bivarient so it is expressed by area. In the point
x’ i.e critical solution temperature, F=0 i.e invariant so this expressed as point. Under constant
pressure, we carry out the experiment by adding water in phenol and for each solubility
temperature will be noted. Then the temperature vs. Weight percentage of phenol will be
plotted. From the graph, obtained maximum solubility temp and its corresponding
composition; those will be critical solution temperature(CST) and con solute composition of
phenol respectively. And this will be studied using the law of rectilinear motion.
You might also like
- Experiment 10: Iodine Clock ReactionDocument11 pagesExperiment 10: Iodine Clock ReactionJohn NdambukiNo ratings yet
- Bio 140 Lab BeetsDocument9 pagesBio 140 Lab Beetsflawson0078970100% (2)
- HWK 5Document1 pageHWK 5Kelly SmithNo ratings yet
- Pcc-Yltyvqza-Police Clearance Certificate p0Document2 pagesPcc-Yltyvqza-Police Clearance Certificate p0John Ndambuki100% (1)
- Temperature Effect On Heart Rate of DaphniaDocument4 pagesTemperature Effect On Heart Rate of DaphniaAparnaDasNo ratings yet
- Lab 5 Phenol-Water SystemDocument5 pagesLab 5 Phenol-Water SystemPaulraj Mosae SelvakumarNo ratings yet
- PhychemDocument10 pagesPhychemMinette PacisNo ratings yet
- Solid-Liquid Phase DiagramDocument4 pagesSolid-Liquid Phase DiagramMichael Go67% (9)
- Deluge System General PDFDocument2 pagesDeluge System General PDFNajam24No ratings yet
- Problems and Solutions in Organic Chemistry: PressDocument21 pagesProblems and Solutions in Organic Chemistry: PressSandipan Saha100% (1)
- Phase Diagram (Two Phase For Water-Phenol)Document8 pagesPhase Diagram (Two Phase For Water-Phenol)Sham J. HamaNo ratings yet
- Water PhenolDocument18 pagesWater PhenolSayd KamalNo ratings yet
- Lab 2Document5 pagesLab 2tariqwaece100% (1)
- Industrial Process Control: Control of Distillation TowersDocument20 pagesIndustrial Process Control: Control of Distillation TowersAmeya DalviNo ratings yet
- Acid Base Titration ExperimentDocument2 pagesAcid Base Titration ExperimentDark_KiroNo ratings yet
- Separo Quiz No. 2Document1 pageSeparo Quiz No. 2Bench GuecoNo ratings yet
- Leaching 7Document37 pagesLeaching 7tesfayregs gebretsadikNo ratings yet
- The Philippine Women'S University Biochemistry Laboratory Experiment/sDocument4 pagesThe Philippine Women'S University Biochemistry Laboratory Experiment/sskyler andradaNo ratings yet
- Isolation of Invertase Formal ReportDocument3 pagesIsolation of Invertase Formal ReportGabbySantosNo ratings yet
- 1.3 Newton's Law of Viscosity and RheologyDocument51 pages1.3 Newton's Law of Viscosity and RheologyfamiliarityNo ratings yet
- 07 Chapter 10 (Compiled)Document86 pages07 Chapter 10 (Compiled)Sofea IzyanNo ratings yet
- GlycerolDocument10 pagesGlycerolAshwani KumarNo ratings yet
- Membrane ProcessesDocument49 pagesMembrane ProcessesNur AmaninaNo ratings yet
- Organic Lab - Distillation PDFDocument2 pagesOrganic Lab - Distillation PDFDaryayBaharNo ratings yet
- Department of Chemistry: Determination of Critical Solution Temperature of Phenol-Water SystemDocument11 pagesDepartment of Chemistry: Determination of Critical Solution Temperature of Phenol-Water Systemzh2607903No ratings yet
- Physical Pharmacy: - Two Component System Containing Liquid Phases - ExpDocument9 pagesPhysical Pharmacy: - Two Component System Containing Liquid Phases - ExpcrtgyhujikNo ratings yet
- Experiment 2 Mutual Solubility of Binary System Phenol-Water A. PurposeDocument11 pagesExperiment 2 Mutual Solubility of Binary System Phenol-Water A. PurposeLinda MartianiNo ratings yet
- Experiment AIM: To Determine The Critical Solution Temperature (CST) and Composition of The SolutionDocument2 pagesExperiment AIM: To Determine The Critical Solution Temperature (CST) and Composition of The SolutionRoshan KumarNo ratings yet
- Practical Physical Chemistry (II) Laboratory ManualDocument25 pagesPractical Physical Chemistry (II) Laboratory Manualabdu30esNo ratings yet
- Water PhenolDocument12 pagesWater PhenolChemistry DepartmentNo ratings yet
- Phychem Lab Notes Ex 5Document3 pagesPhychem Lab Notes Ex 5Skye DiazNo ratings yet
- Determination of Critical Solution Temperature - ThermolabDocument2 pagesDetermination of Critical Solution Temperature - ThermolabindumathijayakaranNo ratings yet
- Title Phase Diagram Mutual Solubility - HTMLDocument12 pagesTitle Phase Diagram Mutual Solubility - HTMLAnoif Naputo AidnamNo ratings yet
- Solubility CurveDocument2 pagesSolubility Curvemohammed mahmoudNo ratings yet
- Laporan Resmi Kesetimbangan Fasa 2 KomponenDocument18 pagesLaporan Resmi Kesetimbangan Fasa 2 KomponenFika Fajariyah ArifinNo ratings yet
- Partially Miscible LiquidsDocument16 pagesPartially Miscible LiquidsYuppie Raj86% (7)
- Experiment 3 Lab ReportDocument10 pagesExperiment 3 Lab ReportVanessa Denise AguilarNo ratings yet
- Phase Diagram of Binary System PDFDocument20 pagesPhase Diagram of Binary System PDFGrgtNo ratings yet
- Applications of Critical Solution TemperatureDocument5 pagesApplications of Critical Solution TemperatureParveen88% (8)
- Basra University College of Science and Technology Pharmacy DepartmentDocument9 pagesBasra University College of Science and Technology Pharmacy DepartmentcrtgyhujikNo ratings yet
- Basra University College of Science and Technology Pharmacy DepartmentDocument9 pagesBasra University College of Science and Technology Pharmacy DepartmentcrtgyhujikNo ratings yet
- Unit 11 PDFDocument15 pagesUnit 11 PDFS M Arshad Rashadi100% (1)
- Physical Chemistry II (TKK-1237) : Review ReviewDocument7 pagesPhysical Chemistry II (TKK-1237) : Review ReviewUlvatus Sa' DiyahNo ratings yet
- Ans To Question + ConclusionDocument5 pagesAns To Question + ConclusionSpearMintNo ratings yet
- Two Component System Containing Liquid PhasesDocument6 pagesTwo Component System Containing Liquid Phasesعلاوي البرشلونيNo ratings yet
- Appendix 2 Lab Report Example 1Document5 pagesAppendix 2 Lab Report Example 1qihashiba523No ratings yet
- Appendix 02. Lab Report Example 1Document5 pagesAppendix 02. Lab Report Example 1Linh LinhNo ratings yet
- APC - Chapter 5 - Part 3 SP23Document11 pagesAPC - Chapter 5 - Part 3 SP23iB13eNo ratings yet
- Chapter 3 Lab ReportDocument5 pagesChapter 3 Lab ReportJabin Sta. TeresaNo ratings yet
- I. Ternary SystemsDocument33 pagesI. Ternary SystemsCatriona BlackNo ratings yet
- Adnan Akhtar Adnan Akhtar: Volumetric Properties of Pure FluidsDocument16 pagesAdnan Akhtar Adnan Akhtar: Volumetric Properties of Pure FluidsAdnan AKhtarNo ratings yet
- Experiment No:1 Determination of Critical Solution Temperature of Phenol-Water SystemDocument1 pageExperiment No:1 Determination of Critical Solution Temperature of Phenol-Water SystemDelin Shaji John100% (1)
- PhenolDocument7 pagesPhenolAkhmad RidhaniNo ratings yet
- Determination of The Differential Heat of SolutionDocument3 pagesDetermination of The Differential Heat of SolutionLoveFreequencyNo ratings yet
- Bin Liq MixDocument6 pagesBin Liq MixUche S AguNo ratings yet
- Volumetric Properties of Pure FluidsDocument40 pagesVolumetric Properties of Pure FluidsAleem Ahmed100% (1)
- Norkus Paper Rev0 FinalDocument18 pagesNorkus Paper Rev0 Finaljlcheefei9258No ratings yet
- Ex.1.2 PhamHongAnh 20201367Document5 pagesEx.1.2 PhamHongAnh 20201367Hồng Anh PhạmNo ratings yet
- Unit-2 Solutions 2 CST and Nernst Distribution LawDocument21 pagesUnit-2 Solutions 2 CST and Nernst Distribution LawSANKARA RAO NEIGAPULANo ratings yet
- Experiment 5: Boiling Point and Melting Point DeterminationDocument7 pagesExperiment 5: Boiling Point and Melting Point Determinationscsa31619No ratings yet
- State of Matter Lec 5Document40 pagesState of Matter Lec 5johnsmithprayNo ratings yet
- Equilibrium-Chapter-7: Pressure Etc Do Not Show Any Change With The Passage of TimeDocument12 pagesEquilibrium-Chapter-7: Pressure Etc Do Not Show Any Change With The Passage of TimeAman SinghNo ratings yet
- Binary Vapour Liquid EquiibrumDocument6 pagesBinary Vapour Liquid Equiibrumnishant shindeNo ratings yet
- Phase Diagram of Binary Mixture and Freezing Point DepressionDocument6 pagesPhase Diagram of Binary Mixture and Freezing Point DepressionAli RostamiNo ratings yet
- Pillars of Society MatrixDocument5 pagesPillars of Society MatrixJohn NdambukiNo ratings yet
- Quality Criteria Checklist For Review PapersDocument3 pagesQuality Criteria Checklist For Review PapersJohn NdambukiNo ratings yet
- Part 3Document2 pagesPart 3John NdambukiNo ratings yet
- Order 4184547 - Anesthetic Care For A Patient JOURNAL ARTICLEDocument11 pagesOrder 4184547 - Anesthetic Care For A Patient JOURNAL ARTICLEJohn NdambukiNo ratings yet
- Order 4184489-How To Succeed at A Job InterviewDocument5 pagesOrder 4184489-How To Succeed at A Job InterviewJohn NdambukiNo ratings yet
- Order 4161202 - Medical EthicsDocument5 pagesOrder 4161202 - Medical EthicsJohn NdambukiNo ratings yet
- Course Report: Grade Number of Pages DetailsDocument17 pagesCourse Report: Grade Number of Pages DetailsJohn Ndambuki100% (1)
- Stablcal Msds 2.16.10Document59 pagesStablcal Msds 2.16.10Cristian VegaNo ratings yet
- Polyrib Catalogue PDF NEW PDFDocument16 pagesPolyrib Catalogue PDF NEW PDFsekarNo ratings yet
- Membrane Cleaning and Support ChemicalsDocument5 pagesMembrane Cleaning and Support ChemicalsMohsin AliNo ratings yet
- Welding Procedure Specification: Company Dodsal Pte Ltd. Approved by KBRT Signature Name DateDocument1 pageWelding Procedure Specification: Company Dodsal Pte Ltd. Approved by KBRT Signature Name DateS GoudaNo ratings yet
- Detection of Coating Defects in Pipelines Using In-Line Inspection ToolsDocument3 pagesDetection of Coating Defects in Pipelines Using In-Line Inspection ToolsmohdrameNo ratings yet
- The Uni-Bell PVC Pipe Association's Guide To Understanding The AWWA C900-16 StandardDocument20 pagesThe Uni-Bell PVC Pipe Association's Guide To Understanding The AWWA C900-16 StandardUnibellNo ratings yet
- Chemical Kinetics: Lecture Notes Edited by John Reif From PPT Lectures byDocument128 pagesChemical Kinetics: Lecture Notes Edited by John Reif From PPT Lectures byFaith Escote100% (1)
- Astm e 140 (2005)Document21 pagesAstm e 140 (2005)henry_zambranoNo ratings yet
- ALUMINIUM LOUVERS CLADDING (O)Document14 pagesALUMINIUM LOUVERS CLADDING (O)Sunitha P. SunithaNo ratings yet
- 1.SolidState KCET PYQsDocument1 page1.SolidState KCET PYQsgangi reddy100% (1)
- Single Aisle Technical Training Manual Maintenance Course - T1 (V2500-A5/Me) OxygenDocument60 pagesSingle Aisle Technical Training Manual Maintenance Course - T1 (V2500-A5/Me) OxygenEvanildoLealNo ratings yet
- New J. Chem., 2012, 36, 2292-2301Document10 pagesNew J. Chem., 2012, 36, 2292-2301yokeshNo ratings yet
- Beacon Monitor Installation and Operations Manual: Tech-205Document10 pagesBeacon Monitor Installation and Operations Manual: Tech-205MerkoNo ratings yet
- Natural Gas Hydrocarbon Contamination in AmineDocument8 pagesNatural Gas Hydrocarbon Contamination in AmineRicardo BecNo ratings yet
- Bertrand Et Al. 2021 - Estimation of Pore Dimensions in Lipid Membranes Induced by Peptides and Other Biomolecules A ReviewDocument18 pagesBertrand Et Al. 2021 - Estimation of Pore Dimensions in Lipid Membranes Induced by Peptides and Other Biomolecules A ReviewAmadorRevillaNo ratings yet
- Newco Cast Steel Valves Tech DataDocument86 pagesNewco Cast Steel Valves Tech Dataeduardo goveaNo ratings yet
- Sewage Treatment PlantDocument56 pagesSewage Treatment Plantjin shodanNo ratings yet
- Che Cost Index Jan 2015Document2 pagesChe Cost Index Jan 2015JulianNo ratings yet
- Apple and Tomato Peels An AlternativeDocument18 pagesApple and Tomato Peels An AlternativeJahara MontesNo ratings yet
- Oxidation of Heterocyclic CompoundsDocument25 pagesOxidation of Heterocyclic CompoundsNam NguyenNo ratings yet
- Casting Inspection PDFDocument8 pagesCasting Inspection PDFamol koteNo ratings yet
- TT 21Document37 pagesTT 21Sahilpreet 5inghNo ratings yet
- Mellowing (6 2019)Document10 pagesMellowing (6 2019)majodvvNo ratings yet
- PG 329-357 PDFDocument29 pagesPG 329-357 PDFfilke100% (1)
- Metric Tube - ParkerDocument6 pagesMetric Tube - ParkerJenny Cecilia Ureña ZuriNo ratings yet
- Toxic Plants in Traditional Indian Systems of Medicine: Thomas M.Walter, Gopi G.RadhaDocument8 pagesToxic Plants in Traditional Indian Systems of Medicine: Thomas M.Walter, Gopi G.Radharajesh_rajesh_rajeshNo ratings yet
- MP Board Class 12 Chemistry Previous Year Paper 2018Document4 pagesMP Board Class 12 Chemistry Previous Year Paper 2018Varun PatidarNo ratings yet
- Msds Msds Soda Caustica LiquidaDocument5 pagesMsds Msds Soda Caustica LiquidaMorelis VelasquezNo ratings yet