Professional Documents
Culture Documents
(Q) Elements Symbols Valances Compounds Formula Common Names
(Q) Elements Symbols Valances Compounds Formula Common Names
Uploaded by
shihab shoronOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
(Q) Elements Symbols Valances Compounds Formula Common Names
(Q) Elements Symbols Valances Compounds Formula Common Names
Uploaded by
shihab shoronCopyright:
Available Formats
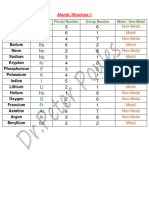
Elements Symbols Valances Table
Atomic Number/ Name of Symbol of Valance Type
Proton Number/ Element Element
Electron Number
1 Hydrogen H 1 Non-metal
2 Helium He Non-metal
5 Boron B 3 Non-metal
6 Carbon C 2, 4 Non-metal
7 Nitrogen N 3 Non-metal
8 Oxygen O 2 Non-metal
9 Fluorine F 1 Non-metal
10 Neon Ne Non-metal
11 Sodium Na (Natrium) 1 Metal
12 Magnesium Mg 2 Metal
13 Aluminium Al 3 Metal
14 Silicon Si 2, 4 Metalloid
15 Phosphorus P 1, 3, 5 Non-metal
16 Sulfur S 2, 4, 6 Non-metal
17 Chlorine Cl 1 Non-metal
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Atomic Number/ Name of Symbol of Valance Type
Proton Number/ Element Element
Electron Number
(Latin name)
18 Argon Ar Non-metal
19 Potassium K (Kalium) 1 Metal
20 Calcium Ca 2 Metal
26 Iron Fe (Ferrum) 2, 3 Metal
29 Copper Cu (Cuprum) 1, 2 Metal
30 Zinc Zn 2 Metal
35 Bromine Br 1 Non-metal
47 Silver Ag (Argentum) 1, 2, 3 Metal
50 Tin Sn (Stannum) 2, 4 Metal
53 Iodine I 1
78 Platinum Pt 1, 2, 3, 4, Metal
79 Gold Au (Aurum) 1, 2, 3 Metal
80 Mercury Hg 1, 2 Metal
(Hydrargyrum)
82 Lead Pb (Plumbum) 2, 4 Metal
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Some Important Chemical Compounds and their Classifications
SL Name of Compound Chemical Type
# Formula
Sodium Chloride NaCl Binary Ionic Compound
Calcium Oxide CaO Binary Ionic Compound
Manganese IV oxide MnO2 Binary Ionic Compound
Silver Oxide Ag2O Binary Ionic Compound
Aluminum Sulfide Al2S3 Binary Ionic Compound
Zinc Chloride ZnCl2 Binary Ionic Compound
Magnesium Iodide MgI2 Binary Ionic Compound
Iron II Oxide FeO Binary Ionic Compound
Iron III Oxide Fe2O3 Binary Ionic Compound
Copper I oxide Cu2O Binary Ionic Compound
Copper II oxide CuO Binary Ionic Compound
Magnesium chloride MgCl2 Binary Ionic Compound
Iron III bromide FeBr3 Binary Ionic Compound
Nitrate NO3-1 Polyatomic Ion
Nitrite NO2-1 Polyatomic Ion
hydroxide OH-1 Polyatomic Ion
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
SL Name of Compound Chemical Type
# Formula
bicarbonate HCO3-1 Polyatomic Ion
carbonate CO3-2 Polyatomic Ion
sulfate SO4-2 Polyatomic Ion
Sulfite SO3-2 Polyatomic Ion
Phosphate PO4-3 Polyatomic Ion
Phosphite PO3-3 Polyatomic Ion
Ammonium NH4+1 Polyatomic Ion
Ammonium Chloride NH4Cl Ternary Ionic Compound
Ammonium phosphate (NH4)3PO4 Ternary Ionic Compound
Calcium phosphate Ca3(PO4)2 Ternary Ionic Compound
Aluminum nitrate Al(NO3)3 Ternary Ionic Compound
Copper I Sulfate Cu2SO4 Ternary Ionic Compound
Calcium hydroxide Ca(OH)2 Ternary Ionic Compound
Magnesium hydroxide Mg(OH)2 Ternary Ionic Compound
Sodium hydroxide NaOH Ternary Ionic Compound
Potassium hydroxide KOH Ternary Ionic Compound
Aluminum hydroxide Al(OH)3 Ternary Ionic Compound
Ammonium hydroxide NH4OH Ternary Ionic Compound
Carbon dioxide CO2 Binary Molecular Compound
Carbon monoxide CO Binary Molecular Compound
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
SL Name of Compound Chemical Type
# Formula
Dinitrogen tetraoxide N2O4 Binary Molecular Compound
Dihydrogen monoxide H2O Binary Molecular Compound
Carbon tetrachloride CCl4 Binary Molecular Compound
Silicon dioxide SiO2 Binary Molecular Compound
Diphosphorous pentachloride P2Cl5 Binary Molecular Compound
Nitrogen monoxide NO Binary Molecular Compound
Dinitrogen pentaoxide N2O5 Binary Molecular Compound
Hydrochloric acid HCl Binary acid
Hydrofluoric acid HF Binary acid
Nitric acid HNO3 Ternary acid
Sulfuric acid H2SO4 Ternary acid
Phosphoric acid H3PO4 Ternary acid
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Important Chemical Compounds with their Molecular Formulas
Aluminium oxide – Al2O3 Lithium bromide – LiBr
Aluminium phosphide – AlP Lithium carbonate– Li2CO3
Aluminium chloride – AlCl3 Lithium chloride – LiCl
Aluminium fluoride – AlF3 Lithium hydroxide – LiOH
Aluminium hydroxide – Al(OH)3 Lithium iodide – LiI
Aluminium nitrate – Al(NO3)3 Lithium nitrate – LiNO3
Aluminium sulfate – Al2(SO4)3
Magnesium carbonate – MgCO3
Ammonia – NH3 Magnesium chloride – MgCl2
Ammonium bicarbonate – NH4HCO3 Magnesium oxide – MgO
Ammonium chloride – NH4Cl Magnesium phosphate – Mg3(PO4)2
Ammonium cyanide – NH4CN Magnesium sulfate – MgSO4
Ammonium dichromate – (NH4)2Cr2O7
Ammonium hydroxide – NH4OH Manganese(IV) oxide– MnO2
Ammonium nitrate – NH4NO3 Manganese(II) chloride – MnCl2
Ammonium sulfite – (NH4)2SO3 Manganese(III) chloride – MnCl3
Ammonium sulfate – (NH4)2SO4 Manganese(IV) fluoride – MnF4
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Manganese(II) phosphate – Mn3(PO4)2
Barium chloride – BaCl2
Barium carbonate – BaCO3 Nitric acid – HNO3
Barium hydroxide – Ba(OH)2 Nitrogen monoxide – NO
Barium iodide – BaI2 Nitrogen dioxide – NO2
Barium nitrate – Ba(NO3)2
Barium sulfate – BaSO4 Iodic acid – HIO3
Barium fluoride – BaF2
Borax – Na2B4O7·10H2O Phosphine – PH3
Boric acid – H3BO3 Phosphoric acid – H3PO4
Boron trichloride – BCl3 Phosphorous acid– H3PO3
Boron trifluoride – BF3 Phosphorus pentabromide – PBr5
Phosphorus pentoxide – P2O5
Calcium carbide – CaC2
Calcium chloride – CaCl2 Potassium bromide – KBr
Calcium hydroxide – Ca(OH)2 Potassium carbonate – K2CO3
Calcium sulfate (Gypsum) – CaSO4 Potassium chloride – KCl
Potassium cyanide – KCN
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Carbon dioxide – CO2 Potassium hydroxide – KOH
Carbon disulfide – CS2 Potassium iodide – KI
Carbon monoxide – CO Potassium nitrate – KNO3
Carbonic acid – H2CO3 Potassium perchlorate – KClO4
Carbon tetrachloride – CCl4 Potassium permanganate – KMnO4
Potassium sulfate – K2SO4
Chloric acid – HClO3 Potassium sulfide – K2S
Copper(II) carbonate – CuCO3 Silica gel – SiO2·nH2O
Copper(I) chloride – CuCl Silicon dioxide – SiO2
Copper(II) chloride – CuCl2 Silicon monoxide – SiO
Copper(II) hydroxide – Cu(OH)2
Copper(II) nitrate – Cu(NO3)2 Silver bromide – AgBr
Copper(I) oxide – Cu2O Silver chloride – AgCl
Copper(II) oxide – CuO Silver hydroxide – AgOH
Copper(II) sulfate – CuSO4 Silver nitrate – AgNO3
Dinitrogen tetroxide – N2O4 Sodium carbonate – Na2CO3
Sodium chloride – NaCl
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Gold(I) chloride – AuCl Sodium hydride – NaH
Gold(III) chloride – AuCl3 Sodium bicarbonate – NaHCO3
Sodium hydroxide – NaOH
Hydrazine – N2H4 Sodium iodide – NaI
Hydrobromic acid – HBr Sodium nitrate – NaNO3
Hydrochloric acid – HCl Sodium nitrite – NaNO2
Hydroiodic acid – HI Sodium sulfate – Na2SO4
Hydrogen bromide – HBr
Hydrogen chloride – HCl Sulfur dioxide – SO2
Hydrogen fluoride – HF Sulfuric acid – H2SO4
Hydrogen peroxide – H2O2 Sulfurous acid – H2SO3
Hydrogen sulfide – H2S
Zinc bromide – ZnBr2
Iron(II) chloride – FeCl2 Zinc carbonate – ZnCO3
Iron(III) chloride – FeCl3 Zinc chloride – ZnCl2
Iron(II) oxide – FeO Zinc fluoride – ZnF2
Iron(III) nitrate – Fe(NO3)3 Zinc iodide – ZnI2
Iron(II,III) oxide – Fe3O4 Zinc oxide – ZnO
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Iron(III) oxide – Fe2O3 Zinc sulfate – ZnSO4
Zinc sulfide – ZnS
Lead(II) carbonate – Pb(CO3)
Lead(II) chloride – PbCl2 Lead(II) phosphate – Pb3(PO4)2
Lead(II) iodide – PbI2 Lead(II) sulfate – Pb(SO4)
Lead(II) nitrate – Pb(NO3)2 Lead(II) sulfide – PbS
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Common Chemicals around Us
Sl# Common Names Chemical Names Chemical Formula
1 Antiperspirant Aluminum chlorohydrate Al2Cl(OH)5
2 Baking powder Sodium bicarbonate NaHCO3
3 Battery acid Sulphuric acid H2SO4
4 Bleaching powder Calcium hypochlorite Ca(ClO)2
5 Blue Vitriol (Fungicide, Herbicide,
Dyeing) Copper Sulphate CuSO4.5H2O
6 Caustic Soda (Cleaning agent, pulping,
water treatment, cement mixing) Sodium Hydroxide NaOH
7 Caustic Potash (Soap making, electrolyte,
food thickener) Potassium Hydroxide KOH
8 Corn starch (Culinary, baby powder, Amylum [C6H10O5]n
adhesive, bioplastic)
9 Carbolic acid (Soap, cosmetic, drug) Phenol C6H5OH
10 Chloroform Trichloromethane CHCl3
11 Dry Ice Solid Carbondioxide CO2
12 Epsom (Medical, Agriculture, Food) Magnesium Sulphate MgSO4.7H2O
13 Formalin 40% solution of CH2O
formaldehyde
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Sl# Common Names Chemical Names Chemical Formula
14 Freon Dichlorodifluoromethane CF2Cl2
15 Glycerine Glycerol C3H5(OH)3
16 Gypsum (Construction) Calcium Sulphate (CaSO4) .2H2O
17 Green Vitriol (Medical, colorant,
Agriculture) Ferrous Sulphate FeSO4.7H2O
18 Hypo Sodium Thiosulphate Na2S2O3.5H2O
19 Laughing gas (Medical, automobile, Dinitrogen N2O
propellant) monoxide/Nitrous oxide
20 Lye Sodium hydroxide NaOH
21 Ammonium Ferrous
Mohr’s Salt (Analytical chemistry) Sulphate FeSO4 (NH4)2 SO4.6H2O
22 Marble Calcium carbonate CaCO3
23 Milk of magnesia Magnesium hydroxide Mg(OH)2
24 Nail polish remover Acetone CH3COCH3
25 Natural / Marsh Gas Methane CH4
26 Plaster of Paris Calcium Sulphate (CaSO4) ½ H2O
27 Potash Ash Potassium Carbonate K2CO3
28 Quick Lime Calcium Oxide CaO
Sl# Common Names Chemical Names Chemical Formula
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
29 Quicksilver Mercury Hg
30 Rubbing alcohol Isopropyl alcohol (CH3)2CHOH
31 Sand/ Quartz Silicon dioxide SiO2
32 Slaked Lime Calcium Hydroxide Ca (OH)2
33 Sour Salt Citric acid C6H8O7
34 Salt Peter Potassium nitrate KNO3
35 Table Sugar Sucrose C12H22O11
36 Teflon Polymer of (C2F4)n
tetrafluoroethylene
37 Talc(um) powder Magnesium silicate Mg3Si4O10(OH)2
38 Tasting salt Monosodium glutamate C5H8NNaO4
39 Table Salt/Rock Salt Sodium chloride NaCl
40 Vinegar Acetic acid C2H4O2
41 Vitamin C Ascorbic acid C6H8O6
42 Washing soda Sodium carbonate Na2CO3⋅10H2O
decahydrate
43 White Vitriol Zinc Sulphate ZnSO4.7H2O
44 Wood alcohol Methyl alcohol CH3OH
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
Dr. Mohammad Harun-Ur-Rashid
Associate Professor
Chemistry, IUBAT
You might also like
- DIscussion of Reactions of Halides in SolutionDocument6 pagesDIscussion of Reactions of Halides in SolutionTingYuan Hoi76% (17)
- Solubility TableDocument1 pageSolubility TableLiolah LiolahNo ratings yet
- Name of Atom Common Ionic ChargeDocument2 pagesName of Atom Common Ionic ChargeMichael Rey MendozaNo ratings yet
- Chemistry Notes PT 3 4Document53 pagesChemistry Notes PT 3 4Eunice Kyla MapisaNo ratings yet
- Nomenclature and Writing Chemical Formula: Binary CompoundsDocument6 pagesNomenclature and Writing Chemical Formula: Binary CompoundsCarl Marco AmonNo ratings yet
- Writing Formula and Nomenclature of Chemical CompoundsDocument3 pagesWriting Formula and Nomenclature of Chemical CompoundsJulie Amor ZantuaNo ratings yet
- Naming CompoundsDocument2 pagesNaming CompoundsTeresa Marie CorderoNo ratings yet
- Common Polyatomic IonsDocument1 pageCommon Polyatomic IonsRoddyNo ratings yet
- Important TablesDocument6 pagesImportant TablesMaheswariNo ratings yet
- 5.9 Polyatomic CompoundsDocument3 pages5.9 Polyatomic Compoundsmichael.delaney8541No ratings yet
- MANTARA - Docx ACTIVITY#5 PART BDocument3 pagesMANTARA - Docx ACTIVITY#5 PART BFarks Mantara0% (1)
- Elements, Compounds and Chemical EquationsDocument11 pagesElements, Compounds and Chemical EquationsKasman Kasonde MumbaNo ratings yet
- GenChem Nomenclature Updated PDFDocument2 pagesGenChem Nomenclature Updated PDFCamille AquinoNo ratings yet
- Language of ChemistryDocument12 pagesLanguage of ChemistryVenkatNo ratings yet
- JRS Tutorials: Chemistry IITDocument58 pagesJRS Tutorials: Chemistry IITtusharr11.mobNo ratings yet
- Chemical Name and FormulasDocument35 pagesChemical Name and FormulasSara HdaifeNo ratings yet
- 10 CBSE ChemistryDocument67 pages10 CBSE ChemistryAlifiyah HussainNo ratings yet
- Chemical Formulas and Nomenclature of CoDocument10 pagesChemical Formulas and Nomenclature of CoAbdullah Sabry AzzamNo ratings yet
- ACTIVITY NO. 2 (CHEMISTRY LABORATORY) - Chemistry For EngineersDocument2 pagesACTIVITY NO. 2 (CHEMISTRY LABORATORY) - Chemistry For EngineersArvhenn BarcelonaNo ratings yet
- CHEM& 161 - Tran - Winter 2021 Elements and Ions: What Does This Tell Us? An Element On This List Exists As ADocument2 pagesCHEM& 161 - Tran - Winter 2021 Elements and Ions: What Does This Tell Us? An Element On This List Exists As AНиколай ЛиксуновNo ratings yet
- Chapter 1 (Answer)Document4 pagesChapter 1 (Answer)MThana BalanNo ratings yet
- SUMMARY Naming and Writing Formulas 1Document10 pagesSUMMARY Naming and Writing Formulas 1TenacityNo ratings yet
- Topic 2 - Microscopic World IDocument12 pagesTopic 2 - Microscopic World IBelladonna LeeNo ratings yet
- General Chemistry 1 NamingDocument108 pagesGeneral Chemistry 1 NamingJolo Allexice R. PinedaNo ratings yet
- Symbols and Charges-Monoatomic IonsDocument20 pagesSymbols and Charges-Monoatomic Ionsjon_kasilagNo ratings yet
- Valency TableDocument2 pagesValency TableZarbEChishtiNo ratings yet
- Laboratory Periodic Table: Gsci1103L-General Chemistry 1 LabDocument6 pagesLaboratory Periodic Table: Gsci1103L-General Chemistry 1 LabAndrea AurielleNo ratings yet
- Chapter 5 Coordination CompoundDocument36 pagesChapter 5 Coordination Compoundammar zakariaNo ratings yet
- Std. 8 Chemistry ProjectDocument8 pagesStd. 8 Chemistry ProjectEnisNo ratings yet
- Writing and Naming Chemical FormulasDocument3 pagesWriting and Naming Chemical FormulasCarlo Joseph Moskito100% (1)
- Lesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li ODocument16 pagesLesson 1.0 - Questions 1. Write Standard Atomic Notation For H Ca F Li OThai NgoNo ratings yet
- Chemistry Info SheetDocument3 pagesChemistry Info SheetClara GreenNo ratings yet
- Summer Assignment For AP Chemistry Class 2020-2021Document5 pagesSummer Assignment For AP Chemistry Class 2020-2021shelly zhangNo ratings yet
- Chm361-Chapter 5Document34 pagesChm361-Chapter 5atikah roshanNo ratings yet
- 10.5. Chemical Nomenclature - Molecular Compounds and AcidsDocument17 pages10.5. Chemical Nomenclature - Molecular Compounds and AcidsNina Anne Marie PascualNo ratings yet
- Symbols and Charges For Monoatomic IonsDocument2 pagesSymbols and Charges For Monoatomic IonsaNo ratings yet
- Booklet On The First 6 Chapters MSDocument47 pagesBooklet On The First 6 Chapters MShalahossam8899No ratings yet
- ApsummerDocument5 pagesApsummerLayleeNo ratings yet
- General Chemistry 2 Module 3Document6 pagesGeneral Chemistry 2 Module 3Jason Vinluan CarinanNo ratings yet
- Chm361 Chapter 5Document34 pagesChm361 Chapter 5syamimiafrinaNo ratings yet
- Name: Date: ..: 10 - Classwork: Topic 5 Chemical Formulas of IonsDocument4 pagesName: Date: ..: 10 - Classwork: Topic 5 Chemical Formulas of Ionsnorule36No ratings yet
- CHM11 3LectureUnit#6Document125 pagesCHM11 3LectureUnit#6Lin Xian XingNo ratings yet
- Common Ions and Their ChargesDocument2 pagesCommon Ions and Their ChargesTristanEvangelistaNo ratings yet
- Monoatomic and Polyatomic IonsDocument1 pageMonoatomic and Polyatomic IonsEstela Bernardette Cortés de HoyosNo ratings yet
- Chemistry - Review On Chemical Formulas With AnswersDocument4 pagesChemistry - Review On Chemical Formulas With AnswersAbdullah HassanNo ratings yet
- Chemical NomenclatureDocument7 pagesChemical NomenclatureQuỳnh NgânNo ratings yet
- Asm 33333333333Document2 pagesAsm 33333333333p5jp29697cNo ratings yet
- MODULE 2tables As ReferencesDocument10 pagesMODULE 2tables As ReferencesJuneyale Padilla100% (1)
- Chemical Formula, Naming & Writing Compound: General Chemistry 1Document30 pagesChemical Formula, Naming & Writing Compound: General Chemistry 1Synne Mae BorneaNo ratings yet
- General Chemistry 1 Atoms, Molecules and IonsDocument37 pagesGeneral Chemistry 1 Atoms, Molecules and IonsSeth CapellanNo ratings yet
- Ionic Compounds and Formula WorksheetDocument4 pagesIonic Compounds and Formula WorksheetKemoy FrancisNo ratings yet
- Notes Chapter 3-StoichiometryDocument31 pagesNotes Chapter 3-StoichiometryHakim AbbasNo ratings yet
- Classification Writing and Naming of Inorganic CompoundsDocument40 pagesClassification Writing and Naming of Inorganic CompoundsEvann Myelle MontejoNo ratings yet
- List of Ions: I. CationsDocument4 pagesList of Ions: I. CationsJamille GamboaNo ratings yet
- Lecture 1 EquationsDocument11 pagesLecture 1 Equationsmerabamoding11No ratings yet
- Write The Names For The Following Compounds: 1. C HDocument4 pagesWrite The Names For The Following Compounds: 1. C HOshauntae FosterNo ratings yet
- AP Chemistry Polyatomic List: Ion Name Ion Name Ion Name Ion NameDocument2 pagesAP Chemistry Polyatomic List: Ion Name Ion Name Ion Name Ion NameHarpreet KaurNo ratings yet
- Common IonsDocument3 pagesCommon IonsabdallaaNo ratings yet
- Symbols and Names For Common Polyatomic IonsDocument1 pageSymbols and Names For Common Polyatomic IonsElixirNo ratings yet
- Modern Supramolecular Gold Chemistry: Gold-Metal Interactions and ApplicationsFrom EverandModern Supramolecular Gold Chemistry: Gold-Metal Interactions and ApplicationsAntonio LagunaNo ratings yet
- 02 - General Chemistry - Atomic Structure and Nuclear Chemistry - P - 02Document236 pages02 - General Chemistry - Atomic Structure and Nuclear Chemistry - P - 02shihab shoronNo ratings yet
- 03 General Chemistry Conduction Bonding Acid-Base Compounds P 03Document242 pages03 General Chemistry Conduction Bonding Acid-Base Compounds P 03shihab shoronNo ratings yet
- CSC-437 CH 01 Basic ConceptsDocument71 pagesCSC-437 CH 01 Basic Conceptsshihab shoronNo ratings yet
- Compiler Design: Lexical AnalysisDocument68 pagesCompiler Design: Lexical Analysisshihab shoronNo ratings yet
- General Chemistry Lab Manual With DEMO DATADocument42 pagesGeneral Chemistry Lab Manual With DEMO DATAshihab shoronNo ratings yet
- Laboratory Periodic Table: Gsci1103L-General Chemistry 1 LabDocument6 pagesLaboratory Periodic Table: Gsci1103L-General Chemistry 1 LabAndrea AurielleNo ratings yet
- Making - Salts - Worksheet Ishita Roy Chemistry Year 9Document2 pagesMaking - Salts - Worksheet Ishita Roy Chemistry Year 9Ishita Roy0% (1)
- Notes On SaltsDocument4 pagesNotes On SaltsFelix S100% (1)
- JURINTDocument12 pagesJURINTOktavina KristaningtyasNo ratings yet
- Salt AnalysisDocument12 pagesSalt AnalysisAditya Verma50% (4)
- Chemical List 2021Document18 pagesChemical List 2021Bootie BootieNo ratings yet
- Products of NeutralisationDocument12 pagesProducts of NeutralisationSumayyah DesaiNo ratings yet
- Acids, Bases and Salts MCQsDocument14 pagesAcids, Bases and Salts MCQsKirthika SNo ratings yet
- Chapter - 17 - Acid, Base and Salt Making PDFDocument4 pagesChapter - 17 - Acid, Base and Salt Making PDFMir Tahsanur RahmanNo ratings yet
- Symbols and Charges-Monoatomic IonsDocument20 pagesSymbols and Charges-Monoatomic Ionsjon_kasilagNo ratings yet
- Ocr As Level Chemistry A: Answer All Questions Max 51 MarksDocument12 pagesOcr As Level Chemistry A: Answer All Questions Max 51 Markswdyi8ugqhNo ratings yet
- IGCSE Chemistry Insoluble Salt PreparationDocument2 pagesIGCSE Chemistry Insoluble Salt PreparationrudywahudiNo ratings yet
- Chemistry Salt AnalysisDocument2 pagesChemistry Salt AnalysisAkshai BalaNo ratings yet
- Neutralisation Reactions (Part 1)Document15 pagesNeutralisation Reactions (Part 1)MihadNo ratings yet
- Fertigation Compatability and Solubility PDFDocument2 pagesFertigation Compatability and Solubility PDFAhmad ZaidiNo ratings yet
- Acids and Bases: MD - Safwat X Riesaf HossainDocument9 pagesAcids and Bases: MD - Safwat X Riesaf HossainMd SafwatNo ratings yet
- P Block Reactions PDFDocument3 pagesP Block Reactions PDFTarundeepNo ratings yet
- Balancing Equations: Practice ProblemsDocument10 pagesBalancing Equations: Practice Problemskhalil rehmanNo ratings yet
- SRL Catalogue 22 23 Excel Version Price ListDocument330 pagesSRL Catalogue 22 23 Excel Version Price ListBidesh M Kirtania100% (1)
- Qualitative AnalysisDocument30 pagesQualitative AnalysisShivaprasadNo ratings yet
- Insoluble Salt Soluble Salt Uses Qualitative Analysis: Na, K and NH, Salts Double Decomposition Reaction Cations AnionsDocument53 pagesInsoluble Salt Soluble Salt Uses Qualitative Analysis: Na, K and NH, Salts Double Decomposition Reaction Cations AnionsPew LingNo ratings yet
- 05-10-2012 - Reactions in Solution IntroDocument2 pages05-10-2012 - Reactions in Solution Introamj1981No ratings yet
- Daily Practice Problems: Compound RepresentationDocument12 pagesDaily Practice Problems: Compound RepresentationrdgsrhdreeNo ratings yet
- Salt Analysis Cheat SheetDocument3 pagesSalt Analysis Cheat Sheetgsg171869No ratings yet
- Chemical: Ciron 302Ss Hastb 416Ss 440Css 17-4Phss Cs 316Ss Ti Monelalloy20 BRDocument27 pagesChemical: Ciron 302Ss Hastb 416Ss 440Css 17-4Phss Cs 316Ss Ti Monelalloy20 BRMubasheer ShariffNo ratings yet
- Practical Chemistry - IocDocument23 pagesPractical Chemistry - Iocdakshanatab255No ratings yet
- Ebook Chemical CompoundsDocument7 pagesEbook Chemical CompoundsRahulNo ratings yet
- Synova ChemicalsDocument201 pagesSynova ChemicalsInnovation InnovationNo ratings yet