Professional Documents
Culture Documents
NUTRI Manipulação Da Microbiota para Tratar Obesidade e DM
NUTRI Manipulação Da Microbiota para Tratar Obesidade e DM
Uploaded by
alegmachadoOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
NUTRI Manipulação Da Microbiota para Tratar Obesidade e DM
NUTRI Manipulação Da Microbiota para Tratar Obesidade e DM
Uploaded by
alegmachadoCopyright:
Available Formats
review article
Diabetes, Obesity and Metabolism 14: 112–120, 2012.
© 2011 Blackwell Publishing Ltd
article
review
The therapeutic potential of manipulating gut microbiota
in obesity and type 2 diabetes mellitus
R. S. Kootte1 , A. Vrieze2 , F. Holleman2 , G. M. Dallinga-Thie1 , E. G. Zoetendal3 , W. M. de Vos3,4 ,
A. K. Groen5 , J. B. L. Hoekstra2 , E. S. Stroes1 & M. Nieuwdorp1,2
1 Department of Vascular Medicine, University of Amsterdam, Amsterdam, the Netherlands
2 Department of Internal Medicine, University of Amsterdam, Amsterdam, the Netherlands
3 Laboratory of Microbiology, Wageningen University, Wageningen, the Netherlands
4 Department of Basic Veterinary Medicine and Bacteriology & Immunology, University of Helsinki, Helsinki, Finland
5 Center for Liver, Digestive, and Metabolic Diseases, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Obesity and type 2 diabetes mellitus (T2DM) are attributed to a combination of genetic susceptibility and lifestyle factors. Their increasing
prevalence necessitates further studies on modifiable causative factors and novel treatment options. The gut microbiota has emerged as an
important contributor to the obesity—and T2DM—epidemic proposed to act by increasing energy harvest from the diet. Although obesity is
associated with substantial changes in the composition and metabolic function of the gut microbiota, the pathophysiological processes remain
only partly understood. In this review we will describe the development of the adult human microbiome and discuss how the composition
of the gut microbiota changes in response to modulating factors. The influence of short-chain fatty acids, bile acids, prebiotics, probiotics,
antibiotics and microbial transplantation is discussed from studies using animal and human models. Ultimately, we aim to translate these
findings into therapeutic pathways for obesity and T2DM in humans.
Keywords: antibiotics, bile acids, gut microbiota, microbial transplantation, obesity, prebiotics, probiotics, SCFA, type 2 diabetes mellitus
Date submitted 31 May 2011; date of first decision 12 July 2011; date of final acceptance 15 July 2011
Introduction as humans were characterized by an altered composition of gut
microbiota compared to their lean counterparts [4,5]. These
The medical community is currently facing an unprecedented
distinct differences in gut microbiota have been shown to result
epidemic of obesity and type 2 diabetes mellitus (T2DM) [1,2].
in a greater capacity to harvest energy from the diet, thereby
These metabolic disorders are believed to be caused by a
contributing to obesity [5,6]. In support, germ-free mice were
combination of genetic susceptibility and lifestyle changes
protected from obesity, insulin resistance, dyslipidemia and
[1–3]. Recently, interest has been drawn towards the role of
fatty liver disease/non-alcoholic steatosis hepatis (NASH) when
the intestinal microbiota as a potential novel contributor to
fed a high-fat Western diet [7,8]. In contrast, following the
this epidemic.
colonization with microbiota from conventionally raised mice,
Until recently, knowledge of the intestinal microbiota was
body fat content in the originally germ-free mice increased
limited mainly because of the lack of analytical methods
up to 60% in 14 days. Moreover, insulin resistance increased
to identify micro-organisms. The introduction of culture-
despite a reduced food intake [9]. Food ingredients otherwise
independent approaches based on 16S ribosomal RNA analysis
indigestible for the hosts were microbially fermented and
(rRNA) and its corresponding gene has profoundly changed absorbed afterwards [6]. Furthermore, the presence of gut
the landscape, allowing to identify a large number of previously microbiota resulted in an increase in hepatic lipogenesis
undetected microbes in a wide variety of ecosystems and has and suppression of the lipoprotein lipase (LPL)-inhibitor
led to a major shift in research on the ecology of microbes angiopoietin-like 4 (ANGPTL4), which was formerly known
including those harbouring the intestinal tract. The application as fasting-induced adipocyte factor (FIAF) [7,9]. On the
of these 16S rRNA-based approaches in researches focusing basis of these and other observations, the gut microbiota
on the intestinal ecosystem has resulted in renewed insights is hypothesized to influence total body energy homeostasis
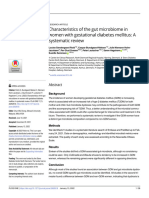
into the pathophysiological roles of the intestinal microbiota in (figure 1).
cardiometabolic diseases. In initial studies, obese mice as well This review portrays modulators that influence the
composition of the gut microbiota. We will also describe how
Correspondence to: M. Nieuwdorp, Department of Internal and Vascular Medicine, the specific changes in gut microbiota affect metabolism and
Academic Medical Centre, Meibergdreef 9, Room F4-159.2, 1105 AZ Amsterdam, the
Netherlands. how these findings could be translated into novel therapeutic
E-mail: m.nieuwdorp@amc.uva.nl pathways for obesity and T2DM.
DIABETES, OBESITY AND METABOLISM review article
Pepsin begins
digestion of Insulin sensitivity
Protein
Cholesterol content
Lipogenesis, glycogenesis
and triglyceride synthesis
Liver
Gall bladder Concentration of bile acids in bile
Triglycerides Stomach
Bile acids
Lipase
Glycerol Peptidase
Pancreas
Fatty acids
Duodenum
Amylase
Protein
Amino acids Levels of conjugated bile acids
Carbohydrates (downregulated deconjugation)
Monosaccharides
Disaccharides
absorbed
Free fatty acids
absorbed
Jejunum
Monoglycerides
absorbed
Ileum
Reabsorption of
Bile acids
(conjugated) bile acids
Levels of short-chain fatty acids
Excretion of triglycerides, cholesterol
and total lipids
Colon Excretion of bile acids
Legend
Increased
Decreased
Mildly increased
Figure 1. Studies comparing germ-free to conventionally raised animals have revealed important effects of gut microbiota on bile acid, fatty liver
[non-alcoholic steatosis hepatis (NASH)] disease as well as glucose, lipid and short-chain fatty acid metabolism. On the right side of this figure is depicted
how metabolism in germ-free animals differs from metabolism in conventionally raised animals. Moreover, absorption of different food-derived nutrients
in different parts of the intestine is depicted.
The Arising of an Adult Human Microbiome: the preferred community composition [14]. How does this
exteriorized organ develop, realizing a foetus is considered
Role of The Epithelial Lining
to be predominantly sterile? At birth, the intestine is
It is estimated that the human adult intestines contain more immediately colonized with bacteria from the mother and the
than 1014 bacteria from over 1000 species. The genetic environment [12,16,17]. Within several years after this initial
material of the intestinal microbes, collectively named the colonization, an adult-like microbiome has succeeded. External
microbiome, exceeds the magnitude of the human genome insults during life can (temporarily) change the composition of
over 100 times [10–13]. Medical literature therefore refers the adult microbiome. Examples of these are dietary changes,
to the gut microbiota as an ‘exteriorized organ’ [13]. The bacterial infections and the use of microbiota-modulating
phyla that account for the vast majority of all gut microbes agents (e.g. antibiotics). Despite continuous external stimuli,
include the Gram-negative Bacteroidetes, Proteobacteria and the composition of a human gut microbiota remains stable
Verrucomicrobia as well as the Gram-positive Firmicutes over time in healthy adults [10,12,13].
and Actinobacteria [10,13–15]. Despite its enormous diversity Host characteristics may contribute to the selection of
and complexity, it has been showed that the microbiota a specific gut microbiota. Epithelial cells throughout the
can be divided into three enterotypes driven by different gastrointestinal tract are covered by an integrated layer of
groups of species that are suggested to contribute to glycocalyx and mucus gel [18]. This layer is continuously
Volume 14 No. 2 February 2012 doi:10.1111/j.1463-1326.2011.01483.x 113
review article DIABETES, OBESITY AND METABOLISM
renewed and varies between different parts of the intestine. diet-induced changes in the composition of the gut microbiota,
Its functions are the selective binding and nourishing of the availability of SCFAs in the intestines changes, thus
commensal intestinal microbes and trapping and clearing of directly modulating energy absorption. In line, therapeutic
invasive pathogens, thereby affecting the composition of the gut modulation like α-glycosidase inhibitors, primarily used as
microbiome [19]. Vice versa, bacteria are capable of adapting to oral antidiabetics, also have the capacity to influence plasma
the surrounding mucus- and glycocalyx layer. Sonnenburg et al. SCFA concentration, underscoring the potential importance of
colonized germ-free mice with one of the Bacteroides species, SCFA production [34].
Bacteroidetes thetaiotaomicron, which prevented its own wash- Indirect pathways by which SCFA might influence
out from the intestines by assembling on partially digested metabolism are suggested. An enteroendocrinological pathway
food particles on shed elements of the mucus gel layer and on is proposed in which SCFAs stimulate the secretion of
exfoliated epithelial lining cells. In addition, B. thetaiotaomicron peptide YY (PYY), a hormone related to obesity [35,36].
showed the capacity to digest endogenous (mucus-derived) Of all SCFAs, butyrate in particular seems to function as
glycans when exogenous polysaccharides were absent [20]. a signalling molecule, although the precise role is unclear.
Similarly, Akkermansia muciniphila was isolated as the only High-fat diets supplemented with butyrate prevented and
cultured Verrucomicrobium species that exclusively grows on reversed insulin resistance in dietary-obese mice. The effects
mucus, has a large glycobiome and is found in healthy intestinal of butyrate action were related to a promotion of both
mucosa [21]. Escherichia coli on the other hand seems to be colonic epithelium [37] and whole body energy expenditure
capable of deploying specific pili to bind to mucus or mannose via a modulation of mitochondria function [38]. At the
sugars on intestinal epithelium [14]. Bearing the variability and same time, butyrate-producing bacteria and faecal butyrate
changing capacities of the mucus and glycocalyx layer in mind, concentrations decline with diets containing decreasing
the symbiotic ‘system’ of host and microbiota at least partially amounts of carbohydrates [30]. Apparently, butyrate can
might determine the composition of the gut microbiota and its beneficially affect metabolism in pathophysiological states.
observed enterotypes in humans [14,20].
The Complex Relationship Between Gut
The Interaction Between Diet and Gut Microbiota and Bile Acid Metabolism
Microbiota Composition: A Role After having travelled through the small intestine, 95% of
for Short-chain Fatty Acids all liver-secreted bile acids are reabsorbed in the ileum to
Diet is one of the multiple factors that can determine the be taken up by the liver: the enterohepatic cycle (EHC) [39].
composition of the gut microbiota [22]. Although diet-induced Only a small part of the bile acid pool escapes the EHC and
changes in gut microbiota occur within a short time frame travels towards the large intestine to be excreted in the faeces.
(within 1 to maximally 3–4 days after a diet switch), the This excretion is accompanied by microbial deconjugation of
changes are readily reversed [23–25]. In animal models, the glycine- and taurine-conjugated bile acids [40,41]. Following
ratio of the most prominent intestinal bacteria, the Bacteroidetes the discovery that germ-free rats tend to accumulate more
and Firmicutes, is altered in response to dietary changes [10,13]. cholesterol than their conventionally raised counterparts [42],
High-fat Western diets in mice resulted in an increase of the Wostmann showed that in the absence of gut microbiota, the
abundance of Firmicutes and a decrease in the abundance bile acid concentration in bile was three times increased and
of Bacteroidetes [23,24,26–28]. To date, human studies have cholesterol absorption was 25% greater [43]. The cholesterol
not been able to fully confirm these observations. Ley et al. accumulation was thought to be due to an increase in intestinal
reported a similar response in the presence of Bacteroidetes bile acid reabsorption [43,44]. Follow-up studies supported
and Firmicutes when obese subjects were put on a low-calorie Wostmann’s hypothesis by showing that germ-free animals
diet [29]. In contrast, others reported no relationship between have elevated levels of conjugated bile acids throughout the
the Bacteroidetes/Firmicutes ratio and low-carbohydrate diets intestine with no deconjugation and strongly decreased faecal
or body mass index (BMI) [14,30]. Hence, more data are excretion [41,45]. More recently, these results were confirmed
needed to show the presence as well as the value of the in experiments with mice treated with antibiotics. Three
Bacteroidetes/Firmicutes ratio in humans. days of treatment with ampicillin increased biliary bile acid
While diet may alter gut microbiota composition, it is only output threefold, while faecal output decreased 70% [46]. The
partly clear how this in turn might influence metabolism. authors concluded that increased expression of the sodium-
Intestinal microbes are capable of generating short-chain dependent bile acid-importer SLC10A2 underlies this response.
fatty acids (SCFAs, i.e. acetate, butyrate, proprionate) by In addition to the increased expression of SLC10A2, Miyata
fermenting dietary carbohydrates that humans cannot digest et al. observed a striking decrease in expression of fibroblast
themselves [5,14,31,32]. Acetate is the dominant SCFA type growth factor 15 (FGF 15) [47]. This autocrine hormone is
in humans [31,32]. SCFAs like proprionate can be utilized a product of intestinal bile-acid-stimulated nuclear hormone
for de novo glucose or lipid synthesis and serve as an energy farnesoid X receptor (FXR), which is apparently deactivated in
source for the host [5]. A recent study showed that germ-free the absence of ileal microbiota. FGF 15 is involved in the cross-
mice are devoid of SCFAs, indicating the importance of gut talk between liver and intestine and under normal physiological
microbiota for production of SCFAs in the intestine [33]. conditions serves to downregulate hepatic bile acid synthesis
From these observations it would appear that following in the postprandial phase. Clearly, the presence of intestinal
114 Kootte et al. Volume 14 No. 2 February 2012
DIABETES, OBESITY AND METABOLISM review article
microbes influences this signalling pathway and may explain the results in greater amounts of SCFAs present to be absorbed
ampicillin-induced increase in expression of 7-α-hydroxylase across the intestinal epithelium [57]. Moreover, a small
(CYP7A1), the key enzyme in bile acid synthesis [47]. pilot study showed that after surgery the amounts of both
Compared to conventionally raised animals, the bile of methanogens and Prevotellacaea were lowered and the initial
germ-free rats and mice contains more hydrophilic muricholic predominance of Firmicutes was mitigated [56]. A recent
acid (MCA) than amphiphatic cholic acid (CA) [43,48]. The animal study partially confirmed these human results. RYGB
ratio of both primary bile acids may play a role in the in non-obese rats diet resulted in decreased amounts of
regulation of the endogenous production and absorption of Firmicutes and Bacteroidetes but a 52-fold higher concentration
cholesterol by the liver, by determining the hydrophobicity of Proteobacteria in comparison with sham-operated rats [58].
and thus level of cholesterol solubilization in mixed micelles With regard to the inflammatory state in obesity, the butyrate-
of the total bile acid pool [49]. Therefore, the decrement of producing Faecalibacterium prausnitzii species was shown to
the CA/MCA ratio in germ-free animals changes cholesterol negatively associate with inflammatory markers before and after
homeostasis. Claus et al. tried to determine which mechanism RYGB, indicating its contribution to a healthy intestine [55].
caused this change [49]. They hypothesized that the enzyme Although bariatric surgery is directly aimed towards
CYP8B1 is a contributing factor, as it is responsible for the improving metabolic disturbances, other surgical interventions
production of CA, whilst MCA may be produced independent within the gastrointestinal tract also seem to influence gut
of this enzyme [48,49]. Germ-free mice indeed showed lower microbiota composition. Patients with short bowel syndrome,
expression levels of CYP8B1 [48–50]. Activation of hepatic for instance, are characterized by nutrient malabsorption and
FXR by the increased bile acid pool in the portal blood may aberrant SCFA metabolism [59]. An invasive therapy for this
be the cause of this phenomenon. However, activation of FXR patient group is a small bowel transplantation (SBT). After SBT,
would also be expected to result in decreased expression of a temporary ileostomy is created to allow monitoring of the
CYP7A1 and this has not been observed [49]. Clearly, the transplanted tissue. During the presence of an ileostomy, the
relationship between activity of the gut microbiota and bile ileal microbiota shifts dramatically with increased colonization
acid homeostasis is complex. Competition between intestinal- by the facultative anaerobic bacteria Lactobacilli, belonging
derived FGF 15-propagated signalling and hepatic FXR may to the Firmicutes phylum, and Enterobacteria, whereas under
underlie this complex behaviour. physiological conditions and after closure of the ileostomy,
Besides FXR, bile acids may signal via a newly discovered Bacteroidetes and Clostridia dominate the ileum [60]. Booijink
signal transduction pathway, mediated by the membrane et al. compared ileal effluent microbiota with faecal microbiota
receptor TGR5. This receptor is strongly expressed in brown in patients with a Brooke ileostomy. Their results confirmed
adipose tissue and the small intestine. In mice, oral gavage with previous data as they found a greater concentration of
exogenous bile acids led to increased energy expenditure in Lactobacilli and a decrease in concentration of Bacteroidetes
brown adipose tissue via activation of TGR5 [51–53]. As such, in the ileal microbiota. More importantly, they found a lesser
obesity and insulin resistance were prevented. In addition, diversity and stability of ileal microbiota when compared to
as L-cells in the distal ileum and colon express TGR5 in faecal microbiota [61]. In conclusion, surgical interventions
high amounts, bile acid-induced stimulation of TGR5 results in the gastrointestinal tract have profound effects on gut
in glucagon-like peptide (GLP-1) release and stimulation of microbiota composition and SCFAs in particular. Nevertheless,
insulin secretion [52,54]. the surgically induced malnutrition status in case of an RYGB
It appears that there is a complex interaction between bile outweighs the fine-tuning effect of the gut microbiota in
acids and gut microbiota. Nevertheless, it is irrefutable that gut reversing obesity and T2DM.
microbiota can influence human metabolism via the bile acid
pathway.
Prebiotics
Prebiotics (mostly oligosaccharides) are non-digestible but
Gastrointestinal Surgery and Gut Microbiota fermentable food ingredients that selectively stimulate the
Bariatric surgery is one of the most efficient procedures growth or activity of one or multiple gut microbes that are
to treat morbid obesity. It results in drastic weight loss beneficial to their human hosts [62,63]. The human intestinal
and improvement of metabolic and inflammatory status, tract can not digest the food ingredient, but the intestinal
but very little is known about the effect of this type of microbes ferment the food particles. Effects of prebiotics
surgery on the gut microbiota [55]. Roux-en-Y gastric bypass have been claimed in all ages, ranging from infants to elderly
(RYGB) is the bariatric major intervention. Detailed studies [62]. Bacteria of the Bifidobacterium, belonging to the Acti-
were able to link both the preoperative metabolic state and nobacteria—and in some cases Lactobacillus spp.—particularly
the postoperative changes to gut microbiota composition. are known for a response to the administration of certain
Before surgery, high levels of an H2 -producing subgroup prebiotics [62,63].
of Bacteroidetes, the Prevotellacaea, next to high levels of The effects of prebiotics on metabolism in part arise by
H2-utilizing methanogens (Archaea) were observed [56]. The reducing a diet-induced inflammatory state. High-fat diets have
transfer of H2 between both bacteria increases the energy the ability to increase lipopolysaccharide (LPS)-containing gut
uptake by the intestine, as methanogens are capable of removing microbiota and subsequently downregulate the amount of
fermentation intermediates (H2 ) from polysaccharides. This Bifidobacteria. The accompanying inflammatory state, called
Volume 14 No. 2 February 2012 doi:10.1111/j.1463-1326.2011.01483.x 115
review article DIABETES, OBESITY AND METABOLISM
metabolic endotoxaemia, is accompanied by insulin resis- T2DM [87–89]. Naito recently described both antidiabetic
tance and weight gain [64,65]. In a physiological situation, and anti-inflammatory effects of Lactobacillus casei in diet-
Bifidobacteria are capable of lowering LPS levels [65,66]. Pre- induced obese mice [90]. In addition to these antidiabetic
biotics containing oligofructose (OFS) specifically stimulate effects, diet-induced obese mice and diet-induced overweight
the growth of these intestinal bacteria [62,67,68]. Administer- rats showed a reduction in body weight gain after they
ing OFS totally restored Bifidobacteria levels and normalized were fed specific Lactobacilli [91,92]. Although these animal
plasma endotoxin levels. It diminished the metabolic endotox- findings are interesting, translation of these beneficial effects
emia, thus leading to improved glucose tolerance, increased of Lactobacilli in humans merits caution. First, none of the
satiety and weight loss in human subjects [64,69,70]. Besides above-mentioned studies directly assessed the relationship
modulating endotoxemia, OFS can alter metabolism in var- between the observed metabolic changes and the gut microbiota
ious other manners. Cani et al. showed that effects of OFS composition. Second, Lactobacilli are widely used within the
were mediated via a GLP1-dependent pathway. High-fat-fed farming industry, resulting in weight gain in animals [93,94]
diabetic mice on OFS treatment exerted improved glucose and this weight-modulating role has also been confirmed both
tolerance, diminished body weight and a decrease in endoge- in children and adults [95–97].
nous glucose production. Either adding the GLP-1 receptor
antagonist exendin 9-39 (Ex-9) or using GLP-1 knockout mice
resulted in a complete lack of the OFS-mediated beneficial Synbiotics
effects [71], thus showing the causal role of GLP-1 in this Studies on probiotics have shown that every probiotic
pathway in animals. Attempts to translate these findings to only benefits from a selected number of carbohydrates [98].
human subjects are ambiguous, showing that OFS tends to Amylase-resistant starch, for example, increases the number of
dose-dependently decrease energy intake and increase PYY intestinal Lactobacilli and Bifidobacteria, whilst decreasing the
plasma concentrations [70,72], but reported effects on sati- number of Enterobacteria [99]. From 40 types of Bifidobacteria,
ety are conflicting [72,73]. Finally, OFS fermentation directly the species Bifidobacterium lactis showed the most growth when
affects butyrate synthesis from extracellular acetate and lactate, combined with this type of starch [100]. These observations
implicating the therapeutic potential of prebiotics [74]. have led to the production of preparations containing
Thus, there is emerging evidence to support the hypothesis both bacteria (probiotics) and saccharides (prebiotics):
that prebiotics can influence gut microbiota composition and, synbiotics [101]. As the prebiotic part of synbiotics is
as such, metabolic disturbances. The amount of evidence specifically selected on the basis of its ability to stimulate
however is limited and a definite beneficial effect on metabolic the growth of the probiotic part, the effects of the probiotics are
disturbances remains to be showed in large prospective enhanced. In certain cases, these combinations show synergistic
randomized controlled trials. effects. Lactobacillus reuteri was more resistant to bile salts
when combined with soygerm powder [102]. In addition, the
combination of fructooligosaccharides (FOS) with Lactobacillus
Probiotics Acidophilus not only led to an expected increased amount of
Probiotics are food supplements that contain living bacteria Lactobacilli. It also caused a growth of Bifidobacteria, to an
such as Bifidobacteria, Lactobacilli, Streptococci and non- even higher extent than the growth of Lactobacilli [103]. Thus,
pathogenic strains of E. coli [75,76]. When adequately adminis- although a possible relationship seems logical, new studies
tered, they confer beneficial effects to the host because of should focus on directly relating probiotic-/synbiotic-induced
changes in the gut microbiota that are both transient and changes in metabolism to changes in gut microbiota.
diminish gradually with time after cessation [77–83]. In 2006,
Goossens et al. compared the effects of consuming Lactobacillus
Plantarum on the microbial colonization of faeces and biopsies Antibiotics
from the ascending colon and rectum [80]. Within faecal Antibiotics normally are prescribed to remove or prevent a
samples, the amount of Lactobacilli was significantly increased. bacterial colonization in the human body, without specifically
However, the biopsies did not confirm a growth of Lactobacilli. targeting certain types of bacteria. As a side effect, antibiotics
These data suggest that probiotics influence the intestinal can influence the composition of the gut microbiota up to
lumen rather than the gut epithelium, possibly explaining the 2 years after administration [104–106]. The decrement of
transient effect of probiotics. Surprisingly, van Baarlen et al. intestinal bacteria after using antibiotics causes alterations
recently described changes in the expression of up to thousands in several bacteria-induced metabolic pathways. Tetracycline
of genes in duodenal biopsies after administration of three types antibiotics resulted in improved glucose tolerance and insulin
of Lactobacilli [84]. sensitivity in diabetic rats and obese mice [107–109]. Further
Alterations in the gut microbiota as a result of probiotics exploring this relationship, ampicillin and norfloxacin caused
are widely acknowledged but evidence showing that probiotic a reduction in hepatic steatosis (NASH), liver triglyceride
administration directly affects inflammatory state has only production and lipogenesis in ob/ob mice. In line, glucose
recently been shown in humans [85,86]. In contrast, studies levels normalized and insulin sensitivity increased. Obesity
on the effects of probiotics on characteristics of T2DM are itself was not a contributing factor to the state of improved
mostly performed in animal models, reporting beneficial glucose tolerance as these results were independent of weight
effects by various strains of Lactobacilli on characteristics of changes [110].
116 Kootte et al. Volume 14 No. 2 February 2012
DIABETES, OBESITY AND METABOLISM review article
Two mechanisms are proposed to bridge metabolism and transplantation, thus offering a new view on the metabolic devi-
antibiotic-induced changes in the gut microbiota composition. ations in obese subjects and a rationale for novel therapeutic
The first is a reduction in low-grade inflammation. Tumour intervention methods directly affecting gut microbiota-induced
necrosis factor (TNF)-α and LPS were shown to negatively metabolism. A follow-up study, the FATLOSE 2-trial, has been
correlate with insulin sensitivity [110]. Cani et al. further initiated to study the long-term effects of single versus multiple
elucidated the role of LPS as they showed that antibiotics lean donor faecal transplants on metabolic measurements.
lowered LPS levels, which inhibited metabolic disorders
from developing. Glucose intolerance, body weight gain and
fat mass development all were reduced [111]. The second Conclusions
possible explanation is that antibiotics directly influence SCFA The gut microbiota may be a major player in maintaining
levels. When female mice were administered vancomycin human metabolism homeostasis. Although interest in humans
orally, microbial fermentation of carbohydrates diminished, has only recently developed, it becomes clear that we first
with a subsequent decrease of faecal SCFA excretion. In need to unravel the underlying mechanisms in the causality of
line with what was expected, faecal samples contained human obesity and T2DM in order to know how to influence
more (unfermented) oligosaccharides [112]. Previous in vitro these gut microbiota-driven processes. Despite our increasing
experiments showed similar results [113]. Although one might knowledge on genetic pathways underlying human obesity
hypothesize that antibiotics result in a diarrhoea-induced and T2DM, specific data on the human gut microbiome are
detrimental nutritional status and thus weight loss, recent scarce. In this review, we tried to answer which potential
observations have suggested otherwise. Early exposure to oral gut microbiota-driven pathways would be worth to study in
antibiotics was found to be associated with overweight in humans and could render novel treatment options in the near
children [114]. Even more interesting is the recent report of an future.
association between intravenous administrated vancomycin
and weight gain in adults subjects, most probably via a
Lactobacilli-mediated pathway, given the intrinsic resistance
Acknowledgements
of Lactobacilli for vancomycin [115]. We would like to thank Tine Thörig for graphic support.
The present data offer an intriguing view into the potential R. S Kootte is supported by a Top Institute Food and Nutrition
beneficial effects of antibiotics on human metabolism. Future grant (2010GH003) and M. Nieuwdorp is supported by a VENI
interventions however should be introduced deliberately, as grant 2008 (016.096.044).
antibiotic resistance is an increasing problem and antibiotics
are associated with Clostridium difficile diarrhoea [116].
Conflict of Interest
All authors helped in writing the manuscript and they have no
Recent Interest: Microbial Transplantation competing interests.
First described in 1958 [117], the transplantation of faecal
microbiota remains a controversial treatment. Until recently, References
this therapy was limited to cases of pseudomembraneous colitis,
1. Bleich S, Cutler D, Murray C, Adams A. Why is the developed world
a bacterial infection often following antibiotic treatment [116]. obese? Annu Rev Public Health 2008; 29: 273–295.
Faecal transplant aids in curing this disease by restoring the
2. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the
gut microbiota of the diseased. This has raised interest in
diabetes epidemic. Nature 2001; 414: 782–787.
the result of faecal transplantation on the modulation of
3. Ahlqvist E, Ahluwalia TS, Groop L. Genetics of type 2 diabetes. Clin Chem
metabolism. Previous studies reported an increase of body fat
2011; 57: 241–254.
up to 60% and an increase in insulin resistance after faeces was
4. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI.
transplanted from conventionally raised mice to germ-free [9].
Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005;
When comparing different faeces donors, lean versus obese, 102: 11070–11075.
the latter caused a greater rise in body fat within germ-free
5. Schwiertz A, Taras D, Schafer K et al. Microbiota and SCFA in lean and
recipients [6,23]. Faecal transplants were even tested between overweight healthy subjects. Obesity (Silver Spring) 2010; 18: 190–195.
different mammals. Gnotobiotic mice, mice conventionalized
6. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An
with human faeces, have shown a preservation of structure and
obesity-associated gut microbiome with increased capacity for energy
diversity of the gut microbiota transplanted from humans. At harvest. Nature 2006; 444: 1027–1031.
the same time, the cholesterol metabolism and adiposity of the
7. Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms
donor were preserved [24,118,119]. underlying the resistance to diet-induced obesity in germ-free mice.
As aforementioned data are hampered by methodological Proc Natl Acad Sci U S A 2007; 104: 979–984.
concerns, we recently performed a study on faecal transplan- 8. Rabot S, Membrez M, Bruneau A et al. Germ-free C57BL/6J mice are
tation in humans (FATLOSE-trial) [120]. In this double-blind resistant to high-fat-diet-induced insulin resistance and have altered
randomized control trial (RCT) we studied the therapeutic cholesterol metabolism. FASEB J 2010; 24: 4948–4959.
effects of allogenic lean donor faeces infusion on insulin resis- 9. Backhed F, Ding H, Wang T et al. The gut microbiota as an environmental
tance in male patients with metabolic syndrome. We observed factor that regulates fat storage. Proc Natl Acad Sci U S A 2004; 101:
beneficial alterations in glucose metabolism after lean donor 15718–15723.
Volume 14 No. 2 February 2012 doi:10.1111/j.1463-1326.2011.01483.x 117
review article DIABETES, OBESITY AND METABOLISM
10. Eckburg PB, Bik EM, Bernstein CN et al. Diversity of the human intestinal 32. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health:
microbial flora. Science 2005; 308: 1635–1638. fermentation and short chain fatty acids. J Clin Gastroenterol 2006; 40:
235–243.
11. Gill SR, Pop M, Deboy RT et al. Metagenomic analysis of the human distal
gut microbiome. Science 2006; 312: 1355–1359. 33. Maslowski KM, Vieira AT, Ng A et al. Regulation of inflammatory
responses by gut microbiota and chemoattractant receptor GPR43. Nature
12. Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of
2009; 461: 1282–1286.
the human infant intestinal microbiota. PLoS Biol 2007; 5: e177.
34. Dehghan-Kooshkghazi M, Mathers JC. Starch digestion, large-bowel
13. Zhu B, Wang X, Li L. Human gut microbiome: the second genome of
fermentation and intestinal mucosal cell proliferation in rats treated with
human body. Protein Cell 2010; 1: 718–725.
the alpha-glucosidase inhibitor acarbose. Br J Nutr 2004; 91: 357–365.
14. Arumugam M, Raes J, Pelletier E et al. Enterotypes of the human gut
35. Grudell AB, Camilleri M. The role of peptide YY in integrative gut
microbiome. Nature 2011; 473: 174–180.
physiology and potential role in obesity. Curr Opin Endocrinol Diabetes
15. Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity Obes 2007; 14: 52–57.
and functionality analysis of the gastrointestinal tract microbiota. Gut
36. Samuel BS, Shaito A, Motoike T et al. Effects of the gut microbiota on
2008; 57: 1605–1615.
host adiposity are modulated by the short-chain fatty-acid binding
16. Dai D, Walker WA. Protective nutrients and bacterial colonization in the G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A 2008; 105:
immature human gut. Adv Pediatr 1999; 46: 353–382. 16767–16772.
17. Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the 37. Donohoe DR, Garge N, Zhang X et al. The microbiome and butyrate
neonatal gastrointestinal tract. Am J Clin Nutr 1999; 69: 1035S–1045S. regulate energy metabolism and autophagy in the Mammalian colon.
Cell Metab 2011; 13: 517–526.
18. Sonnenburg JL, Angenent LT, Gordon JI. Getting a grip on things: how do
communities of bacterial symbionts become established in our intestine? 38. Gao Z, Yin J, Zhang J et al. Butyrate improves insulin sensitivity and
Nat Immunol 2004; 5: 569–573. increases energy expenditure in mice. Diabetes 2009; 58: 1509–1517.
19. Moran AP, Gupta A, Joshi L. Sweet-talk: role of host glycosylation in 39. Keitel V, Kubitz R, Haussinger D. Endocrine and paracrine role of bile
bacterial pathogenesis of the gastrointestinal tract. Gut 2011; 60: acids. World J Gastroenterol 2008; 14: 5620–5629.
1412–1425. 40. Claus SP, Tsang TM, Wang Y et al. Systemic multicompartmental effects
20. Sonnenburg JL, Xu J, Leip DD et al. Glycan foraging in vivo by an intestine- of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol
adapted bacterial symbiont. Science 2005; 307: 1955–1959. 2008; 4: 219.
21. Png CW, Linden SK, Gilshenan KS et al. Mucolytic bacteria with increased 41. Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and
prevalence in IBD mucosa augment in vitro utilization of mucin by other comparative metagenomic analysis of bile salt hydrolase activity in
bacteria. Am J Gastroenterol 2010; 105: 2420–2428. the human gut microbiome. Proc Natl Acad Sci U S A 2008; 105:
13580–13585.
22. Muegge BD, Kuczynski J, Knights D et al. Diet drives convergence in gut
microbiome functions across mammalian phylogeny and within humans. 42. Kellogg TF, Wostmann BS. Lipid metabolism. In: Coates ME, Gordon HA,
Wostmann BS (eds). The Germfree Animal in Research. New York:
Science 2011; 332: 970–974.
Academic Press, 1968; 181–196.
23. Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is
43. Wostmann BS. Intestinal bile acids and cholesterol absorption in the
linked to marked but reversible alterations in the mouse distal gut
germfree rat. J Nutr 1973; 103: 982–990.
microbiome. Cell Host Microbe 2008; 3: 213–223.
44. Abrams GD, Bishop JE. Effect of the normal microbial flora on
24. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect
gastrointestinal motility. Proc Soc Exp Biol Med 1967; 126: 301–304.
of diet on the human gut microbiome: a metagenomic analysis in
humanized gnotobiotic mice. Sci Transl Med 2009; 1: 6ra14. 45. Madsen D, Beaver M, Chang L, Bruckner-Kardoss E, Wostmann B. Analy-
sis of bile acids in conventional and germfree rats. J Lipid Res 1976; 17:
25. Walker AW, Ince J, Duncan SH et al. Dominant and diet-responsive groups
107–111.
of bacteria within the human colonic microbiota. ISME J 2011; 5: 220–230.
46. Miyata M, Yamakawa H, Hamatsu M, Kuribayashi H, Takamatsu Y,
26. Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Absence
Yamazoe Y. Enterobacteria modulate intestinal bile acid transport and
of intestinal microbiota does not protect mice from diet-induced obesity.
homeostasis through apical sodium-dependent bile acid transporter
Br J Nutr 2010; 104: 919–929. (SLC10A2) expression. J Pharmacol Exp Ther 2011; 336: 188–196.
27. Hildebrandt MA, Hoffmann C, Sherrill-Mix SA et al. High-fat diet deter- 47. Miyata M, Takamatsu Y, Kuribayashi H, Yamazoe Y. Administration of
mines the composition of the murine gut microbiome independently of ampicillin elevates hepatic primary bile acid synthesis through
obesity. Gastroenterology 2009; 137: 1716–1724. suppression of ileal fibroblast growth factor 15 expression. J Pharmacol
28. Murphy EF, Cotter PD, Healy S et al. Composition and energy harvesting Exp Ther 2009; 331: 1079–1085.
capacity of the gut microbiota: relationship to diet, obesity and time in 48. Vlahcevic ZR, Eggertsen G, Bjorkhem I, Hylemon PB, Redford K, Pandak
mouse models. Gut 2010; 59: 1635–1642. WM. Regulation of sterol 12alpha-hydroxylase and cholic acid
29. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut biosynthesis in the rat. Gastroenterology 2000; 118: 599–607.
microbes associated with obesity. Nature 2006; 444: 1022–1023. 49. Claus SP, Ellero SL, Berger B et al. Colonization-induced host-gut micro-
30. Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. bial metabolic interaction. MBio 2011; 2: e00271–10.
Reduced dietary intake of carbohydrates by obese subjects results in 50. Mataki C, Magnier BC, Houten SM et al. Compromised intestinal lipid
decreased concentrations of butyrate and butyrate-producing bacteria in absorption in mice with a liver-specific deficiency of liver receptor
feces. Appl Environ Microbiol 2007; 73: 1073–1078. homolog 1. Mol Cell Biol 2007; 27: 8330–8339.
31. Pouteau E, Nguyen P, Ballevre O, Krempf M. Production rates and 51. Iguchi Y, Yamaguchi M, Sato H, Kihira K, Nishimaki-Mogami T, Une M.
metabolism of short-chain fatty acids in the colon and whole body Bile alcohols function as the ligands of membrane-type bile acid-
using stable isotopes. Proc Nutr Soc 2003; 62: 87–93. activated G protein-coupled receptor. J Lipid Res 2010; 51: 1432–1441.
118 Kootte et al. Volume 14 No. 2 February 2012
DIABETES, OBESITY AND METABOLISM review article
52. Thomas C, Gioiello A, Noriega L et al. TGR5-mediated bile acid sensing 72. Verhoef SP, Meyer D, Westerterp KR. Effects of oligofructose on appetite
controls glucose homeostasis. Cell Metab 2009; 10: 167–177. profile, glucagon-like peptide 1 and peptide YY3-36 concentrations and
53. Watanabe M, Houten SM, Mataki C et al. Bile acids induce energy energy intake. Br J Nutr 2011; 106: 1757–1762.
expenditure by promoting intracellular thyroid hormone activation. 73. Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes
Nature 2006; 439: 484–489. satiety in healthy human: a pilot study. Eur J Clin Nutr 2006; 60: 567–572.
54. Maruyama T, Tanaka K, Suzuki J et al. Targeted disruption of G protein- 74. Morrison DJ, Mackay WG, Edwards CA, Preston T, Dodson B, Weaver LT.
coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol 2006; Butyrate production from oligofructose fermentation by the human faecal
191: 197–205. flora: what is the contribution of extracellular acetate and lactate? Br
55. Furet JP, Kong LC, Tap J et al. Differential adaptation of human gut J Nutr 2006; 96: 570–577.
microbiota to bariatric surgery-induced weight loss: links with metabolic 75. Jia W, Li H, Zhao L, Nicholson JK. Gut microbiota: a potential new territory
and low-grade inflammation markers. Diabetes 2010; 59: 3049–3057. for drug targeting. Nat Rev Drug Discov 2008; 7: 123–129.
56. Zhang H, DiBaise JK, Zuccolo A et al. Human gut microbiota in obesity 76. Rastall RA, Gibson GR, Gill HS et al. Modulation of the microbial ecology
and after gastric bypass. Proc Natl Acad Sci U S A 2009; 106: 2365–2370. of the human colon by probiotics, prebiotics and synbiotics to
57. Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host- enhance human health: an overview of enabling science and potential
archaeal-bacterial mutualism. Proc Natl Acad Sci U S A 2006; 103: applications. FEMS Microbiol Ecol 2005; 52: 145–152.
10011–10016. 77. Chen RM, Wu JJ, Lee SC, Huang AH, Wu HM. Increase of intestinal
58. Li JV, Ashrafian H, Bueter M et al. Metabolic surgery profoundly influences Bifidobacterium and suppression of coliform bacteria with short-term
gut microbial-host metabolic cross-talk. Gut 2011; 60: 1214–1223. yogurt ingestion. J Dairy Sci 1999; 82: 2308–2314.
59. Jeejeebhoy KN. Short bowel syndrome: a nutritional and medical 78. Fuentes S, Egert M, Jimenez-Valera M et al. Administration of Lactobacil-
approach. CMAJ 2002; 166: 1297–1302. lus casei and Lactobacillus plantarum affects the diversity of murine
intestinal lactobacilli, but not the overall bacterial community structure.
60. Hartman AL, Lough DM, Barupal DK et al. Human gut microbiome adopts Res Microbiol 2008; 159: 237–243.
an alternative state following small bowel transplantation. Proc Natl
Acad Sci U S A 2009; 106: 17187–17192. 79. Gardiner GE, Casey PG, Casey G et al. Relative ability of orally
administered Lactobacillus murinus to predominate and persist in
61. Booijink CC, El-Aidy S, Rajilic-Stojanovic M et al. High temporal and inter- the porcine gastrointestinal tract. Appl Environ Microbiol 2004; 70:
individual variation detected in the human ileal microbiota. Environ 1895–1906.
Microbiol 2010; 12: 3213–3227.
80. Goossens DA, Jonkers DM, Russel MG, Stobberingh EE, Stockbrugger RW.
62. Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and The effect of a probiotic drink with Lactobacillus plantarum 299v on
oligofructose and its consequences for gut health. Eur J Clin Nutr 2009; the bacterial composition in faeces and mucosal biopsies of rectum and
63: 1277–1289. ascending colon. Aliment Pharmacol Ther 2006; 23: 255–263.
63. Roberfroid M. Prebiotics: the concept revisited. J Nutr 2007; 137: 81. Martin FP, Wang Y, Sprenger N et al. Probiotic modulation of symbiotic
830S–837S. gut microbial-host metabolic interactions in a humanized microbiome
64. Cani PD, Amar J, Iglesias MA et al. Metabolic endotoxemia initiates mouse model. Mol Syst Biol 2008; 4: 157.
obesity and insulin resistance. Diabetes 2007; 56: 1761–1772. 82. Tannock GW, Munro K, Harmsen HJ, Welling GW, Smart J, Gopal PK.
65. Cani PD, Neyrinck AM, Fava F et al. Selective increases of bifidobacteria Analysis of the fecal microflora of human subjects consuming a probiotic
in gut microflora improve high-fat-diet-induced diabetes in mice through product containing Lactobacillus rhamnosus DR20. Appl Environ Microbiol
a mechanism associated with endotoxaemia. Diabetologia 2007; 50: 2000; 66: 2578–2588.
2374–2383. 83. Vahjen W, Taras D, Simon O. Effect of the probiotic Enterococcus faecium
66. Griffiths EA, Duffy LC, Schanbacher FL et al. In vivo effects of bifidobacteria NCIMB10415 on cell numbers of total Enterococcus spp., E. faecium and
and lactoferrin on gut endotoxin concentration and mucosal immunity in E. faecalis in the intestine of piglets. Curr Issues Intest Microbiol 2007; 8:
Balb/c mice. Dig Dis Sci 2004; 49: 579–589. 1–7.
67. Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects 84. van Baarlen P, Troost F, van der Meer C et al. Human mucosal in vivo
of a trans-galactooligosaccharide prebiotic on faecal microbiota and transcriptome responses to three lactobacilli indicate how probiotics
symptoms in irritable bowel syndrome. Aliment Pharmacol Ther 2009; may modulate human cellular pathways. Proc Natl Acad Sci U S A 2011;
29: 508–518. 108(Suppl. 1): 4562–4569.
68. Tuohy KM, Rouzaud GC, Bruck WM, Gibson GR. Modulation of the human 85. Konstantinov SR, Smidt H, de Vos WM et al. S layer protein A of
gut microflora towards improved health using prebiotics–assessment of Lactobacillus acidophilus NCFM regulates immature dendritic cell and
efficacy. Curr Pharm Des 2005; 11: 75–90. T cell functions. Proc Natl Acad Sci U S A 2008; 105: 19474–19479.
69. Cani PD, Lecourt E, Dewulf EM et al. Gut microbiota fermentation of 86. van Baarlen P, Troost FJ, van HS et al. Differential NF-kappaB pathways
prebiotics increases satietogenic and incretin gut peptide production induction by Lactobacillus plantarum in the duodenum of healthy humans
with consequences for appetite sensation and glucose response after a correlating with immune tolerance. Proc Natl Acad Sci U S A 2009; 106:
meal. Am J Clin Nutr 2009; 90: 1236–1243. 2371–2376.
70. Parnell JA, Reimer RA. Weight loss during oligofructose supplementation 87. Matsuzaki T, Yamazaki R, Hashimoto S, Yokokura T. Antidiabetic effects of
is associated with decreased ghrelin and increased peptide YY in an oral administration of Lactobacillus casei in a non-insulin-dependent
overweight and obese adults. Am J Clin Nutr 2009; 89: 1751–1759. diabetes mellitus (NIDDM) model using KK-Ay mice. Endocr J 1997; 44:
71. Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. 357–365.
Improvement of glucose tolerance and hepatic insulin sensitivity by 88. Tabuchi M, Ozaki M, Tamura A et al. Antidiabetic effect of Lactobacillus
oligofructose requires a functional glucagon-like peptide 1 receptor. GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem
Diabetes 2006; 55: 1484–1490. 2003; 67: 1421–1424.
Volume 14 No. 2 February 2012 doi:10.1111/j.1463-1326.2011.01483.x 119
review article DIABETES, OBESITY AND METABOLISM
89. Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing 104. Antonopoulos DA, Huse SM, Morrison HG, Schmidt TM, Sogin ML,
Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Young VB. Reproducible community dynamics of the gastrointestinal
Nutrition 2007; 23: 62–68. microbiota following antibiotic perturbation. Infect Immun 2009; 77:
90. Naito E, Yoshida Y, Makino K et al. Beneficial effect of oral administration 2367–2375.
of Lactobacillus casei strain Shirota on insulin resistance in diet-induced 105. Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term ecological impacts
obesity mice. J Appl Microbiol 2011; 110: 650–657. of antibiotic administration on the human intestinal microbiota. ISME J
91. Lee HY, Park JH, Seok SH et al. Human originated bacteria, Lactobacillus 2007; 1: 56–66.
rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity 106. Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of
effects in diet-induced obese mice. Biochim Biophys Acta 2006; 1761: antibiotic exposure on the human intestinal microbiota. Microbiology
736–744. 2010; 156: 3216–3223.
92. Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on 107. Begin-Heck N, Bourassa M, Heick HM. The effect of oxytetracycline on
body weight and adipose tissue mass in diet-induced overweight rats. insulin resistance in obese mice. Biochem J 1974; 142: 465–475.
J Microbiol 2010; 48: 712–714. 108. Dalpe-Scott M, Heick HM, Begin-Heick N. Oxytetracycline treatment
93. Angelakis E, Raoult D. The increase of Lactobacillus species in the gut improves the response to insulin in the spontaneously diabetic (BB)
flora of newborn broiler chicks and ducks is associated with weight gain. rat. Diabetes 1982; 31: 53–59.
PLoS One 2010; 5: e10463. 109. Dubuc PU, Willis PL. Age dependent effects of oxytetracycline in ob/ob
94. Khan M, Raoult D, Richet H, Lepidi H, La SB. Growth-promoting effects of mice. Diabetologia 1978; 14: 129–133.
single-dose intragastrically administered probiotics in chickens. Br Poult 110. Membrez M, Blancher F, Jaquet M et al. Gut microbiota modulation with
Sci 2007; 48: 732–735. norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J
95. Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring 2008; 22: 2416–2426.
bacterial community of human gut microbiota reveals an increase in 111. Cani PD, Bibiloni R, Knauf C et al. Changes in gut microbiota control
Lactobacillus in obese patients and Methanogens in anorexic patients. metabolic endotoxemia-induced inflammation in high-fat diet-induced
PLoS One 2009; 4: e7125. obesity and diabetes in mice. Diabetes 2008; 57: 1470–1481.
96. Chouraqui JP, Grathwohl D, Labaune JM et al. Assessment of the safety, 112. Yap IK, Li JV, Saric J et al. Metabonomic and microbiological analysis of
tolerance, and protective effect against diarrhea of infant formulas the dynamic effect of vancomycin-induced gut microbiota modification
containing mixtures of probiotics or probiotics and prebiotics in a in the mouse. J Proteome Res 2008; 7: 3718–3728.
randomized controlled trial. Am J Clin Nutr 2008; 87: 1365–1373.
113. Bender A, Breves G, Stein J, Leonhard-Marek S, Schroder B, Winckler C.
97. Guandalini S, Pensabene L, Zikri MA et al. Lactobacillus GG administered Colonic fermentation as affected by antibiotics and acidic pH: Application
in oral rehydration solution to children with acute diarrhea: a multicenter of an in vitro model. Z Gastroenterol 2001; 39: 911–918.
European trial. J Pediatr Gastroenterol Nutr 2000; 30: 54–60.
114. Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood
98. Bielecka M, Biedrzycka A, Majkowska A. Selection of probiotics and overweight after establishment of the gut microbiota: the role of delivery
prebiotics for synbiotics and confirmation of their in vivo effectiveness. mode, pre-pregnancy weight and early administration of antibiotics. Int
Food Res Int 2002; 35: 125–131. J Obes (Lond) 2011; 35: 522–529.
99. Silvi S, Rumney CJ, Cresci A, Rowland IR. Resistant starch modifies gut 115. Thuny F, Richet H, Casalta JP, Angelakis E, Habib G, Raoult D. Vancomycin
microflora and microbial metabolism in human flora-associated rats treatment of infective endocarditis is linked with recently acquired
inoculated with faeces from Italian and UK donors. J Appl Microbiol 1999; obesity. PLoS One 2010; 5: e9074.
86: 521–530.
116. Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-
100. Crittenden RG, Morris LF, Harvey ML, Tran LT, Mitchell HL, Playne MJ. associated enteric disease. Ann Intern Med 2006; 145: 758–764.
Selection of a Bifidobacterium strain to complement resistant starch
in a synbiotic yoghurt. J Appl Microbiol 2001; 90: 268–278. 117. Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct
in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:
101. Rastall RA, Maitin V. Prebiotics and synbiotics: towards the next 854–859.
generation. Curr Opin Biotechnol 2002; 13: 490–496.
118. Gerard P, Beguet F, Lepercq P et al. Gnotobiotic rats harboring human
102. De BP, Wouters R, Verstraete W. Combined use of Lactobacillus reuteri intestinal microbiota as a model for studying cholesterol-to-coprostanol
and soygerm powder as food supplement. Lett Appl Microbiol 2001; 33: conversion. FEMS Microbiol Ecol 2004; 47: 337–343.
420–424.
119. Pang X, Hua X, Yang Q et al. Inter-species transplantation of gut
103. Gmeiner M, Kneifel W, Kulbe KD et al. Influence of a synbiotic mixture microbiota from human to pigs. ISME J 2007; 1: 156–162.
consisting of Lactobacillus acidophilus 74–2 and a fructooligosaccharide
preparation on the microbial ecology sustained in a simulation of the 120. Vrieze A, Holleman F, Serlie MJ et al. Metabolic effects of transplanting
human intestinal microbial ecosystem (SHIME reactor). Appl Microbiol gut microbiota from lean donors to subjects with metabolic syndrome.
Biotechnol 2000; 53: 219–223 Diabetologia 2010; 53: S44.
120 Kootte et al. Volume 14 No. 2 February 2012
Copyright of Diabetes, Obesity & Metabolism is the property of Wiley-Blackwell and its content may not be
copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written
permission. However, users may print, download, or email articles for individual use.
You might also like
- Production of Insulin Using Recombinant Dna TechnologyDocument16 pagesProduction of Insulin Using Recombinant Dna Technologyravindrababu2908No ratings yet
- BP203T Bichemistry 100 MCQsDocument16 pagesBP203T Bichemistry 100 MCQsShubhrat MaheshwariNo ratings yet
- 1 s2.0 S1521691813000619 Main Obezitatea Si MicrobiotaDocument11 pages1 s2.0 S1521691813000619 Main Obezitatea Si MicrobiotaLUTICHIEVICI NATALIANo ratings yet
- Importance of Nutritional Therapy in The Management of Intestinal Diseases: Beyond Energy and Nutrient SupplyDocument12 pagesImportance of Nutritional Therapy in The Management of Intestinal Diseases: Beyond Energy and Nutrient SupplyIsabella María GantivarNo ratings yet
- Fibras e MicrobiotaDocument10 pagesFibras e Microbiotataelesoliveira.tNo ratings yet
- Xie Et Al 2023 A Major Mechanism For ImmunomodulationDocument13 pagesXie Et Al 2023 A Major Mechanism For Immunomodulationtaha242004No ratings yet
- Linking Gut Microbiota and Inflammation To Obesity and Insulin ResistanceDocument11 pagesLinking Gut Microbiota and Inflammation To Obesity and Insulin ResistanceDr LoloNo ratings yet
- Up-Regulating The Human Intestinal Microbiome Using Whole Plant Foods, Polyphenols, And/or FiberDocument7 pagesUp-Regulating The Human Intestinal Microbiome Using Whole Plant Foods, Polyphenols, And/or FiberjosetelhadoNo ratings yet
- Nutrients 15 00332 v2Document17 pagesNutrients 15 00332 v2koehler.thereseNo ratings yet
- Os Efeitos Do Comportamento Murino de Lactobacillus Helveticuson Dependem Da Dieta e GenótipoDocument10 pagesOs Efeitos Do Comportamento Murino de Lactobacillus Helveticuson Dependem Da Dieta e Genótipoj238111No ratings yet
- Hiel 2019 Effects of A Diet Based On Inulin-Rich Vegetables On Gut Health and Nutritional Behavior in Healthy Humans.Document13 pagesHiel 2019 Effects of A Diet Based On Inulin-Rich Vegetables On Gut Health and Nutritional Behavior in Healthy Humans.maria alejandraNo ratings yet
- When To Eat The Influence of Circadian Rhythms On Metabolic Health Are Animal Studies Providing The EvidenceDocument14 pagesWhen To Eat The Influence of Circadian Rhythms On Metabolic Health Are Animal Studies Providing The EvidenceAndré LeiteNo ratings yet
- Gut-Microbiome-Obesity-And-Metabolic-DysfunctionDocument8 pagesGut-Microbiome-Obesity-And-Metabolic-DysfunctionSandraosoNo ratings yet
- The Role of Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Pathways of MechanismsDocument8 pagesThe Role of Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Pathways of MechanismsMuliaNo ratings yet
- Artigo 1Document13 pagesArtigo 1Ana Cláudia MatiasNo ratings yet
- Metabolites 13 00410 v2Document15 pagesMetabolites 13 00410 v2Nejc KovačNo ratings yet
- 3 - You Are What You Eat, Diet, HealthDocument22 pages3 - You Are What You Eat, Diet, HealthPaula CantalapiedraNo ratings yet
- Ayuno Intermitente 2021Document15 pagesAyuno Intermitente 2021draingridfeijooNo ratings yet
- Reviews: You Are What You Eat: Diet, Health and The Gut MicrobiotaDocument22 pagesReviews: You Are What You Eat: Diet, Health and The Gut MicrobiotaHarry YucraNo ratings yet
- NIH Public Access: Author ManuscriptDocument19 pagesNIH Public Access: Author ManuscriptEdmar SilvaNo ratings yet
- Nutrients: Can Gut Microbiota Composition Predict Response To Dietary Treatments?Document15 pagesNutrients: Can Gut Microbiota Composition Predict Response To Dietary Treatments?Meisya Nur'ainiNo ratings yet
- Gut Kidney 1Document10 pagesGut Kidney 1AlfirahmatikaNo ratings yet
- Acellular CarbsDocument15 pagesAcellular CarbsstarmittNo ratings yet
- The Kidney Gut Axis Implications For Nutrition Care 2015 Megan RossiDocument5 pagesThe Kidney Gut Axis Implications For Nutrition Care 2015 Megan RossiWobik HopeNo ratings yet
- Gut Microbiota in The Pathogenesis and TherapeuticDocument13 pagesGut Microbiota in The Pathogenesis and TherapeuticDaniel SilvaNo ratings yet
- Review Article Crosstalk Between The Ketogenic Diet and Epilepsy: From The Perspective of Gut MicrobiotaDocument10 pagesReview Article Crosstalk Between The Ketogenic Diet and Epilepsy: From The Perspective of Gut Microbiotarince restivionaNo ratings yet
- Gut MicrobiomeDocument28 pagesGut MicrobiomeIce burgNo ratings yet
- Nutrition, The Gut Microbiome and The Metabolic SyndromeDocument14 pagesNutrition, The Gut Microbiome and The Metabolic SyndromeM. Tasrif MansurNo ratings yet
- Diabetic Cats and Lack of Butyrate 2019Document13 pagesDiabetic Cats and Lack of Butyrate 2019Paola LorrayneNo ratings yet
- (1479683X - European Journal of Endocrinology) MECHANISMS in ENDOCRINOLOGY - Gut Microbiota in Patients With Type 2 Diabetes MellitusDocument11 pages(1479683X - European Journal of Endocrinology) MECHANISMS in ENDOCRINOLOGY - Gut Microbiota in Patients With Type 2 Diabetes MellitusIryaniNo ratings yet
- Obesity and The Human Microbiome: Ruth E. LeyDocument7 pagesObesity and The Human Microbiome: Ruth E. Leyanka_mihaelaNo ratings yet
- Gut Microbiota-Derived Metabolites As Central Regulators in Metabolic DisordersDocument9 pagesGut Microbiota-Derived Metabolites As Central Regulators in Metabolic DisordersZaira TJNo ratings yet
- The Role of The FODMAP Diet in IBSDocument24 pagesThe Role of The FODMAP Diet in IBSMércia FiuzaNo ratings yet
- Muilwijk 2023 The Entero Endocrine Response FolloDocument18 pagesMuilwijk 2023 The Entero Endocrine Response FolloMuôngNo ratings yet
- Fnut 08 758518Document19 pagesFnut 08 758518HitmanNo ratings yet
- Aliment Pharmacol Ther - 2020 - Logan - Analysis of 61 Exclusive Enteral Nutrition Formulas Used in The Management ofDocument13 pagesAliment Pharmacol Ther - 2020 - Logan - Analysis of 61 Exclusive Enteral Nutrition Formulas Used in The Management ofRohma YuliaNo ratings yet
- Bioensayo PDFDocument7 pagesBioensayo PDFSilvia FernándezNo ratings yet
- International Archives of Medicine: Fat Feeding Potentiates The Diabetogenic Effect of Dexamethasone in Wistar RatsDocument12 pagesInternational Archives of Medicine: Fat Feeding Potentiates The Diabetogenic Effect of Dexamethasone in Wistar RatsShanmugam SivabalanNo ratings yet
- NBHsDocument7 pagesNBHsBram KNo ratings yet
- Beneficial Effects of Exercise On Gut Microbiota.. and Gut-Liver 2019Document20 pagesBeneficial Effects of Exercise On Gut Microbiota.. and Gut-Liver 2019Vera Brok-VolchanskayaNo ratings yet
- CLN 65p635Document10 pagesCLN 65p635Priady Wira PrasetiaNo ratings yet
- Diet-Microbiota Interactions and Personalized NutritionDocument12 pagesDiet-Microbiota Interactions and Personalized NutritionTelmoNo ratings yet
- Mechanistic and Therapeutic Advances in Non-Alcoholic Fatty 2018Document13 pagesMechanistic and Therapeutic Advances in Non-Alcoholic Fatty 2018Vera Brok-VolchanskayaNo ratings yet
- Sciencedirect: Caloric Dose-Responsive Genes in Blood Cells Differentiate The Metabolic Status of Obese MenDocument10 pagesSciencedirect: Caloric Dose-Responsive Genes in Blood Cells Differentiate The Metabolic Status of Obese MenWa Ode Umrawati LatifNo ratings yet
- 1 s2.0 S0753332222010496 MainDocument10 pages1 s2.0 S0753332222010496 MainHiếu Nguyễn TrọngNo ratings yet
- Áteresztő Bél Sy És DMDocument12 pagesÁteresztő Bél Sy És DMDaniella FülöpNo ratings yet
- Fimmu 2017 01159Document17 pagesFimmu 2017 01159THAIS LENINE DE ALBUQUERQUENo ratings yet
- Microbiota y GlutenDocument15 pagesMicrobiota y GlutenAlexandra Mondragón SernaNo ratings yet
- 1 s2.0 S1550413114000655 MainDocument13 pages1 s2.0 S1550413114000655 MainDesi AdestiNo ratings yet
- The Beneficial Effects of Dietary Interventions On Gut MicrobiotaDocument33 pagesThe Beneficial Effects of Dietary Interventions On Gut MicrobiotaEdwin Giancarlo Hidalgo ArroyoNo ratings yet
- 2020 Bascuñán K. Dietary Gluten As A Conditioning Factor of The Gut Microbiota in Celiac DiseaseDocument15 pages2020 Bascuñán K. Dietary Gluten As A Conditioning Factor of The Gut Microbiota in Celiac DiseaseJimena AyalaNo ratings yet
- Intestinal Permeability in Children With CeliacDocument15 pagesIntestinal Permeability in Children With CeliacNejc KovačNo ratings yet
- Nutrients 14 03985 v2Document17 pagesNutrients 14 03985 v2Lisa GonçalvesNo ratings yet
- Mecanismo Dos ProbioticosDocument10 pagesMecanismo Dos ProbioticosFernanda ToledoNo ratings yet
- Diet Influences The Functions of The HumDocument15 pagesDiet Influences The Functions of The HumJULIETANo ratings yet
- Microorganisms 10 00452 v2Document15 pagesMicroorganisms 10 00452 v2V.EricSson BaPtisTaNo ratings yet
- DietaDocument12 pagesDietaSilviaNo ratings yet
- Pulse Crop Effects On Gut Microbial Populations, Intestinal Function, and Adiposity in A Mouse Model of Diet-Induced Obesity John N. McginleyDocument40 pagesPulse Crop Effects On Gut Microbial Populations, Intestinal Function, and Adiposity in A Mouse Model of Diet-Induced Obesity John N. Mcginleyzora.mendez637100% (9)
- Angelova 2013 Review Models of Obesity and Metabolic SyndromeDocument8 pagesAngelova 2013 Review Models of Obesity and Metabolic SyndromePaul SimononNo ratings yet
- 1 s2.0 S0271531712000176 MainDocument8 pages1 s2.0 S0271531712000176 MainEdwin RizoNo ratings yet
- Canfora 2019Document13 pagesCanfora 2019salamiNo ratings yet
- Dietary Fibre Functionality in Food and Nutraceuticals: From Plant to GutFrom EverandDietary Fibre Functionality in Food and Nutraceuticals: From Plant to GutFarah HosseinianNo ratings yet
- NUTRI Jejum Curto Melhora Regeneração Do Intestino Moscas CELL 2018Document16 pagesNUTRI Jejum Curto Melhora Regeneração Do Intestino Moscas CELL 2018alegmachadoNo ratings yet
- Gut Microbiota Modulation As A Possible Mediating Mechanism For Fasting-Induced Alleviation of Metabolic Complications: A Systematic ReviewDocument17 pagesGut Microbiota Modulation As A Possible Mediating Mechanism For Fasting-Induced Alleviation of Metabolic Complications: A Systematic ReviewalegmachadoNo ratings yet
- NUTRI Microbiota e Doença Metabólica 2018 OPENDocument15 pagesNUTRI Microbiota e Doença Metabólica 2018 OPENalegmachadoNo ratings yet
- Descarboxilação - CinéticaDocument9 pagesDescarboxilação - CinéticaalegmachadoNo ratings yet
- Urea Berthelot KitDocument2 pagesUrea Berthelot KitDinesh SreedharanNo ratings yet
- HEMOSTASISDocument12 pagesHEMOSTASISRyan PedregosaNo ratings yet
- Okra The Thesis PDFDocument62 pagesOkra The Thesis PDFAfia SayeedNo ratings yet
- Energy Balance and Body Composition PPT 2Document5 pagesEnergy Balance and Body Composition PPT 2Nisa MustafaNo ratings yet
- Carbohydrate MetabolismDocument19 pagesCarbohydrate MetabolismAbhithNo ratings yet
- Bio 307 Outline 21-22Document2 pagesBio 307 Outline 21-22Metehan KaraNo ratings yet
- Mucosta Drug Interactions - MIMS MalaysiaDocument4 pagesMucosta Drug Interactions - MIMS MalaysiaEsy LNo ratings yet
- Worksheet Q3 Week 4&5 PDFDocument4 pagesWorksheet Q3 Week 4&5 PDFJaybie TejadaNo ratings yet
- Enzyme/Receptor-Ligand Interactions and Its AnalysisDocument20 pagesEnzyme/Receptor-Ligand Interactions and Its AnalysisRaman ChandelNo ratings yet
- (Latest) Proforma STBP2203 Amali Biokimia Asas Sem 2 20182019Document4 pages(Latest) Proforma STBP2203 Amali Biokimia Asas Sem 2 20182019Koay Kian YewNo ratings yet
- Bone Marrow-Derived Progenitors, 2007 PDFDocument293 pagesBone Marrow-Derived Progenitors, 2007 PDFFabrício CamargoNo ratings yet
- ImrulDocument5 pagesImrulJobaer ShaonNo ratings yet
- A Level Biology Essay Model AnswersDocument4 pagesA Level Biology Essay Model AnswersKFCStadivari100% (1)
- BIO 11 Lab 02 2004 Enz. Copy - DoDocument10 pagesBIO 11 Lab 02 2004 Enz. Copy - DoKizzy Anne Boatswain CarbonNo ratings yet
- Chapter 5 Molecular Diffusion in Biological Solutions & GelsDocument14 pagesChapter 5 Molecular Diffusion in Biological Solutions & Gelsrushdi100% (2)
- Ijms 20 01358Document24 pagesIjms 20 01358CHETAN SOMUNo ratings yet
- Lecture 23 - EicosanoidsDocument22 pagesLecture 23 - Eicosanoidsapi-3703352100% (1)
- Page4 Rnaseq SampleDocument12 pagesPage4 Rnaseq SampleZhang ChengNo ratings yet
- Bacterial Endotoxin Testing in The LaboratoryDocument16 pagesBacterial Endotoxin Testing in The LaboratoryAvnish0003No ratings yet
- Biological Membranes and Transport and BiosignalingDocument34 pagesBiological Membranes and Transport and BiosignalingBONAVENTURA ALVINO DESMONDANo ratings yet
- 2 Introduction To MetabolismDocument17 pages2 Introduction To MetabolismBryan DellomosNo ratings yet
- RNA and Protein Synthesis QuizDocument6 pagesRNA and Protein Synthesis QuizJeremy Guggenheim100% (2)
- BC 367 Experiment 4 Kinetic Properties of Acid Phosphatase: Roh + Hpo Phosphatase + H ODocument8 pagesBC 367 Experiment 4 Kinetic Properties of Acid Phosphatase: Roh + Hpo Phosphatase + H OAliceKeikoNo ratings yet
- Artigo de ImunoDocument8 pagesArtigo de ImunoEverlyn Batista Gregório LimaNo ratings yet
- Environmental Biotechnology - BBTechDocument5 pagesEnvironmental Biotechnology - BBTechBekele OljiraNo ratings yet
- NEJM vonWDDocument14 pagesNEJM vonWDMonica Aleja FernándezNo ratings yet
- CORONA-SARS Detection BASED ON SPOT-CHECK PCR - PSA-PASTEUR TEST - V.0120Document22 pagesCORONA-SARS Detection BASED ON SPOT-CHECK PCR - PSA-PASTEUR TEST - V.0120Thanh DoNo ratings yet
- Fat MetabolismDocument14 pagesFat MetabolismDimakatso Nkanyezi DubazaneNo ratings yet