Professional Documents
Culture Documents
2.9 Eutectic Phase Diagrams
2.9 Eutectic Phase Diagrams
Uploaded by
Guilherme Dos Santos Moreira0 ratings0% found this document useful (0 votes)
2 views2 pagesOriginal Title
r_se16
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
2 views2 pages2.9 Eutectic Phase Diagrams
2.9 Eutectic Phase Diagrams
Uploaded by
Guilherme Dos Santos MoreiraCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 2
2.
9 Eutectic phase diagrams 33
2.9 Eutectic phase diagrams
Materials showing a eutectic phase diagram often are technically highly relevant.
Eutectic is Greek and means low/well melting. One of the
most important eutectic’s is tin-lead (Sn-Pb) used for solder-
ing. Another interesting example is indium-gallium (InGa)
which is liquid just above room temperature and allows for
easy ohmic contacts on semiconductors like Si. The tin lead
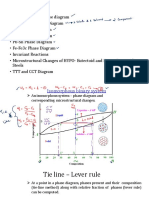
phase diagram is shown in Fig. 2.12 a). The eutectic point
(marked by E) has a temperature below 200o C and a Sn con-
centration somewhat above 70%. The eutectic composition
is melting completely at the lowest temperature. The melt
will solidify directly without splitting up into two phases. As
a consequence the eutectic composition does not have to be
cooled down extremely slowly in order to get a reasonably
homogeneous solid. We will find nicely ordered lamellas of
Pb and Sn as shown in Fig. 2.12 b).
In order to understand a eutectic phase diagram we have
to combine the g-plots of mixtures with a miscibility gap as
shown in Fig. 2.1 b) and the g-plots of mixtures defining the
a) liquidus and solidus lines as shown in Fig. 2.11.
As discussed in section 2.1 a miscibility gap occurs for a
dominant repulsive interaction between the two components
Figure 2.12: a) Eutectic phase diagram of PbSn. b) of a mixture. With increasing temperature the entropy gets
lamellar structure of solidified Pb Sn with eutectic more dominant, reducing the regime of phase separation un-
composition. til at a certain high temperature (Ω/R T = β = 2) phase
separation vanishes and complete miscibility exists. A eu-
tectic phase diagram is found if the Gibbs potential of the solid phase with a miscibility gap becomes larger than
the Gibbs potential of the liquid phase. The relation between the eutectic phase diagram and the g-plots for several
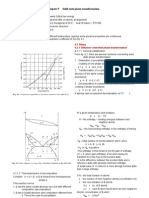
temperatures is shown in Fig. 2.13.
First we will repeat some basics about labeling phases. As usual homogeneous phases are labeled by Greek char-
acters. Here α is a Pb rich phase with a small amount of solved Sn. Correspondingly β is a Sn rich phase with
a small fraction of solved Pb. In between we have a phase separation of both phases α + β with a composition
following the lever rule. The phase boundaries here therefore mark the limits of solubility in solid solution. At
eutectic temperature the Gibbs potential of the liquid phase L tangents the Maxwell line of phase separation α + β
implying the beginning of a phase separation between L +α and L +β. At the eutectic point we have on common
tangent (representing one chemical potential) between three points with fixed compositions at a fixed temperature
Teut . Therefore the horizontal straight line connection the three phases in the phase diagram has F = 0, i.e. no
degrees of freedom.
Above the eutectic temperature two regimes with phase separation exist representing the two common tangents in
the g-plots for T1 and T2 indicating mixtures of liquid and solid phases α resp. β. For different temperatures the
thermodynamically stable composition of the phases α resp. β change. To reach always this stable composition
takes long times within a solid. This is the reason why cooling down at a non eutectic composition results in a
solid with a very inhomogeneous concentration distribution of Pb and Sn (if not cooled down infinitely slowly). In
strong contrast cooling down at eutectic composition leads to a solidification at lowest possible temperature with
a constant eutectic composition of the solid and a micro structure as illustrated in Fig. 2.12 b). Here the lamellar
structure basically reflects just the repulsive interaction between Pb and Sn.
So it is the eutectic reaction
L→α+β (2.66)
which allows for an efficient stable soldering process.
34 Advanced properties of mixing
Figure 2.13: Schematic eutectic phase diagram. For the 5 temperatures the corresponding Gibbs potentials and
Maxwell constructions for phase separation are shown.
You might also like
- QA/QC ManualDocument42 pagesQA/QC ManualGuilherme Dos Santos Moreira100% (1)
- Lab 05 Modeling of Mechanical Systems Using Simscap Multibody 2nd Generation Part 1Document19 pagesLab 05 Modeling of Mechanical Systems Using Simscap Multibody 2nd Generation Part 1Reem GheithNo ratings yet
- Question & Answer Set-7Document12 pagesQuestion & Answer Set-7eeng.ali651550% (2)
- Basics of The CMM 120Document4 pagesBasics of The CMM 120cqi9nNo ratings yet
- Lecture No.17 Binary Phase DiagramsDocument16 pagesLecture No.17 Binary Phase DiagramsNorma Luzmila Chambilla FirataNo ratings yet
- MSE 260 Assignment 3Document3 pagesMSE 260 Assignment 3michael ananaNo ratings yet
- Department of MME BUET, DhakaDocument18 pagesDepartment of MME BUET, DhakaHussain Mohammad ImranNo ratings yet
- Note CHP 2-Material Science 281 Uitm Em110Document74 pagesNote CHP 2-Material Science 281 Uitm Em110bino_rye100% (1)
- Materials Science - Alloys System - 2Document16 pagesMaterials Science - Alloys System - 2Sumit JoshiNo ratings yet
- Ch. 9 Phase DiagramsDocument9 pagesCh. 9 Phase Diagramsravi hargassnerNo ratings yet
- Monotectic AlloysDocument8 pagesMonotectic Alloysandrea usugaNo ratings yet
- MSM-3 Phases in Solids (Part - II Invariant Systems)Document13 pagesMSM-3 Phases in Solids (Part - II Invariant Systems)Shashank SinghNo ratings yet
- N The V: Organometallic CompoundsDocument12 pagesN The V: Organometallic CompoundslaythNo ratings yet
- MM235 - Phase Diagram - SMDocument18 pagesMM235 - Phase Diagram - SMUtkarsh MishraNo ratings yet
- Lesson 5 PDFDocument32 pagesLesson 5 PDFMajak MarialNo ratings yet
- Phase Diagram ExDocument23 pagesPhase Diagram ExTey KaijingNo ratings yet
- Eutectic Phase Diagram PDFDocument2 pagesEutectic Phase Diagram PDFEugene0% (1)
- Biomaterials II Lec 5Document13 pagesBiomaterials II Lec 5m9trdk92ksNo ratings yet
- Chapter-7: The Iron-Iron Carbide Equilibrium DiagramDocument9 pagesChapter-7: The Iron-Iron Carbide Equilibrium DiagramHeaven in Home StudentNo ratings yet
- QED Coherence and Electrolyte SolutionsDocument7 pagesQED Coherence and Electrolyte SolutionsScribd PipottNo ratings yet
- Me8491 QB 02Document30 pagesMe8491 QB 02Dr.A.Maniram KumarNo ratings yet
- Intermediate Phases or CompoundsDocument3 pagesIntermediate Phases or CompoundsRaja AKNo ratings yet
- PhaseDocument6 pagesPhasefarooq_bagbanNo ratings yet
- Chemistry PQMS2Document9 pagesChemistry PQMS2Shahid SayeedNo ratings yet
- MetalesDocument58 pagesMetalesHéctor UnzuetaNo ratings yet
- 3 Phase DiagramsDocument27 pages3 Phase Diagramskrishna tejaNo ratings yet
- Diffused Double LayerDocument4 pagesDiffused Double LayerRaja GNo ratings yet
- Phase Diagram - : Dr. Aneela Wakeel 02-01-2018Document42 pagesPhase Diagram - : Dr. Aneela Wakeel 02-01-2018Hassan KhanNo ratings yet
- PQT Chapter 9a Phase DiagramsDocument53 pagesPQT Chapter 9a Phase DiagramsDương Hữu PhươngNo ratings yet
- Diagramas de FaseDocument20 pagesDiagramas de FaseRuben Jose VARGASNo ratings yet
- Phase Diagram - : Dr. Aneela WakeelDocument21 pagesPhase Diagram - : Dr. Aneela WakeelHassan KhanNo ratings yet
- Lab 7 - Phase DiagramsDocument7 pagesLab 7 - Phase Diagramsabd333No ratings yet
- Chapter9 p2c PDFDocument9 pagesChapter9 p2c PDFAbdillah ShalihNo ratings yet
- Eutectic Solution PDFDocument2 pagesEutectic Solution PDFMelissaNo ratings yet
- Atkins, Solution, 7th EdDocument15 pagesAtkins, Solution, 7th Edapi-3723327No ratings yet
- Wk7 (2) - Phase Diagrams (Case 2)Document10 pagesWk7 (2) - Phase Diagrams (Case 2)saeed khaledNo ratings yet
- Richard S. Treptowed-The Lead-Acid Battery Its Voltage in Theory and in Practice 079p334 PDFDocument5 pagesRichard S. Treptowed-The Lead-Acid Battery Its Voltage in Theory and in Practice 079p334 PDFJohanMonNo ratings yet
- A Comparative Study of The Bonding of N and CO To Ru (001) and The Role of 5 Orbital in Their Molecular Vibrational Frequency ChangesDocument3 pagesA Comparative Study of The Bonding of N and CO To Ru (001) and The Role of 5 Orbital in Their Molecular Vibrational Frequency ChangesTrần Duy TânNo ratings yet
- Undercooling ListDocument16 pagesUndercooling List이상벽No ratings yet
- Eng Mat Chapter 4Document126 pagesEng Mat Chapter 4VC Chua Yee LeongNo ratings yet
- 2 5391019718461098174Document7 pages2 5391019718461098174Motaz TharwatNo ratings yet
- Carlini 2004Document6 pagesCarlini 2004Raira San JoseNo ratings yet
- 2 Metal and Semiconductor Electrodes: 2.1 MetalsDocument11 pages2 Metal and Semiconductor Electrodes: 2.1 MetalsPopescu Viorel-MihaiNo ratings yet
- Beam With Settlement 2Document14 pagesBeam With Settlement 2Soro PenoNo ratings yet
- Carbon Nanotube: Properties and ApplicationsDocument22 pagesCarbon Nanotube: Properties and ApplicationsmanuNo ratings yet
- Che 414Document21 pagesChe 414Looking forwardNo ratings yet
- 4Document63 pages4ARJUN PESARU 19BME0161No ratings yet
- Class Notes For Making RugsDocument2 pagesClass Notes For Making RugsdakNo ratings yet
- Chapter 9 Solid State Phase TransformationDocument18 pagesChapter 9 Solid State Phase Transformationpoom2007No ratings yet
- Catalyst Nilkel-MainDocument7 pagesCatalyst Nilkel-Mainsyarif hidayatNo ratings yet
- Sheet 5 - Phase Diagrams IIDocument2 pagesSheet 5 - Phase Diagrams IIroro98syamNo ratings yet
- P-T Effects On Melting and CrystallizationDocument17 pagesP-T Effects On Melting and CrystallizationhimeNo ratings yet
- Phase Rule 2Document17 pagesPhase Rule 2SarojNo ratings yet
- PH4211 Statistical Mechanics: Problem Sheet 1Document129 pagesPH4211 Statistical Mechanics: Problem Sheet 1Abhigyan HazarikaNo ratings yet
- Hsslive Xi Chem Model 2020 KeyDocument5 pagesHsslive Xi Chem Model 2020 KeyAndrewNo ratings yet
- Wang - 2022 - PRL - Gigantic Magnetochiral Anisotropy in The Topological Semimetal (ZrTe5)Document5 pagesWang - 2022 - PRL - Gigantic Magnetochiral Anisotropy in The Topological Semimetal (ZrTe5)A SINo ratings yet
- Wave Propagation and Dispersion Characteristics in Anisotropic MediumDocument15 pagesWave Propagation and Dispersion Characteristics in Anisotropic MediumqwerrfsNo ratings yet
- 104 PhaseDiags QS2AnsDocument6 pages104 PhaseDiags QS2Ansnilanga123No ratings yet
- Redox (Housecroft) PDFDocument22 pagesRedox (Housecroft) PDFMark Adam FerryNo ratings yet
- Electronic Structure of Molecules: Diatomic Molecules, Small Molecules, Saturated Hydrocarbons, Conjugated Molecules, Molecules of Biochemical InterestFrom EverandElectronic Structure of Molecules: Diatomic Molecules, Small Molecules, Saturated Hydrocarbons, Conjugated Molecules, Molecules of Biochemical InterestNo ratings yet
- Supercapacitors Based on Carbon or Pseudocapacitive MaterialsFrom EverandSupercapacitors Based on Carbon or Pseudocapacitive MaterialsNo ratings yet
- Soils as a Key Component of the Critical Zone 3: Soils and Water CirculationFrom EverandSoils as a Key Component of the Critical Zone 3: Soils and Water CirculationGuilhem BourriéNo ratings yet
- Brochure Gpe Qpol-Accessories enDocument8 pagesBrochure Gpe Qpol-Accessories enGuilherme Dos Santos MoreiraNo ratings yet
- Caustic Cracking - Incidents in RefineriesDocument2 pagesCaustic Cracking - Incidents in RefineriesGuilherme Dos Santos MoreiraNo ratings yet
- 3D Inspection + SMTDocument4 pages3D Inspection + SMTGuilherme Dos Santos MoreiraNo ratings yet
- The Chemical Elements of A Smartphone: Click To EnlargeDocument4 pagesThe Chemical Elements of A Smartphone: Click To EnlargeGuilherme Dos Santos MoreiraNo ratings yet
- SPI Inspection Techniques in The SMT LineDocument5 pagesSPI Inspection Techniques in The SMT LineGuilherme Dos Santos MoreiraNo ratings yet
- SMD-PComponents Soldering Practice BoardDocument20 pagesSMD-PComponents Soldering Practice BoardGuilherme Dos Santos MoreiraNo ratings yet
- 11 Abstract Lya OkulovDocument1 page11 Abstract Lya OkulovGuilherme Dos Santos MoreiraNo ratings yet
- ARTIGO - Cross-Section Preparation For Solder JointsDocument7 pagesARTIGO - Cross-Section Preparation For Solder JointsGuilherme Dos Santos MoreiraNo ratings yet
- Welding Defects Causes and Remedies NptelDocument3 pagesWelding Defects Causes and Remedies NptelGuilherme Dos Santos MoreiraNo ratings yet
- ARTIGO - Meeting The Challenges of High Volume PCB Cross-Section Sample PreparatioDocument4 pagesARTIGO - Meeting The Challenges of High Volume PCB Cross-Section Sample PreparatioGuilherme Dos Santos MoreiraNo ratings yet
- ARTIGO Overview of Selected Issues Related To SolderingDocument17 pagesARTIGO Overview of Selected Issues Related To SolderingGuilherme Dos Santos MoreiraNo ratings yet
- Micromachines 13 00860 v2Document15 pagesMicromachines 13 00860 v2Guilherme Dos Santos MoreiraNo ratings yet
- ARTIGO - PCB Cross Section White PaperDocument3 pagesARTIGO - PCB Cross Section White PaperGuilherme Dos Santos MoreiraNo ratings yet
- Metallographic Preparation Materials: Lead Alloys of Soft: K. H. EdnieDocument13 pagesMetallographic Preparation Materials: Lead Alloys of Soft: K. H. EdnieGuilherme Dos Santos MoreiraNo ratings yet
- Arfmtsv46 N1 P147 152Document6 pagesArfmtsv46 N1 P147 152Guilherme Dos Santos MoreiraNo ratings yet
- SMT DictionaryDocument7 pagesSMT DictionaryGuilherme Dos Santos MoreiraNo ratings yet
- Die Casting Parameters and SimulationsDocument9 pagesDie Casting Parameters and SimulationsGuilherme Dos Santos Moreira100% (1)
- Corrosion Inhibitors-1Document24 pagesCorrosion Inhibitors-1Guilherme Dos Santos MoreiraNo ratings yet
- This Content Downloaded From 200.129.187.61 On Tue, 02 Aug 2022 12:59:55 UTC:56 UTCDocument4 pagesThis Content Downloaded From 200.129.187.61 On Tue, 02 Aug 2022 12:59:55 UTC:56 UTCGuilherme Dos Santos MoreiraNo ratings yet
- Solder Paste Specification: No-Clean Xjs-638Document6 pagesSolder Paste Specification: No-Clean Xjs-638Guilherme Dos Santos MoreiraNo ratings yet
- Unit Cell Cubic StructuresDocument8 pagesUnit Cell Cubic StructuresGuilherme Dos Santos MoreiraNo ratings yet
- High Speed Tensile Test of High Strength SteelDocument2 pagesHigh Speed Tensile Test of High Strength SteelGuilherme Dos Santos MoreiraNo ratings yet
- Geometry Questions For CAT ExamDocument41 pagesGeometry Questions For CAT ExamGuilherme Dos Santos MoreiraNo ratings yet
- TA UserCom 39 en LRDocument28 pagesTA UserCom 39 en LRGuilherme Dos Santos MoreiraNo ratings yet
- How To Choise Refractory For Metallurgy FurnacesDocument15 pagesHow To Choise Refractory For Metallurgy FurnacesGuilherme Dos Santos MoreiraNo ratings yet
- Phase Transformation 12Document20 pagesPhase Transformation 12Guilherme Dos Santos MoreiraNo ratings yet
- Uasa Year 1Document10 pagesUasa Year 1vatsalkrishnasamyNo ratings yet
- A Level Biology Statistics Summary Test Formula Use Degrees of Freedom Accept/reject Null Hypothesis Extra InformationDocument12 pagesA Level Biology Statistics Summary Test Formula Use Degrees of Freedom Accept/reject Null Hypothesis Extra InformationmohammedNo ratings yet
- Project Report On Indian OilDocument80 pagesProject Report On Indian OilPayal PatidarNo ratings yet
- Abruptio PlacentaDocument20 pagesAbruptio PlacentaHizkia Mangaraja Hasiholan LimNo ratings yet
- Netflix ReportDocument9 pagesNetflix ReportMarijuana MomentNo ratings yet
- Good and Evil of Moby Dick and Captain AhabDocument4 pagesGood and Evil of Moby Dick and Captain AhabCismaru ElenaNo ratings yet
- Bridge Axi AhbDocument22 pagesBridge Axi Ahbkrishnaav100% (1)
- Robb Et Al 2013 A New Species of Strix Owl From OmanDocument36 pagesRobb Et Al 2013 A New Species of Strix Owl From OmanMagnus RobbNo ratings yet
- Mosaic TRD2 Tests U7 1Document3 pagesMosaic TRD2 Tests U7 1Patricia Alfaro GarijoNo ratings yet
- SLG Chem2 LG 4.9 BuffersDocument6 pagesSLG Chem2 LG 4.9 BuffersIman SontousidadNo ratings yet
- DSTV PDFDocument4 pagesDSTV PDFEddy TangaNo ratings yet
- Manual Haier HYC-390 enDocument24 pagesManual Haier HYC-390 enDaniel BuitragoNo ratings yet
- Cleanupdrive Project ProposalDocument13 pagesCleanupdrive Project ProposalAllyn Chabill AlmiranteNo ratings yet
- Carta Das Novas Que Vieram A El-Rei Nosso Senhor Do Descobrimento Do Prestes JoãoDocument41 pagesCarta Das Novas Que Vieram A El-Rei Nosso Senhor Do Descobrimento Do Prestes JoãoMaria do Rosário MonteiroNo ratings yet
- Garden Staff Position Description 2015Document1 pageGarden Staff Position Description 2015congressheightsontheriseNo ratings yet
- 4MA1 2HR Que 20220118Document32 pages4MA1 2HR Que 20220118Bruce RussellNo ratings yet
- Half Value Layer CalculationsDocument52 pagesHalf Value Layer Calculationsshabbir62675% (4)
- GRSS - FINAL LIST - 1 June 2011 - 2Document26 pagesGRSS - FINAL LIST - 1 June 2011 - 2Robel TadesseNo ratings yet
- Wastewater Treatment: D. Jim Livingston Asst. Prof. of ChemistryDocument22 pagesWastewater Treatment: D. Jim Livingston Asst. Prof. of ChemistryJim LivingstonNo ratings yet
- Annotated BibliographyDocument15 pagesAnnotated Bibliographyapi-190312542No ratings yet
- ICT 1105 Assignment 2Document23 pagesICT 1105 Assignment 2Rachel NgNo ratings yet
- HP 100 Series Rack v142: Key Features and BenefitsDocument2 pagesHP 100 Series Rack v142: Key Features and Benefitsevangelista7940No ratings yet
- Class 10 National Genius Search Examination: Advanced: Check The Correctness of The Roll No. With The Answer SheetDocument4 pagesClass 10 National Genius Search Examination: Advanced: Check The Correctness of The Roll No. With The Answer SheetPPNo ratings yet
- Lesson 2 Discrete Probability DistributionsDocument11 pagesLesson 2 Discrete Probability DistributionsReign SaplacoNo ratings yet
- Science 7 Q4 SLM11Document15 pagesScience 7 Q4 SLM11Seen Tuna-doughNo ratings yet
- TN Panchayat Building Rules 1997Document22 pagesTN Panchayat Building Rules 1997krish1979No ratings yet
- UNIT - 2 - Radio Link Features in GSM SystemsDocument18 pagesUNIT - 2 - Radio Link Features in GSM SystemsPrakhar ParasharNo ratings yet
- Rtaf SVX001C en - 11252015Document106 pagesRtaf SVX001C en - 11252015Thiraviam AyyappanNo ratings yet