Professional Documents
Culture Documents
Laboratory Test Report: 16 Years/Female
Laboratory Test Report: 16 Years/Female
Uploaded by
kl karthikeyaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Laboratory Test Report: 16 Years/Female
Laboratory Test Report: 16 Years/Female
Uploaded by
kl karthikeyaCopyright:
Available Formats
Vijaya Diagnostic Centre
Plot no: 43/198, Old Rainbow Hospital Lane, NRPET, Kurnool-518004.
Email : info@vijayadiagnostic.com
www.vijayadiagnostic.com
LABORATORY TEST REPORT
Regn Date :20/08/2021 11:35 Sample Collection : 20/08/2021 11:40
Name : MS. V MEGHANA /ID:1674530 Print Date : 21/08/2021 20:31
Regn No :802023205 Age / Sex : 16 Years/Female
Ref By :SELF Regn Centre : NR Pet 1-KRNL - 80
Sample Type :Swab Ref no. :
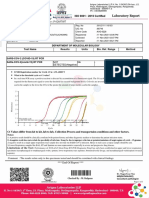
COVID -19 TESTING - SARS -CoV-2 RNA
TEST NAME RESULT
SARS-CoV-2 (RdRp gene) : NEGATIVE
Method: Real Time RT-PCR
--------------------------
INTERPRETATION:
--------------------------
Result | Remarks
------------------------------------------------------------------------
Positive | RNA specific to SARS-CoV-2 Detected.
------------------------------------------------------------------------
Negative | RNA specific to SARS-CoV-2 NOT Detected.
------------------------------------------------------------------------
Limit of Detection:
-------------------
• Analytical lower unit of detection <150 viral genome equivalents/mL
Comments:
-------------------
• Covid 19 Qualitative RT PCR test is an in vitro qualitative PCR assay for the qualitative detection
of Novel Corona Virus 2019 in respiratory specimens
• Test is conducted on Nasopharyngeal swab/ Oropharyngeal swabs and other respiratory specimens collected
in viral transport media.
• Detection of confirmatory(RdRp) genes indicates presence of SARS-CoV-2 RNA in the specimen tested.
• Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole basis for
patient management decisions. They must be correlated with clinical observations, patient history
and epidemiological information.
• Mutations or polymorphisms in the primer and probe binding sites, presence of PCR inhibition due to host
factors may also cause false negative results.
• Fresh sample for RT PCR can be considered after a gap of 2-4 days if there is a strong clinical
suspicion/contact of Covid 19 patient
• Repeat sampling and testing of lower respiratory specimen is strongly recommended in a severe or progressive disease.
• This test is a qualitative assay and does not quantify viral load. Various host factors, variability in
sample collection / site and techniques used by the laboratories can affect CT values. Therfore, CT values are not
an absolute indication of viral load and should be interpreted with caution.
Note:
• ICMR-Registration Number : VIJAY001
• COVID-19 test is conducted with a kit approved by ICMR/CE-IVD/US-FDA.
• Kindly consult Referring Physician/Authorized Government Hospital for appropriate follow up
DR.AKSHATHA P
CONSULTANT MICROBIOLOGIST
Released Date 21/08/2021 14:14 Page 1 of 1
You might also like
- TyphoidDocument3 pagesTyphoidShivam Tomar83% (6)
- Laboratory Test Report: Test Name Result Sars-Cov-2Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2Karthikeya MoorthyNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDarpan NegandhiNo ratings yet
- Cellular Basis of LifeDocument4 pagesCellular Basis of Lifemarc7victor7salesNo ratings yet
- I DCD 0067262239Document1 pageI DCD 0067262239abhinavNo ratings yet
- Gopu.R:::: Patient Age / Sex 30 Y / Male BranchDocument1 pageGopu.R:::: Patient Age / Sex 30 Y / Male BranchGopu RNo ratings yet
- MrsSNIGDHA 43Y FemaleDocument3 pagesMrsSNIGDHA 43Y FemalePathkind LabNo ratings yet
- Laboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Raja SekharNo ratings yet
- Laboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Marupudi SaikrishnaNo ratings yet
- Laboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Emmr RajNo ratings yet
- Laboratory Test Report: Sars-Cov-2Document1 pageLaboratory Test Report: Sars-Cov-2SubbuNo ratings yet
- KSXN7228Document1 pageKSXN7228sri sainathNo ratings yet
- Test Report: Investigation Observed Value Unit Biological Ref. Interval Specimen Covid-19 Virus Qualitative PCRDocument2 pagesTest Report: Investigation Observed Value Unit Biological Ref. Interval Specimen Covid-19 Virus Qualitative PCRRahul DesardaNo ratings yet
- XWOS8716Document5 pagesXWOS8716Aesthetic CoachNo ratings yet
- Laboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)hasan aNo ratings yet
- Report 2112051392Document1 pageReport 2112051392kashish singhNo ratings yet
- Laboratory Test Report: Test Name Result Sars-Cov-2Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2NareshNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range Methodmaneesh babuNo ratings yet
- Laboratory Test Report: Mr. Shiva RatnakarDocument1 pageLaboratory Test Report: Mr. Shiva RatnakarRatnakar YeluripatiNo ratings yet
- Inik4750Document1 pageInik4750Ratnakar YeluripatiNo ratings yet
- Lab Report: Mr. Atul KumarDocument1 pageLab Report: Mr. Atul KumarOCILABS Origin & Cause InvestigationNo ratings yet
- GC129053 (1) - 210804 - 061549Document2 pagesGC129053 (1) - 210804 - 061549anuragNo ratings yet
- Sandeep Walunj 01 04 2021 02 07 16 PMDocument2 pagesSandeep Walunj 01 04 2021 02 07 16 PMAbhijeet PatilNo ratings yet
- Department of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodanilsgaikwadNo ratings yet
- MR - Saurabh Vinaykumar Shukla-1Document1 pageMR - Saurabh Vinaykumar Shukla-1KAUSHAL KUMAR SHUKLANo ratings yet
- Laboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Charith ReddyNo ratings yet
- Babu Sankar:::: Patient Age / Sex 47 Y / Male BranchDocument1 pageBabu Sankar:::: Patient Age / Sex 47 Y / Male BranchRahul GNo ratings yet
- TARUN SETHI 28Y - M-CDocument1 pageTARUN SETHI 28Y - M-CArun SethiNo ratings yet
- Covid ReportDocument1 pageCovid ReportGourima BabbarNo ratings yet
- Railway TicketsDocument1 pageRailway TicketssaurabhNo ratings yet
- Sami Khaled Khazaal - 105732 - 2020Document1 pageSami Khaled Khazaal - 105732 - 2020iKoalaNo ratings yet
- Rakesh SainiDocument4 pagesRakesh SainiAdarsh SonkarNo ratings yet
- Laboratory Test Report: Sars-Cov-2Document1 pageLaboratory Test Report: Sars-Cov-2sandeep yadavNo ratings yet
- N. Boominathan:::: Patient Age / Sex 58 Y / Male BranchDocument1 pageN. Boominathan:::: Patient Age / Sex 58 Y / Male BranchBoomi Nathan NatarajanNo ratings yet
- Final Laboratory Report: 17 Years 2163032321Document1 pageFinal Laboratory Report: 17 Years 2163032321TanmayiVanageNo ratings yet
- Laboratory Test Report: Test Name Result Biological Reference Interval HIV 1 & 2 AntibodiesDocument1 pageLaboratory Test Report: Test Name Result Biological Reference Interval HIV 1 & 2 AntibodiesMohamed YaseenNo ratings yet
- Sars-Cov-2 by RT PCR (Qualitative) : Icmr Reg .No. - SanpalagDocument1 pageSars-Cov-2 by RT PCR (Qualitative) : Icmr Reg .No. - SanpalagHaimanti NathNo ratings yet
- Report 2Document2 pagesReport 2vipultrivedi9049No ratings yet
- Molecular Biology Sars-Cov-2 (Covid 19) Detection by Real Time PCRDocument2 pagesMolecular Biology Sars-Cov-2 (Covid 19) Detection by Real Time PCRMithileshNo ratings yet
- RahulDocument3 pagesRahulCOVID-19 ProjectNo ratings yet
- Hanuman Badabanala StotramDocument4 pagesHanuman Badabanala Stotrampramod yelagonda0% (1)
- Covidreportrtpcrtest YashDocument2 pagesCovidreportrtpcrtest YashYash ShahiNo ratings yet
- Laboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)srinathNo ratings yet
- Real Time Qualitative RT-PCR Detection of 2019-nCOV RNA / COVID-19 RNADocument1 pageReal Time Qualitative RT-PCR Detection of 2019-nCOV RNA / COVID-19 RNAHemendra RaiNo ratings yet
- Department of Laboratory Medicine: Critical Care ServicesDocument2 pagesDepartment of Laboratory Medicine: Critical Care ServicesRTI ACTNo ratings yet
- Sars-Cov-2 (Covid-19) by RT-PCR: Molecular TestingDocument3 pagesSars-Cov-2 (Covid-19) by RT-PCR: Molecular TestingAfro GumNo ratings yet
- VM211511105 Masterayushgupta719848319509 20211115092858002Document2 pagesVM211511105 Masterayushgupta719848319509 20211115092858002pmirzapure420No ratings yet
- Medical Laboratory Report: Specimen Nasopharyngeal / Oropharyngeal Swab Covid-19 Qualitative PCRDocument1 pageMedical Laboratory Report: Specimen Nasopharyngeal / Oropharyngeal Swab Covid-19 Qualitative PCRVivek SahNo ratings yet
- Laboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Vishwam ChilumulaNo ratings yet
- Covid-19 by Real Time RT PCRDocument2 pagesCovid-19 by Real Time RT PCRArun AntonyNo ratings yet
- MR Amit Thakkar - 7028246333Document5 pagesMR Amit Thakkar - 7028246333Mahesh PallaviNo ratings yet
- Moleculer Biology: Lab ID MRN Reference No. DOBDocument1 pageMoleculer Biology: Lab ID MRN Reference No. DOBAfkar inteNo ratings yet
- Specialist Hospital: Molecular BiologyDocument2 pagesSpecialist Hospital: Molecular BiologyIT MalurNo ratings yet
- Sivaprasath.S:::: Patient Age / Sex 18 Y / Male BranchDocument1 pageSivaprasath.S:::: Patient Age / Sex 18 Y / Male BranchSibi SivaNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDevi Sri PrasadNo ratings yet
- MR - Santosh Kumar MahasethDocument1 pageMR - Santosh Kumar MahasethMukesh MistriNo ratings yet
- PdfText - 2021-11-11T202745.532Document1 pagePdfText - 2021-11-11T202745.532Murtaza AhmarNo ratings yet
- TestReport - 22 06 2021 - Apollo 2471624375836407Document2 pagesTestReport - 22 06 2021 - Apollo 2471624375836407thakuryaNo ratings yet
- Divya Bangera MBBS, MD Microbiology MME Team LeadDocument2 pagesDivya Bangera MBBS, MD Microbiology MME Team LeadRajavardhanNo ratings yet
- Covid-19 Test Report: PATIENT NAME: Indu Gupta Raj BalaDocument1 pageCovid-19 Test Report: PATIENT NAME: Indu Gupta Raj BalaTarun BhatnagarNo ratings yet
- Atlas of Early Neoplasias of the Gastrointestinal Tract: Endoscopic Diagnosis and Therapeutic DecisionsFrom EverandAtlas of Early Neoplasias of the Gastrointestinal Tract: Endoscopic Diagnosis and Therapeutic DecisionsFrieder BerrNo ratings yet
- Class Xii SHHW 2024 2025Document126 pagesClass Xii SHHW 2024 2025Manish MishraNo ratings yet
- African History Notes BookletsDocument86 pagesAfrican History Notes BookletsJus DaisyNo ratings yet
- 1st Quarter ReviewerDocument4 pages1st Quarter ReviewerallanNo ratings yet
- Bio Topic 4 Notes PDFDocument11 pagesBio Topic 4 Notes PDFdgfhjNo ratings yet
- LearnEnglish Reading C1 How Humans Evolved LanguageDocument4 pagesLearnEnglish Reading C1 How Humans Evolved LanguageYasa ReyNo ratings yet
- Answer The Following Questions About CladogramsDocument2 pagesAnswer The Following Questions About CladogramsSabrine BenzakourNo ratings yet
- Blood Basics-1Document3 pagesBlood Basics-1api-355865052No ratings yet
- Color Plates (1-60)Document12 pagesColor Plates (1-60)Gayle BocalaNo ratings yet
- Science 7-2ND QUATER EXAMDocument4 pagesScience 7-2ND QUATER EXAMVincent S. RedolosaNo ratings yet
- Lesson 5Document3 pagesLesson 5Angela Meiko MalateNo ratings yet
- Molecular Basis of Inheritance PYQS Ans Key - 19189643 - 2023 - 06 - 05 - 12 - 38Document25 pagesMolecular Basis of Inheritance PYQS Ans Key - 19189643 - 2023 - 06 - 05 - 12 - 38Arsh DhawanNo ratings yet
- Unit 2-Topic 1-Physical SelfDocument9 pagesUnit 2-Topic 1-Physical SelfMab ShiNo ratings yet
- NCERT Solutions For Class 11 Biology Chapter 8 - Cell The Unit of Life - .Document10 pagesNCERT Solutions For Class 11 Biology Chapter 8 - Cell The Unit of Life - .susilmaji92No ratings yet
- Jay Tugend - Mitosis Performance TaskDocument4 pagesJay Tugend - Mitosis Performance Taskapi-673971695No ratings yet
- Joram Case StudyDocument15 pagesJoram Case StudyHoney Bee S. PlatolonNo ratings yet
- Resume Abstrak Artikel Internasional 2020Document371 pagesResume Abstrak Artikel Internasional 2020Irwina eka derayaNo ratings yet
- Pengaruh EMS Terhadap Pertumbuhan Dan Variasi Tanaman MarigoldDocument6 pagesPengaruh EMS Terhadap Pertumbuhan Dan Variasi Tanaman MarigoldWahyu Putra PermanaNo ratings yet
- НҰСҚА 4092 Mathematics: A) B) C) D)Document10 pagesНҰСҚА 4092 Mathematics: A) B) C) D)ADKIT ChannelNo ratings yet
- Biology Is TheDocument3 pagesBiology Is TheAbegail Jean Naces BarcenaNo ratings yet
- Efficient Recovery of Transgenic Plants Through Organogenesis and Embryogenesis Using A Cryptic Promoter To Drive Marker Gene ExpressionDocument7 pagesEfficient Recovery of Transgenic Plants Through Organogenesis and Embryogenesis Using A Cryptic Promoter To Drive Marker Gene ExpressionAfandynibandera AfandynibanderaNo ratings yet
- Forensics EvidenceDocument80 pagesForensics EvidenceSony BaniyaNo ratings yet
- Grade 11 Learners Unit 1 EnglishDocument5 pagesGrade 11 Learners Unit 1 EnglishvhczuluNo ratings yet
- Microbiology Related Thesis TopicsDocument7 pagesMicrobiology Related Thesis Topicsjensantiagosyracuse100% (2)
- Unit Bryophyta (Paper Code 502)Document47 pagesUnit Bryophyta (Paper Code 502)Abhishek Singh Chandel0% (1)
- Claimed Dark Breeds of The LycansDocument60 pagesClaimed Dark Breeds of The LycansElle OfbbkNo ratings yet
- Bio SummaryDocument3 pagesBio SummaryDeepak SinghNo ratings yet
- Platanus Orientalis CreticaDocument4 pagesPlatanus Orientalis CreticaCarles JiménezNo ratings yet
- Metabolic Engineering J. Nielsen PDFDocument21 pagesMetabolic Engineering J. Nielsen PDFAlberttNo ratings yet
- From Stardust To First CellsDocument273 pagesFrom Stardust To First CellsManci PeterNo ratings yet