Professional Documents
Culture Documents
Chemi
Chemi
Uploaded by
timothyCopyright:
Available Formats
You might also like
- Chapter 2 ExerciseDocument2 pagesChapter 2 ExerciseMegan Louisse VictorioNo ratings yet
- Chapter # 03: Periodic Table and Periodicity of Properties (Topic Wise Questions)Document9 pagesChapter # 03: Periodic Table and Periodicity of Properties (Topic Wise Questions)husain aliNo ratings yet
- Ionisation EnergyDocument12 pagesIonisation Energyhahajing1980No ratings yet
- Ionization Energy Trend in The Periodic TableDocument3 pagesIonization Energy Trend in The Periodic TableOluwabamire OreoluwaNo ratings yet
- Chemical BondingDocument49 pagesChemical Bondingध्रुव पांडेयNo ratings yet
- Ionization EnergyDocument69 pagesIonization EnergyVisalakshi Venkat100% (2)
- Ionization Energy and Electron AffinityDocument9 pagesIonization Energy and Electron AffinityKhan AaghaNo ratings yet
- STH - 20200419 - Plasma Chemistry Ch2Document21 pagesSTH - 20200419 - Plasma Chemistry Ch2Si Thu HanNo ratings yet
- Plasma Chemistry: Chapter 2 Elementary Plasma-Chemical ReactionsDocument21 pagesPlasma Chemistry: Chapter 2 Elementary Plasma-Chemical ReactionsSi Thu HanNo ratings yet
- 3.0 Chemical BondingDocument50 pages3.0 Chemical Bondingparkinsondilys7No ratings yet
- LP Notess EF-IDocument41 pagesLP Notess EF-Ipratibha suryawanshiNo ratings yet
- Chemistry Chapter 6 ReviewDocument4 pagesChemistry Chapter 6 ReviewSirena GutierrezNo ratings yet
- IonizationDocument9 pagesIonizationAttaUrRahmanNo ratings yet
- Ionic BondingDocument7 pagesIonic BondingDr. Sheelu SharmaNo ratings yet
- Wa0041.Document4 pagesWa0041.Benson ShayoNo ratings yet
- 5.physical Chemistry (A Level Only)Document176 pages5.physical Chemistry (A Level Only)TendaiNo ratings yet
- Classification of ElementsDocument74 pagesClassification of ElementsJimit Patel BankNo ratings yet
- Worksheet BenzenaDocument5 pagesWorksheet BenzenaRizky KurniawatiNo ratings yet
- HL Electron Configuration and The Periodic TableDocument12 pagesHL Electron Configuration and The Periodic TableMarilee HuntNo ratings yet
- Ion EnergiesDocument4 pagesIon EnergiesNamratha NairNo ratings yet
- Ionization EnergyDocument2 pagesIonization EnergynapilgabethNo ratings yet
- Ions and Ionic Bonding Pre-AICEDocument4 pagesIons and Ionic Bonding Pre-AICEKelsey MarieNo ratings yet
- Module 1 - Nature of ElectricityDocument14 pagesModule 1 - Nature of ElectricityEd Carlo RamisNo ratings yet
- AS Level Summary-Unit 1Document11 pagesAS Level Summary-Unit 1Filiz Kocayazgan ShanablehNo ratings yet
- Chemical Bonding Class11th by PS Sir IIT JEEDocument44 pagesChemical Bonding Class11th by PS Sir IIT JEEMahendra PandaNo ratings yet
- 6 Chapter Chemical Bonding Text Book ExerciseDocument20 pages6 Chapter Chemical Bonding Text Book ExerciseSajid Azeem100% (2)
- Che Chapter 3Document7 pagesChe Chapter 3lisaNo ratings yet
- Electron Configurations LessonDocument19 pagesElectron Configurations LessonWARREN ESCAPENo ratings yet
- Ebenezer Matric .Hr. Sec. School: 1.what Is Ionization Energy?Document3 pagesEbenezer Matric .Hr. Sec. School: 1.what Is Ionization Energy?r karthickNo ratings yet
- Module 2 - Conduction and Breakdown in GasesDocument58 pagesModule 2 - Conduction and Breakdown in GasesFah Rukh100% (1)
- PRB Set 2Document3 pagesPRB Set 2Aya HachanaNo ratings yet
- Chemguide - Answers: Electron AffinitiesDocument1 pageChemguide - Answers: Electron AffinitiesS K MishraNo ratings yet
- CIE Chemistry A Level: 2: Atomic StructureDocument6 pagesCIE Chemistry A Level: 2: Atomic StructureahumanbeinginearthNo ratings yet
- IB Chemistry Notes On Periodic TableDocument32 pagesIB Chemistry Notes On Periodic TableYasser Khairy AbdelghaniNo ratings yet
- CIE Chemistry A Level: 2: Atomic StructureDocument6 pagesCIE Chemistry A Level: 2: Atomic StructureMyraNo ratings yet
- Chapter 1 Periodic PropertiesDocument34 pagesChapter 1 Periodic PropertiesMayank Mourya100% (1)
- Periodic Table - Periodic Properties & Variations of PropertiesDocument34 pagesPeriodic Table - Periodic Properties & Variations of PropertieskumarvaradarajanNo ratings yet
- 2017-02-06 Chemical BondingDocument37 pages2017-02-06 Chemical BondingAbrar Mubasshir RahmanNo ratings yet
- Periodic Table Questions and AnswersDocument5 pagesPeriodic Table Questions and AnswersVISWANATHAN GNo ratings yet
- 5 Trends in The Periodic TableDocument4 pages5 Trends in The Periodic TableCris CorsinoNo ratings yet
- 2 13 Ionisation EnergiesDocument6 pages2 13 Ionisation EnergiesRobertLiu100% (2)
- Ionisation Energy ClassDocument13 pagesIonisation Energy ClassKordell McShineNo ratings yet
- Electrons in Atoms.Document62 pagesElectrons in Atoms.Md.Tanjim reza TurjoNo ratings yet
- Chemistry Nucleus-F: Theory Notes On Chemical Bonding-IDocument1 pageChemistry Nucleus-F: Theory Notes On Chemical Bonding-IRaju SinghNo ratings yet
- 10 Senyawa Kompleks AplikomDocument4 pages10 Senyawa Kompleks AplikomFatimatuz zahrohNo ratings yet
- Electron Groups-Electron Geometry: Example?Document12 pagesElectron Groups-Electron Geometry: Example?Cat CNo ratings yet
- Engineering Material Presentation: Presented To: Engr. Nadeem Hassan Presented By: Abdul Rauf 2k17-Che-17Document70 pagesEngineering Material Presentation: Presented To: Engr. Nadeem Hassan Presented By: Abdul Rauf 2k17-Che-17Abdul Rauf ChNo ratings yet
- Ion Pairing and Electron Transfer: R. A. MarcusDocument7 pagesIon Pairing and Electron Transfer: R. A. MarcusHỗn ĐộnNo ratings yet
- Worksheet 11 - Periodic TrendsDocument7 pagesWorksheet 11 - Periodic TrendsAvocodo FotovatNo ratings yet
- Chem 111 Week Three Notes BDocument7 pagesChem 111 Week Three Notes BnyachiosirNo ratings yet
- General Chemistry Lesson 9Document17 pagesGeneral Chemistry Lesson 9dreih MadrigNo ratings yet
- Chemical Bonding: (A Step Towards Smart Education)Document33 pagesChemical Bonding: (A Step Towards Smart Education)RANJEET SHARMANo ratings yet
- Study Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!Document26 pagesStudy Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!Bhupinder KaurNo ratings yet
- Introduction To Chemical Bonding: Ionic Bond OR Electrovalent BondDocument12 pagesIntroduction To Chemical Bonding: Ionic Bond OR Electrovalent Bondanon_397573801No ratings yet
- Atomic Structure and Bonding v3cDocument34 pagesAtomic Structure and Bonding v3cBirdii97No ratings yet
- Chapter 8 Worked ExamplesDocument23 pagesChapter 8 Worked ExamplesSarah GonzalesNo ratings yet
- ELECTROPHORESISDocument19 pagesELECTROPHORESISAmit SahNo ratings yet
- Electrical Circuits 1Document11 pagesElectrical Circuits 1Joanna Cristine NedicNo ratings yet
- Unit 2 HL (Atomic Structure HL)Document11 pagesUnit 2 HL (Atomic Structure HL)ellhamnasserNo ratings yet
Chemi
Chemi
Uploaded by
timothyOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemi
Chemi
Uploaded by
timothyCopyright:
Available Formats
Name: Sutanto, Timothy
Class: 11C3
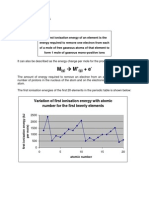
A. First ionization energy is a kind of energy that takes place or required in order to remove one
mole of electrons from one mole of gaseous atoms so that it can produce 1+ ion, and the
element present in the reaction should in gas form.
B. As we can see from the ionization energy that is provided there, I choose the element B and C in
one group. My observation is the change of the ionization energy that happens in both
elements. As we observe the four elements, elements B and C greatest change in the ionization
energy is in the transition from IE1 to IE2. This observation strongly indicates that both element
B and element C belong to the same group in the periodic table.
C. Element B because it needs 4563 kJ mol^-1 to do that process.
D. Element A will reacts and forms an ion with a charge of 3+ because in the ionization energy
given, element A in the IE 3 going to IE 4, if we compare the change on that state with the other
element, element A will give the highest difference in which it is the highest jump. The change in
IE3 and IE4 is the highest jump in element A that this indicates that they will be going to the next
energy level. The change in IE is higher that means the previous 3 electron are no longer in one
shell. It also needs high energy to move from shells especially they belong to different shell.
E. In addition, the changes in
You might also like
- Chapter 2 ExerciseDocument2 pagesChapter 2 ExerciseMegan Louisse VictorioNo ratings yet
- Chapter # 03: Periodic Table and Periodicity of Properties (Topic Wise Questions)Document9 pagesChapter # 03: Periodic Table and Periodicity of Properties (Topic Wise Questions)husain aliNo ratings yet
- Ionisation EnergyDocument12 pagesIonisation Energyhahajing1980No ratings yet
- Ionization Energy Trend in The Periodic TableDocument3 pagesIonization Energy Trend in The Periodic TableOluwabamire OreoluwaNo ratings yet
- Chemical BondingDocument49 pagesChemical Bondingध्रुव पांडेयNo ratings yet
- Ionization EnergyDocument69 pagesIonization EnergyVisalakshi Venkat100% (2)
- Ionization Energy and Electron AffinityDocument9 pagesIonization Energy and Electron AffinityKhan AaghaNo ratings yet
- STH - 20200419 - Plasma Chemistry Ch2Document21 pagesSTH - 20200419 - Plasma Chemistry Ch2Si Thu HanNo ratings yet
- Plasma Chemistry: Chapter 2 Elementary Plasma-Chemical ReactionsDocument21 pagesPlasma Chemistry: Chapter 2 Elementary Plasma-Chemical ReactionsSi Thu HanNo ratings yet
- 3.0 Chemical BondingDocument50 pages3.0 Chemical Bondingparkinsondilys7No ratings yet
- LP Notess EF-IDocument41 pagesLP Notess EF-Ipratibha suryawanshiNo ratings yet
- Chemistry Chapter 6 ReviewDocument4 pagesChemistry Chapter 6 ReviewSirena GutierrezNo ratings yet
- IonizationDocument9 pagesIonizationAttaUrRahmanNo ratings yet
- Ionic BondingDocument7 pagesIonic BondingDr. Sheelu SharmaNo ratings yet
- Wa0041.Document4 pagesWa0041.Benson ShayoNo ratings yet
- 5.physical Chemistry (A Level Only)Document176 pages5.physical Chemistry (A Level Only)TendaiNo ratings yet
- Classification of ElementsDocument74 pagesClassification of ElementsJimit Patel BankNo ratings yet
- Worksheet BenzenaDocument5 pagesWorksheet BenzenaRizky KurniawatiNo ratings yet
- HL Electron Configuration and The Periodic TableDocument12 pagesHL Electron Configuration and The Periodic TableMarilee HuntNo ratings yet
- Ion EnergiesDocument4 pagesIon EnergiesNamratha NairNo ratings yet
- Ionization EnergyDocument2 pagesIonization EnergynapilgabethNo ratings yet
- Ions and Ionic Bonding Pre-AICEDocument4 pagesIons and Ionic Bonding Pre-AICEKelsey MarieNo ratings yet
- Module 1 - Nature of ElectricityDocument14 pagesModule 1 - Nature of ElectricityEd Carlo RamisNo ratings yet
- AS Level Summary-Unit 1Document11 pagesAS Level Summary-Unit 1Filiz Kocayazgan ShanablehNo ratings yet
- Chemical Bonding Class11th by PS Sir IIT JEEDocument44 pagesChemical Bonding Class11th by PS Sir IIT JEEMahendra PandaNo ratings yet
- 6 Chapter Chemical Bonding Text Book ExerciseDocument20 pages6 Chapter Chemical Bonding Text Book ExerciseSajid Azeem100% (2)
- Che Chapter 3Document7 pagesChe Chapter 3lisaNo ratings yet
- Electron Configurations LessonDocument19 pagesElectron Configurations LessonWARREN ESCAPENo ratings yet
- Ebenezer Matric .Hr. Sec. School: 1.what Is Ionization Energy?Document3 pagesEbenezer Matric .Hr. Sec. School: 1.what Is Ionization Energy?r karthickNo ratings yet
- Module 2 - Conduction and Breakdown in GasesDocument58 pagesModule 2 - Conduction and Breakdown in GasesFah Rukh100% (1)
- PRB Set 2Document3 pagesPRB Set 2Aya HachanaNo ratings yet
- Chemguide - Answers: Electron AffinitiesDocument1 pageChemguide - Answers: Electron AffinitiesS K MishraNo ratings yet
- CIE Chemistry A Level: 2: Atomic StructureDocument6 pagesCIE Chemistry A Level: 2: Atomic StructureahumanbeinginearthNo ratings yet
- IB Chemistry Notes On Periodic TableDocument32 pagesIB Chemistry Notes On Periodic TableYasser Khairy AbdelghaniNo ratings yet
- CIE Chemistry A Level: 2: Atomic StructureDocument6 pagesCIE Chemistry A Level: 2: Atomic StructureMyraNo ratings yet
- Chapter 1 Periodic PropertiesDocument34 pagesChapter 1 Periodic PropertiesMayank Mourya100% (1)
- Periodic Table - Periodic Properties & Variations of PropertiesDocument34 pagesPeriodic Table - Periodic Properties & Variations of PropertieskumarvaradarajanNo ratings yet
- 2017-02-06 Chemical BondingDocument37 pages2017-02-06 Chemical BondingAbrar Mubasshir RahmanNo ratings yet
- Periodic Table Questions and AnswersDocument5 pagesPeriodic Table Questions and AnswersVISWANATHAN GNo ratings yet
- 5 Trends in The Periodic TableDocument4 pages5 Trends in The Periodic TableCris CorsinoNo ratings yet
- 2 13 Ionisation EnergiesDocument6 pages2 13 Ionisation EnergiesRobertLiu100% (2)
- Ionisation Energy ClassDocument13 pagesIonisation Energy ClassKordell McShineNo ratings yet
- Electrons in Atoms.Document62 pagesElectrons in Atoms.Md.Tanjim reza TurjoNo ratings yet
- Chemistry Nucleus-F: Theory Notes On Chemical Bonding-IDocument1 pageChemistry Nucleus-F: Theory Notes On Chemical Bonding-IRaju SinghNo ratings yet
- 10 Senyawa Kompleks AplikomDocument4 pages10 Senyawa Kompleks AplikomFatimatuz zahrohNo ratings yet
- Electron Groups-Electron Geometry: Example?Document12 pagesElectron Groups-Electron Geometry: Example?Cat CNo ratings yet
- Engineering Material Presentation: Presented To: Engr. Nadeem Hassan Presented By: Abdul Rauf 2k17-Che-17Document70 pagesEngineering Material Presentation: Presented To: Engr. Nadeem Hassan Presented By: Abdul Rauf 2k17-Che-17Abdul Rauf ChNo ratings yet
- Ion Pairing and Electron Transfer: R. A. MarcusDocument7 pagesIon Pairing and Electron Transfer: R. A. MarcusHỗn ĐộnNo ratings yet
- Worksheet 11 - Periodic TrendsDocument7 pagesWorksheet 11 - Periodic TrendsAvocodo FotovatNo ratings yet
- Chem 111 Week Three Notes BDocument7 pagesChem 111 Week Three Notes BnyachiosirNo ratings yet
- General Chemistry Lesson 9Document17 pagesGeneral Chemistry Lesson 9dreih MadrigNo ratings yet
- Chemical Bonding: (A Step Towards Smart Education)Document33 pagesChemical Bonding: (A Step Towards Smart Education)RANJEET SHARMANo ratings yet
- Study Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!Document26 pagesStudy Materials: Vedantu Innovations Pvt. Ltd. Score High With A Personal Teacher, Learn LIVE Online!Bhupinder KaurNo ratings yet
- Introduction To Chemical Bonding: Ionic Bond OR Electrovalent BondDocument12 pagesIntroduction To Chemical Bonding: Ionic Bond OR Electrovalent Bondanon_397573801No ratings yet
- Atomic Structure and Bonding v3cDocument34 pagesAtomic Structure and Bonding v3cBirdii97No ratings yet
- Chapter 8 Worked ExamplesDocument23 pagesChapter 8 Worked ExamplesSarah GonzalesNo ratings yet
- ELECTROPHORESISDocument19 pagesELECTROPHORESISAmit SahNo ratings yet
- Electrical Circuits 1Document11 pagesElectrical Circuits 1Joanna Cristine NedicNo ratings yet
- Unit 2 HL (Atomic Structure HL)Document11 pagesUnit 2 HL (Atomic Structure HL)ellhamnasserNo ratings yet