Professional Documents
Culture Documents
4th Quarter Module 1
4th Quarter Module 1
Uploaded by
jackielou trongcoso0 ratings0% found this document useful (0 votes)
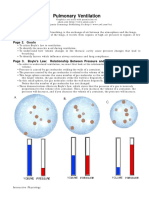
11 views2 pagesBoyle's law states that for a fixed amount of an ideal gas kept at a constant temperature, pressure and volume are inversely proportional. The law is demonstrated through several examples:
1) When the plunger is pulled out of a syringe, the volume increases and pressure decreases, allowing fluid to be drawn in. Pushing the plunger decreases volume and increases pressure to force fluid out.
2) During plane travel or diving, decreasing pressure due to changes in altitude or depth causes gas volumes like in sinuses or lungs to increase due to Boyle's law.
3) Sitting on a balloon decreases its volume, increasing pressure until the balloon pops, again demonstrating the inverse relationship between pressure and volume
Original Description:
skience
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentBoyle's law states that for a fixed amount of an ideal gas kept at a constant temperature, pressure and volume are inversely proportional. The law is demonstrated through several examples:
1) When the plunger is pulled out of a syringe, the volume increases and pressure decreases, allowing fluid to be drawn in. Pushing the plunger decreases volume and increases pressure to force fluid out.
2) During plane travel or diving, decreasing pressure due to changes in altitude or depth causes gas volumes like in sinuses or lungs to increase due to Boyle's law.
3) Sitting on a balloon decreases its volume, increasing pressure until the balloon pops, again demonstrating the inverse relationship between pressure and volume
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
11 views2 pages4th Quarter Module 1
4th Quarter Module 1
Uploaded by
jackielou trongcosoBoyle's law states that for a fixed amount of an ideal gas kept at a constant temperature, pressure and volume are inversely proportional. The law is demonstrated through several examples:
1) When the plunger is pulled out of a syringe, the volume increases and pressure decreases, allowing fluid to be drawn in. Pushing the plunger decreases volume and increases pressure to force fluid out.
2) During plane travel or diving, decreasing pressure due to changes in altitude or depth causes gas volumes like in sinuses or lungs to increase due to Boyle's law.
3) Sitting on a balloon decreases its volume, increasing pressure until the balloon pops, again demonstrating the inverse relationship between pressure and volume
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 2
Science 4th quarter Module 1
Assessment
1.D
2.B
3.C
4.D
5.A
6.D
7.A
8.D
9.C
10.A
Enrichment (A.)
1. Given: P1=760 torr
V1= 3.0 L
V2= 12.0 L
Required: P2(Final Pressure) =?
2. Given: P1= 6.0 atm
P2= 0.50 at,
V1= 25.0 L
Required: V2(Final volume) =?
(B.)
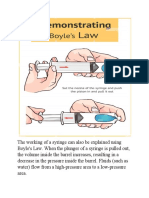
1. Invention of syringes
The working of a syringe can also be explained using Boyle's Law. When the plunger of a
syringe is pulled out, the volume inside the barrel increases, resulting in a decrease in the
pressure inside the barrel. Fluids (such as water) flow from a high pressure area to a low
pressure area. The opposite is also true. When the plunger is pushed back in, the volume
decreases and the pressure increases. Once the pressure is greater than that outside the
syringe, the fluid inside the barrel will flow out.
2. Plane travel
Boyle's law states that for a given temperature the volume is inversely proportional to the
pressure. This law explains why sinuses or middle ear (which are normally fixed volume gas-
filled spaces) may hurt when during altitude or pressure changes. At a constant temperature,
the volume of gas is inversely proportional to its pressure. Basically, as you ascend in altitude
(or to the surface if diving), gas expands to a greater volume due to decreased pressure
exerted on it.
3. Popping a balloon
When you sit on a balloon you decrease the volume . Therefore, the pressure must increase
and, when it is too high, the balloon cannot hold it anymore and pops. The above law is
named Boyle's Law. It holds if the temperature of an ideal gas remains constant. When the
temperature changes as well, the following two ideal gas laws are involved.
4. Breathing
The operation of your lungs also can be explained using Boyle’s Law. When you inhale
(breathe in), your diaphragm (a large muscle below your lungs) lowers, which increases the
volume inside your lungs. This makes the air pressure inside your lungs lower than the air
pressure outside your lungs (and your body); therefore, the outside air is drawn into your
lungs (much like the syringe). When you exhale (breathe out), your diaphragm pushes
upwards, reducing the volume inside your lungs, increasing the pressure and forcing the air
outwards.
5. SCUBA diving
Boyle's Law is also important to divers because it means that if a diver takes a lung- ful of air
while he is underwater, that air will expand in his lungs as he rises to the surface. If he holds
his breath, or ascends too rapidly (like a cork) the expanding air can rupture his lungs.Boyle's
Law describes the role of water pressure in the dive environment. It applies and affects many
aspects of scuba diving. Consider the following examples:Descent - As a diver descends, the
water pressure around him increases, causing air in his scuba equipment and body to occupy a
smaller volume (compress).Ascent - As a diver ascends, water pressure decreases, so Boyle's
Law states that the air in his gear and body expand to occupy a greater volume.
You might also like
- Oma Suomi 1 PDFDocument131 pagesOma Suomi 1 PDFCONSTANZA VALERIA FLORES RIOS0% (3)
- Egans Fundamentals of Respiratory Care 11th Edition Kacmarek Test BankDocument22 pagesEgans Fundamentals of Respiratory Care 11th Edition Kacmarek Test Bankhillyobsidian8hi42g100% (29)
- Test Bank For Pilbeams Mechanical Ventilation 6th Edition by CairoDocument24 pagesTest Bank For Pilbeams Mechanical Ventilation 6th Edition by Cairojenniferhoustonmjcfngpbow100% (41)
- HAPS Pulmonary Ventilation ActivityDocument4 pagesHAPS Pulmonary Ventilation ActivityLucelle AdanteNo ratings yet
- DD43.1 Assembly GuideDocument17 pagesDD43.1 Assembly Guideohsweet jeremyNo ratings yet
- Fire Requirements - UBBLDocument47 pagesFire Requirements - UBBLchipon3150% (2)
- Uses and Applications of The Different Gas LawsDocument8 pagesUses and Applications of The Different Gas LawsWin7Addict100% (30)
- DocumentDocument1 pageDocumentcristinemaelumanao18No ratings yet
- Definition of Boyle's Law: What Is An Ideal Gas?Document4 pagesDefinition of Boyle's Law: What Is An Ideal Gas?حسين كاظم ياسينNo ratings yet
- Science ScriptDocument2 pagesScience ScriptTrixie San DiegoNo ratings yet
- Gas Laws Opt OutDocument39 pagesGas Laws Opt OutTahir HussainNo ratings yet
- Boyle's Law LESSON PLANDocument5 pagesBoyle's Law LESSON PLANQueencess Ara TorresNo ratings yet
- Entropy 03 00116Document2 pagesEntropy 03 00116Ali MpkNo ratings yet
- Boyles LawDocument6 pagesBoyles LawMara TillesNo ratings yet
- Application of BoyleDocument10 pagesApplication of BoyleRuthcel BautistaNo ratings yet
- Scuba Diving and Gas LawDocument17 pagesScuba Diving and Gas LawTie Ing HuiNo ratings yet
- ANP1105B Topic 6B Respiratory Physiology 2014Document42 pagesANP1105B Topic 6B Respiratory Physiology 2014mdhNo ratings yet
- Gas Laws Form 4 Exercise and ExamplesDocument6 pagesGas Laws Form 4 Exercise and ExampleszahidahoseinNo ratings yet
- GAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidDocument9 pagesGAS LAWS: Boyle's Law, Charles's Law and Gay-Lussac's Law: Waste-Face-Masks-Ppe-CovidMichiiee BatallaNo ratings yet
- Lesson 1: Boyle's LawDocument16 pagesLesson 1: Boyle's LawKen ArellanoNo ratings yet
- Gas Laws Boyle'S Law: Presented By: Raymar V. Villarama Althea Lynn B. CallantaDocument17 pagesGas Laws Boyle'S Law: Presented By: Raymar V. Villarama Althea Lynn B. CallantaAlthea Lynn B. CallantaNo ratings yet
- The Science of The Hyperbaric ChamberDocument48 pagesThe Science of The Hyperbaric Chamberopan kursusNo ratings yet
- 循环呼吸器潜水的呼吸生理学Document14 pages循环呼吸器潜水的呼吸生理学彭亦荣No ratings yet
- ExamplesDocument12 pagesExamplesMoldir SerikNo ratings yet
- Boyles Law CompleteDocument4 pagesBoyles Law CompleteLincoln PiaoanNo ratings yet
- Real Life Application of Boyles LawDocument12 pagesReal Life Application of Boyles LawChelsie Rose GalangcoNo ratings yet
- Recall The Functions of The Organs in The Gas Exchange SystemDocument14 pagesRecall The Functions of The Organs in The Gas Exchange SystemAhmad AhmadNo ratings yet
- Sport Diving-CompletedDocument66 pagesSport Diving-CompletedMuthu KumarNo ratings yet
- Respiratory System: Dr. Agung Kurniawan, MkesDocument31 pagesRespiratory System: Dr. Agung Kurniawan, MkesJojo BodhoNo ratings yet
- Siyensikula-Boyle's LawDocument3 pagesSiyensikula-Boyle's Lawrolando dumlaoNo ratings yet
- Pulmonary Ventilation PDFDocument11 pagesPulmonary Ventilation PDFSrsblackieNo ratings yet
- Formal Report 7ADocument6 pagesFormal Report 7ANico YowNo ratings yet
- Modules On Gas LawsDocument10 pagesModules On Gas LawsJudith Cambri CueNo ratings yet
- Boyle's LawDocument24 pagesBoyle's LawRalph Jasil Esparagoza EscanillasNo ratings yet
- Roselyn 1Document5 pagesRoselyn 1Lecsos clyde Pang-agNo ratings yet
- Study Guide 4: Diaphragm External Intercostal MusclesDocument37 pagesStudy Guide 4: Diaphragm External Intercostal MusclesItadori YujiNo ratings yet
- Topic 5 (Part 1)Document25 pagesTopic 5 (Part 1)DjemKurt GagatamNo ratings yet
- Boyle's LawDocument9 pagesBoyle's LawGel AmihanNo ratings yet
- Q4 Behavior of GasesDocument31 pagesQ4 Behavior of Gasesfsean2008No ratings yet
- Respiration NotesDocument4 pagesRespiration NotesJana AldourNo ratings yet
- Gas Laws Lesson 2 PPT For StudentDocument50 pagesGas Laws Lesson 2 PPT For Studentchlovelain1No ratings yet
- Lesson Plan - Boyle's LawDocument3 pagesLesson Plan - Boyle's LawQueencess Ara TorresNo ratings yet
- LoveubabykoDocument2 pagesLoveubabykoSheila Mae NimNo ratings yet
- Just Breathe Activity-Lungs WooksheetDocument1 pageJust Breathe Activity-Lungs WooksheetJhade Rio GadinganNo ratings yet
- Boyle's LawDocument31 pagesBoyle's LawDaryl CadanillaNo ratings yet
- Week5 AbcdDocument7 pagesWeek5 AbcdCookie MonsterNo ratings yet
- Use The Law To Explain The Following ObservationsDocument6 pagesUse The Law To Explain The Following ObservationsCedric Adrian AblogNo ratings yet
- Physiology, Boyle's Law - StatPearls - NCBI BookshelfDocument3 pagesPhysiology, Boyle's Law - StatPearls - NCBI BookshelfChris IidaNo ratings yet
- 24 - Physiology of The Respiratory System PDFDocument38 pages24 - Physiology of The Respiratory System PDFlovelyc95No ratings yet
- Gas Exchange in Humans Grade 9Document49 pagesGas Exchange in Humans Grade 9harliv2.5chandhokNo ratings yet
- Breathing - and - Gas Exchange Igcse BiologyDocument88 pagesBreathing - and - Gas Exchange Igcse BiologyEllie HousenNo ratings yet
- 37 AnswersDocument10 pages37 AnswersJasmine Nicole EnriquezNo ratings yet
- Respiration - VentilationDocument102 pagesRespiration - VentilationSodeinde SimeonNo ratings yet
- If You Play-WPS OfficeDocument1 pageIf You Play-WPS Officeawitg031No ratings yet
- Descriptions PagesDocument31 pagesDescriptions PageskhairulNo ratings yet
- BoyleDocument1 pageBoylePASTEURSYNCH GELBOLINGO, ERNIE IINo ratings yet
- Presentation1 Boyles LawDocument40 pagesPresentation1 Boyles LawErnest S. AbiertasNo ratings yet
- The Human Breathing System Part2Document23 pagesThe Human Breathing System Part2Delfin Jr UrbanoNo ratings yet
- Gas Exchange in Humans CIE IGCSE Biology 610 Classified Paster Paper 2Document21 pagesGas Exchange in Humans CIE IGCSE Biology 610 Classified Paster Paper 2IGCSE Physics & ChemistryNo ratings yet
- Behavior of GasesDocument13 pagesBehavior of GasesSharalyn P. SiaNo ratings yet
- 4th Quarter Week 1 1Document59 pages4th Quarter Week 1 1Jonathan MayoNo ratings yet
- Q4-WEEK 1-Boyle's LawDocument34 pagesQ4-WEEK 1-Boyle's LawAdonis SanielNo ratings yet
- Module 4Document28 pagesModule 424E&CSSniddhi MalankarNo ratings yet
- Is.13944.1994 Window RetensionDocument13 pagesIs.13944.1994 Window Retensionrpagarwal2No ratings yet
- PDFlib in PHP HowTo PDFDocument11 pagesPDFlib in PHP HowTo PDFJordi BaleNo ratings yet
- Chapter 5 Machine 1Document44 pagesChapter 5 Machine 1martazemeduNo ratings yet
- Ramdump Wcss Msa0 2023-12-24 18-17-34 PropsDocument14 pagesRamdump Wcss Msa0 2023-12-24 18-17-34 Propschaimaefadil3No ratings yet
- HP Color Laserjet Cp5220: Service ManualDocument51 pagesHP Color Laserjet Cp5220: Service ManualBelkisNo ratings yet
- Discovery 20: Configure Syslog: Task 1: Configure The Router To Log To ServerDocument2 pagesDiscovery 20: Configure Syslog: Task 1: Configure The Router To Log To ServerIonut StanciuNo ratings yet
- Software Development Learning Path - Board InfinityDocument22 pagesSoftware Development Learning Path - Board InfinityNirupam NishantNo ratings yet
- Types of Graphs in Data StructuresDocument18 pagesTypes of Graphs in Data StructuresJahangir AliNo ratings yet
- Steam PWRDocument3 pagesSteam PWRIsuru OvinNo ratings yet
- FF0329 01 Pyramid Concept Template PowerpointDocument7 pagesFF0329 01 Pyramid Concept Template PowerpointCoach Felipe FarthNo ratings yet
- Briana L. Harvath ResumeDocument3 pagesBriana L. Harvath ResumeBriana HarvathNo ratings yet
- WFG 2013 enDocument28 pagesWFG 2013 enIhcene BoudaliNo ratings yet
- Mtu - Oil & Gas Sales ProgramDocument63 pagesMtu - Oil & Gas Sales Programalamen ZayidNo ratings yet
- Information Sheet 1.2-1docxDocument4 pagesInformation Sheet 1.2-1docxMarc GelacioNo ratings yet
- V-ZUG LTD Spare Parts List V-ZUG LTD Spare Parts List V-ZUG LTD Spare Parts ListDocument21 pagesV-ZUG LTD Spare Parts List V-ZUG LTD Spare Parts List V-ZUG LTD Spare Parts ListChristian LaffinNo ratings yet
- Assessing Concrete Strength Variability in Existing Structures Based On The Results of NDTs - 2018Document15 pagesAssessing Concrete Strength Variability in Existing Structures Based On The Results of NDTs - 2018Carlos MartinsNo ratings yet
- Offline Coding Activities - Computational Thinking - Discovery Education Coding PDFDocument14 pagesOffline Coding Activities - Computational Thinking - Discovery Education Coding PDFShakila ShakiNo ratings yet
- Spesifikasi Somatom Go - AllDocument5 pagesSpesifikasi Somatom Go - AllRadiologi RSUD KilisuciNo ratings yet
- Instructional Material FOR EETE 10013 Philippine Electrical CodeDocument58 pagesInstructional Material FOR EETE 10013 Philippine Electrical CodeRobert ApiladoNo ratings yet
- How To Write Shared LibrariesDocument47 pagesHow To Write Shared LibrariesPeng XiaoNo ratings yet
- Document 2820181.2 - Enterprise Manager 13.5 Cloud Control Upgrade Run BookDocument15 pagesDocument 2820181.2 - Enterprise Manager 13.5 Cloud Control Upgrade Run BookEhsan BabarNo ratings yet
- Project PumpDocument4 pagesProject PumpPrajwal WakhareNo ratings yet
- Atde 16 Atde210093Document10 pagesAtde 16 Atde210093Josip StjepandicNo ratings yet
- Software Engineering PPT - 3Document73 pagesSoftware Engineering PPT - 3Mithil JogiNo ratings yet
- C# Database Connection Tutorial With ExampleDocument35 pagesC# Database Connection Tutorial With ExampleSebastian100% (2)
- DC Convolution CodesDocument136 pagesDC Convolution CodesARAVINDNo ratings yet