Professional Documents
Culture Documents
Xavier M Gil 610 S 800 W #30 Payson, UT 84651-2625: Test Report
Xavier M Gil 610 S 800 W #30 Payson, UT 84651-2625: Test Report
Uploaded by
FranciscoCopyright:
Available Formats
You might also like
- Positive: What Does It Mean To Have A Test Result?Document2 pagesPositive: What Does It Mean To Have A Test Result?lelo lamo100% (1)
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?liz100% (2)
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Todd EddyNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?robertoNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Gabriela GuardaNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Kelli Belli100% (1)
- Forensic MedicineDocument199 pagesForensic MedicineJannat Zahra100% (1)
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesVivek VinuNo ratings yet
- Result 24657 00757Document1 pageResult 24657 00757margarita echeverryNo ratings yet
- Result 27686 00261Document1 pageResult 27686 00261Tracee GoffNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Seiyi KohayagawaNo ratings yet
- Jean Durant 1940 Biarritz DR Apt 8 Miami Beach, FL 33141: Test ReportDocument1 pageJean Durant 1940 Biarritz DR Apt 8 Miami Beach, FL 33141: Test ReportStacy KestwickNo ratings yet
- Result 32285 00269Document1 pageResult 32285 00269Paz BianchiNo ratings yet
- Report F28b66a8 4cdc 4f63 B84a 0bd8cefafae9Document2 pagesReport F28b66a8 4cdc 4f63 B84a 0bd8cefafae9romyludonadoNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Ajmel Azad EliasNo ratings yet
- Aragaw 206714-1 364272Document1 pageAragaw 206714-1 364272zeine omerNo ratings yet
- El Arte de DelegarDocument2 pagesEl Arte de DelegarGreen DusterNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Mr. Banavathu Gopi Kiran NaikDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Mr. Banavathu Gopi Kiran NaikGopi Kiran NaikNo ratings yet
- Covid-19 Qualitative Real Time PCR:: DR - SELFDocument1 pageCovid-19 Qualitative Real Time PCR:: DR - SELFRajdeep DeyNo ratings yet
- Covid Test 6Document1 pageCovid Test 6DjibzlaeNo ratings yet
- ResultDocument1 pageResultNandini Pritesh PatelNo ratings yet
- Ivd Report Mn5gp - IndividualDocument2 pagesIvd Report Mn5gp - IndividualToni MirosanuNo ratings yet
- 07 - SAMPLE COVID Molecular Negative Not Detected Sample ReportDocument1 page07 - SAMPLE COVID Molecular Negative Not Detected Sample ReportuzairNo ratings yet
- Covid-19 Qualitative Real Time PCR:: DR - Pradip Kumar DasDocument1 pageCovid-19 Qualitative Real Time PCR:: DR - Pradip Kumar Dasdebabrata maitraNo ratings yet
- Covid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedDocument2 pagesCovid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedRonni PriceNo ratings yet
- MithunDocument1 pageMithunMithun MukherjeeNo ratings yet
- Mr. Ravtej Singh: Test Description Observed Value Biological Reference RangeDocument2 pagesMr. Ravtej Singh: Test Description Observed Value Biological Reference Rangevasu jamwalNo ratings yet
- Index PHPDocument1 pageIndex PHPvlera.jashari2No ratings yet
- Research & Development: Test Name Status Result Unit Reference Interval SARS-COV-2 Real-Time PCR, QualitativeDocument2 pagesResearch & Development: Test Name Status Result Unit Reference Interval SARS-COV-2 Real-Time PCR, QualitativeakashNo ratings yet
- SR3802595Document1 pageSR3802595om agencyNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Brian MagallanesNo ratings yet
- L Hi Appt Results 06252021Document7 pagesL Hi Appt Results 06252021C RealNo ratings yet
- KiyaraDocument1 pageKiyaraUpadhayayAnkurNo ratings yet
- HERMANO, Nicolas JR. Anay: COVID-19 PCR (C19T1)Document1 pageHERMANO, Nicolas JR. Anay: COVID-19 PCR (C19T1)QuizaNo ratings yet
- PopulationHealth PDFDocument1 pagePopulationHealth PDFMatar DrameNo ratings yet
- 21122557122c Mr. Devki Nandan PunethaDocument2 pages21122557122c Mr. Devki Nandan PunethaDevkinandan PunethaNo ratings yet
- Prophasedx Laboratory Phone: (855) 982-1100Document2 pagesProphasedx Laboratory Phone: (855) 982-1100ommanon15 aNo ratings yet
- April Jane: Cellular Immunology and ImmunogeneticsDocument2 pagesApril Jane: Cellular Immunology and ImmunogeneticsAya BeeNo ratings yet
- Personal Information: Molecular BiologyDocument2 pagesPersonal Information: Molecular BiologyAmandeep SinghNo ratings yet
- Clinical Significance:: Conditions of Laboratory Testing & ReportingDocument2 pagesClinical Significance:: Conditions of Laboratory Testing & ReportingAashwin PoovankunnilNo ratings yet
- Take Care Sa GensanDocument1 pageTake Care Sa GensanAya BeeNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Sophy SvecNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Master Dodla Venkata SanjeethDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Master Dodla Venkata SanjeethDv ScNo ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRDRSM QAUNo ratings yet
- SR4750118 1Document1 pageSR4750118 1ac9467593No ratings yet
- Sars-Cov-2 Rna, QL, RT PCR (Covid-19) - DetailsDocument1 pageSars-Cov-2 Rna, QL, RT PCR (Covid-19) - DetailsimlimitededitionNo ratings yet
- Kartik RTPCRDocument2 pagesKartik RTPCRMahesh PatilNo ratings yet
- Not Infected (Negative) : ResultDocument1 pageNot Infected (Negative) : Resultعبد الله ريانتو أحمدNo ratings yet
- Department of Molecular Biology Covid-19 Virus Qualitative PCRDocument2 pagesDepartment of Molecular Biology Covid-19 Virus Qualitative PCRpooja sharmaNo ratings yet
- Madhan - 642161200148401 2Document2 pagesMadhan - 642161200148401 2madhanNo ratings yet
- Ma'ruf SabilanDocument1 pageMa'ruf SabilanManga MinNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Mr. Machineni Sai KrishnaDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Mr. Machineni Sai KrishnaVenkat Sai Dhilli Engg. 2020No ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRUmar BadshahNo ratings yet
- Aed2020-27745 MR - Tejashwin Ravishankar 129334Document1 pageAed2020-27745 MR - Tejashwin Ravishankar 129334sadhanaNo ratings yet
- Dean Anthony Priore-2021120815-8-12-2021Document1 pageDean Anthony Priore-2021120815-8-12-2021Dean PrioreNo ratings yet
- RapidCare - RT PCR - September 5th 3Document1 pageRapidCare - RT PCR - September 5th 3দীপা পালNo ratings yet
- KRISHNADocument1 pageKRISHNAUpadhayayAnkurNo ratings yet
- Lab-Result - Ronal Saisayado - 2871970 - 21209876Document1 pageLab-Result - Ronal Saisayado - 2871970 - 21209876Kalam ManaluNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Miss. Dodla GaganamokshaDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Miss. Dodla GaganamokshaDv ScNo ratings yet
- La Batalla Por Tu MenteDocument2 pagesLa Batalla Por Tu MenteAda Milagros Meléndez DíazNo ratings yet
- SARS-CoV-2 Viral Outbreak Investigation: Laboratory PerspectiveFrom EverandSARS-CoV-2 Viral Outbreak Investigation: Laboratory PerspectiveNo ratings yet
- Spinal Cord Injury Rehabilitation by Karen Whalley HammellDocument366 pagesSpinal Cord Injury Rehabilitation by Karen Whalley Hammellasllo100% (1)
- 6 Plugin 047974Document32 pages6 Plugin 047974Sutardi DiharjoNo ratings yet
- TROPiCS-02 - JCO 2022Document16 pagesTROPiCS-02 - JCO 2022Nicola CrestiNo ratings yet
- Newer Formulations of The Triptans: Advances in Migraine ManagementDocument21 pagesNewer Formulations of The Triptans: Advances in Migraine ManagementShivam BhadauriaNo ratings yet
- Antibiotic Resistance and Evolution Case Study GR 11Document7 pagesAntibiotic Resistance and Evolution Case Study GR 11Petro BricalliNo ratings yet
- Module 15 - Treatment Planning SlideShow 080306Document45 pagesModule 15 - Treatment Planning SlideShow 080306ishtiiiNo ratings yet
- Pedia Old eDocument11 pagesPedia Old eDivynne MadeloNo ratings yet
- Abdulraheem Et AlDocument10 pagesAbdulraheem Et AlLaoye Abdulrahman AdewaleNo ratings yet
- Effectiveness and Feasibility of Limberg and Karydakis FlapDocument69 pagesEffectiveness and Feasibility of Limberg and Karydakis Flapnaveena reddyNo ratings yet
- Nursing Responsibilities in Delivery RoomDocument23 pagesNursing Responsibilities in Delivery RoomNica Sue67% (3)
- Southampton Grading SystemDocument5 pagesSouthampton Grading SystemswestyNo ratings yet
- Pediatric HerniaDocument4 pagesPediatric HerniaRudi haris munandar0% (1)
- CovidDocument6 pagesCovidMahmoud ElmahdyNo ratings yet
- A Case of Retained Placenta in A Dairy Cow: December 2016Document4 pagesA Case of Retained Placenta in A Dairy Cow: December 2016Andi HasrawatiNo ratings yet
- Leung and Lam 2018Document9 pagesLeung and Lam 2018Wahida MuntazaNo ratings yet
- GMP FinalDocument37 pagesGMP FinalekramNo ratings yet
- Community Health Nursing 1 Lecture Week 4Document7 pagesCommunity Health Nursing 1 Lecture Week 4Cherry Louise O. SanvictoresNo ratings yet
- Alteration in ComfortDocument2 pagesAlteration in Comfortbchouman777No ratings yet
- Alsangedy Bullets For PacesDocument317 pagesAlsangedy Bullets For PacesSagit Nauman81No ratings yet
- Community Health Nursing PortfolioDocument42 pagesCommunity Health Nursing PortfolioAljane BistoNo ratings yet
- Severe Cutaneous Adverse Drug ReactionDocument87 pagesSevere Cutaneous Adverse Drug ReactionEpi PanjaitanNo ratings yet
- Clinical Presentation in SyringomyeliaDocument7 pagesClinical Presentation in SyringomyeliaRajkamal SarmaNo ratings yet
- Sickle Cell Disease ED Vasocclusive Crises Pain Management GuidelineDocument1 pageSickle Cell Disease ED Vasocclusive Crises Pain Management GuidelineLakshmanan KrishnamurtiNo ratings yet
- Trans SaVi Oto Lec 03 Diseases of The External and Middle EarDocument13 pagesTrans SaVi Oto Lec 03 Diseases of The External and Middle EarJoherNo ratings yet
- Ramachari Anaesthesiologist-Brief BiodataDocument1 pageRamachari Anaesthesiologist-Brief Biodatadr_saketram6146No ratings yet
- Sample Medical Incident Report: Section 1Document2 pagesSample Medical Incident Report: Section 1DS SystemsNo ratings yet
- Maria Stadelman: Current AddressDocument2 pagesMaria Stadelman: Current AddressMaria StadelmanNo ratings yet
- Myofunctional Therapy CapstoneDocument21 pagesMyofunctional Therapy Capstoneapi-673020353No ratings yet
- Congenital-Abnormalities-and-Preterm-Birth-Related-to-Maternal-Illnesses-During-Pregnancy (2010) PDFDocument542 pagesCongenital-Abnormalities-and-Preterm-Birth-Related-to-Maternal-Illnesses-During-Pregnancy (2010) PDFPuspa Sekar MentariNo ratings yet
Xavier M Gil 610 S 800 W #30 Payson, UT 84651-2625: Test Report
Xavier M Gil 610 S 800 W #30 Payson, UT 84651-2625: Test Report
Uploaded by
FranciscoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Xavier M Gil 610 S 800 W #30 Payson, UT 84651-2625: Test Report
Xavier M Gil 610 S 800 W #30 Payson, UT 84651-2625: Test Report
Uploaded by
FranciscoCopyright:
Available Formats
11 1 3 ite 1
alt a e it 41 4
stome e vi e: support@nomitesting.com
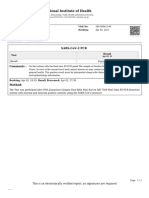
Xavier M Gil
610 S 800 W #30
Payson, UT 84651-2625
Test Report
Patient Date of Birth Report Date/Time Collection Date/Time Medical Record Number
Xavier M Gil 11/21/2010 1/16/2022 10:06AM 1/16/2022 9:51AM 26395-00343
Test Employee ID Result Type of
Test
SARS-CoV-2 N/A Negative COVID-Ag
Name and Address of Lab Where Test Was Performed
Provo Town Centre Mall (Appointment Only) Open M-Su 7am-7pm: 1200 Towne Centre Blvd., Provo,
lfUT
your is NEGATIVE for SARS-CoV-2 (coronavirus 2), the virus that causes COVID-19 this may mean you were
resultUSA
84601,
not infected at the time your test was performed or there was not enough virus present to be detected.
If your result is POSITIVE for SARS-CoV-2 (Coronavirus 2), the virus that causes COVID-19, you were infected with
COVID-19 at the time of testing.
If you receive an INVALID or REJECTED test result, it means that the sample you provided was non-optimal and could
not be tested or could not produce appropriate data to determine whether or not you are infected with the virus. If you
receive an invalid/rejected test result, please re-collect and re-submit a new sample.
CDC guidelines for COVID-19 are updated regularly. For the most up-to-date information on testing, quarantining, and
isolation, please visit the Centers for Disease Control and Prevention websites:
https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html
https://www.cdc.gov/coronavirus/2019-ncov/testing/diagnostic-testing.html
Method: The GenBody COVID-19 Ag test is an immunochromatographic rapid diagnostic test (RDT) intended for the
qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) or anterior nasal
(AN) swab specimens from individuals who are suspected of COVID-19 by their healthcare provider within the first six
days of symptom onset, or from individuals without symptoms or other epidemiological reasons to suspect COVID-19
when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
Limitations: There are different kinds of tests for COVID-19. Molecular tests (also known as PCR tests) detect genetic
material from the virus. Antigen tests detect proteins from the virus. Antigen tests are specific for the virus but are not as
sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not rule out
infection.
Disclaimer: The GenBody COVID-19 Ag test has not been FDA cleared or approved. It has been authorized by the FDA
under an emergency use authorization for use by authorized entities. The test has been authorized only for the detection
of proteins from SARS-CoV-2, not for any other viruses or pathogens, and is only authorized for the duration of the
declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection
and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is
terminated or revoked sooner.
Fact Sheets
https://www.fda.gov/media/150786/download
https://www.fda.gov/media/150787/download
You might also like
- Positive: What Does It Mean To Have A Test Result?Document2 pagesPositive: What Does It Mean To Have A Test Result?lelo lamo100% (1)
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?liz100% (2)
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Todd EddyNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?robertoNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Gabriela GuardaNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Kelli Belli100% (1)
- Forensic MedicineDocument199 pagesForensic MedicineJannat Zahra100% (1)
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesVivek VinuNo ratings yet
- Result 24657 00757Document1 pageResult 24657 00757margarita echeverryNo ratings yet
- Result 27686 00261Document1 pageResult 27686 00261Tracee GoffNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Seiyi KohayagawaNo ratings yet
- Jean Durant 1940 Biarritz DR Apt 8 Miami Beach, FL 33141: Test ReportDocument1 pageJean Durant 1940 Biarritz DR Apt 8 Miami Beach, FL 33141: Test ReportStacy KestwickNo ratings yet
- Result 32285 00269Document1 pageResult 32285 00269Paz BianchiNo ratings yet
- Report F28b66a8 4cdc 4f63 B84a 0bd8cefafae9Document2 pagesReport F28b66a8 4cdc 4f63 B84a 0bd8cefafae9romyludonadoNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Ajmel Azad EliasNo ratings yet
- Aragaw 206714-1 364272Document1 pageAragaw 206714-1 364272zeine omerNo ratings yet
- El Arte de DelegarDocument2 pagesEl Arte de DelegarGreen DusterNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Mr. Banavathu Gopi Kiran NaikDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Mr. Banavathu Gopi Kiran NaikGopi Kiran NaikNo ratings yet
- Covid-19 Qualitative Real Time PCR:: DR - SELFDocument1 pageCovid-19 Qualitative Real Time PCR:: DR - SELFRajdeep DeyNo ratings yet
- Covid Test 6Document1 pageCovid Test 6DjibzlaeNo ratings yet
- ResultDocument1 pageResultNandini Pritesh PatelNo ratings yet
- Ivd Report Mn5gp - IndividualDocument2 pagesIvd Report Mn5gp - IndividualToni MirosanuNo ratings yet
- 07 - SAMPLE COVID Molecular Negative Not Detected Sample ReportDocument1 page07 - SAMPLE COVID Molecular Negative Not Detected Sample ReportuzairNo ratings yet
- Covid-19 Qualitative Real Time PCR:: DR - Pradip Kumar DasDocument1 pageCovid-19 Qualitative Real Time PCR:: DR - Pradip Kumar Dasdebabrata maitraNo ratings yet
- Covid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedDocument2 pagesCovid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedRonni PriceNo ratings yet
- MithunDocument1 pageMithunMithun MukherjeeNo ratings yet
- Mr. Ravtej Singh: Test Description Observed Value Biological Reference RangeDocument2 pagesMr. Ravtej Singh: Test Description Observed Value Biological Reference Rangevasu jamwalNo ratings yet
- Index PHPDocument1 pageIndex PHPvlera.jashari2No ratings yet
- Research & Development: Test Name Status Result Unit Reference Interval SARS-COV-2 Real-Time PCR, QualitativeDocument2 pagesResearch & Development: Test Name Status Result Unit Reference Interval SARS-COV-2 Real-Time PCR, QualitativeakashNo ratings yet
- SR3802595Document1 pageSR3802595om agencyNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Brian MagallanesNo ratings yet
- L Hi Appt Results 06252021Document7 pagesL Hi Appt Results 06252021C RealNo ratings yet
- KiyaraDocument1 pageKiyaraUpadhayayAnkurNo ratings yet
- HERMANO, Nicolas JR. Anay: COVID-19 PCR (C19T1)Document1 pageHERMANO, Nicolas JR. Anay: COVID-19 PCR (C19T1)QuizaNo ratings yet
- PopulationHealth PDFDocument1 pagePopulationHealth PDFMatar DrameNo ratings yet
- 21122557122c Mr. Devki Nandan PunethaDocument2 pages21122557122c Mr. Devki Nandan PunethaDevkinandan PunethaNo ratings yet
- Prophasedx Laboratory Phone: (855) 982-1100Document2 pagesProphasedx Laboratory Phone: (855) 982-1100ommanon15 aNo ratings yet
- April Jane: Cellular Immunology and ImmunogeneticsDocument2 pagesApril Jane: Cellular Immunology and ImmunogeneticsAya BeeNo ratings yet
- Personal Information: Molecular BiologyDocument2 pagesPersonal Information: Molecular BiologyAmandeep SinghNo ratings yet
- Clinical Significance:: Conditions of Laboratory Testing & ReportingDocument2 pagesClinical Significance:: Conditions of Laboratory Testing & ReportingAashwin PoovankunnilNo ratings yet
- Take Care Sa GensanDocument1 pageTake Care Sa GensanAya BeeNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?Sophy SvecNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Master Dodla Venkata SanjeethDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Master Dodla Venkata SanjeethDv ScNo ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRDRSM QAUNo ratings yet
- SR4750118 1Document1 pageSR4750118 1ac9467593No ratings yet
- Sars-Cov-2 Rna, QL, RT PCR (Covid-19) - DetailsDocument1 pageSars-Cov-2 Rna, QL, RT PCR (Covid-19) - DetailsimlimitededitionNo ratings yet
- Kartik RTPCRDocument2 pagesKartik RTPCRMahesh PatilNo ratings yet
- Not Infected (Negative) : ResultDocument1 pageNot Infected (Negative) : Resultعبد الله ريانتو أحمدNo ratings yet
- Department of Molecular Biology Covid-19 Virus Qualitative PCRDocument2 pagesDepartment of Molecular Biology Covid-19 Virus Qualitative PCRpooja sharmaNo ratings yet
- Madhan - 642161200148401 2Document2 pagesMadhan - 642161200148401 2madhanNo ratings yet
- Ma'ruf SabilanDocument1 pageMa'ruf SabilanManga MinNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Mr. Machineni Sai KrishnaDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Mr. Machineni Sai KrishnaVenkat Sai Dhilli Engg. 2020No ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRUmar BadshahNo ratings yet
- Aed2020-27745 MR - Tejashwin Ravishankar 129334Document1 pageAed2020-27745 MR - Tejashwin Ravishankar 129334sadhanaNo ratings yet
- Dean Anthony Priore-2021120815-8-12-2021Document1 pageDean Anthony Priore-2021120815-8-12-2021Dean PrioreNo ratings yet
- RapidCare - RT PCR - September 5th 3Document1 pageRapidCare - RT PCR - September 5th 3দীপা পালNo ratings yet
- KRISHNADocument1 pageKRISHNAUpadhayayAnkurNo ratings yet
- Lab-Result - Ronal Saisayado - 2871970 - 21209876Document1 pageLab-Result - Ronal Saisayado - 2871970 - 21209876Kalam ManaluNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Miss. Dodla GaganamokshaDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Miss. Dodla GaganamokshaDv ScNo ratings yet
- La Batalla Por Tu MenteDocument2 pagesLa Batalla Por Tu MenteAda Milagros Meléndez DíazNo ratings yet
- SARS-CoV-2 Viral Outbreak Investigation: Laboratory PerspectiveFrom EverandSARS-CoV-2 Viral Outbreak Investigation: Laboratory PerspectiveNo ratings yet
- Spinal Cord Injury Rehabilitation by Karen Whalley HammellDocument366 pagesSpinal Cord Injury Rehabilitation by Karen Whalley Hammellasllo100% (1)
- 6 Plugin 047974Document32 pages6 Plugin 047974Sutardi DiharjoNo ratings yet
- TROPiCS-02 - JCO 2022Document16 pagesTROPiCS-02 - JCO 2022Nicola CrestiNo ratings yet
- Newer Formulations of The Triptans: Advances in Migraine ManagementDocument21 pagesNewer Formulations of The Triptans: Advances in Migraine ManagementShivam BhadauriaNo ratings yet
- Antibiotic Resistance and Evolution Case Study GR 11Document7 pagesAntibiotic Resistance and Evolution Case Study GR 11Petro BricalliNo ratings yet
- Module 15 - Treatment Planning SlideShow 080306Document45 pagesModule 15 - Treatment Planning SlideShow 080306ishtiiiNo ratings yet
- Pedia Old eDocument11 pagesPedia Old eDivynne MadeloNo ratings yet
- Abdulraheem Et AlDocument10 pagesAbdulraheem Et AlLaoye Abdulrahman AdewaleNo ratings yet
- Effectiveness and Feasibility of Limberg and Karydakis FlapDocument69 pagesEffectiveness and Feasibility of Limberg and Karydakis Flapnaveena reddyNo ratings yet
- Nursing Responsibilities in Delivery RoomDocument23 pagesNursing Responsibilities in Delivery RoomNica Sue67% (3)
- Southampton Grading SystemDocument5 pagesSouthampton Grading SystemswestyNo ratings yet
- Pediatric HerniaDocument4 pagesPediatric HerniaRudi haris munandar0% (1)
- CovidDocument6 pagesCovidMahmoud ElmahdyNo ratings yet
- A Case of Retained Placenta in A Dairy Cow: December 2016Document4 pagesA Case of Retained Placenta in A Dairy Cow: December 2016Andi HasrawatiNo ratings yet
- Leung and Lam 2018Document9 pagesLeung and Lam 2018Wahida MuntazaNo ratings yet
- GMP FinalDocument37 pagesGMP FinalekramNo ratings yet
- Community Health Nursing 1 Lecture Week 4Document7 pagesCommunity Health Nursing 1 Lecture Week 4Cherry Louise O. SanvictoresNo ratings yet
- Alteration in ComfortDocument2 pagesAlteration in Comfortbchouman777No ratings yet
- Alsangedy Bullets For PacesDocument317 pagesAlsangedy Bullets For PacesSagit Nauman81No ratings yet
- Community Health Nursing PortfolioDocument42 pagesCommunity Health Nursing PortfolioAljane BistoNo ratings yet
- Severe Cutaneous Adverse Drug ReactionDocument87 pagesSevere Cutaneous Adverse Drug ReactionEpi PanjaitanNo ratings yet
- Clinical Presentation in SyringomyeliaDocument7 pagesClinical Presentation in SyringomyeliaRajkamal SarmaNo ratings yet
- Sickle Cell Disease ED Vasocclusive Crises Pain Management GuidelineDocument1 pageSickle Cell Disease ED Vasocclusive Crises Pain Management GuidelineLakshmanan KrishnamurtiNo ratings yet
- Trans SaVi Oto Lec 03 Diseases of The External and Middle EarDocument13 pagesTrans SaVi Oto Lec 03 Diseases of The External and Middle EarJoherNo ratings yet
- Ramachari Anaesthesiologist-Brief BiodataDocument1 pageRamachari Anaesthesiologist-Brief Biodatadr_saketram6146No ratings yet
- Sample Medical Incident Report: Section 1Document2 pagesSample Medical Incident Report: Section 1DS SystemsNo ratings yet
- Maria Stadelman: Current AddressDocument2 pagesMaria Stadelman: Current AddressMaria StadelmanNo ratings yet
- Myofunctional Therapy CapstoneDocument21 pagesMyofunctional Therapy Capstoneapi-673020353No ratings yet
- Congenital-Abnormalities-and-Preterm-Birth-Related-to-Maternal-Illnesses-During-Pregnancy (2010) PDFDocument542 pagesCongenital-Abnormalities-and-Preterm-Birth-Related-to-Maternal-Illnesses-During-Pregnancy (2010) PDFPuspa Sekar MentariNo ratings yet