Professional Documents
Culture Documents
Acids, Alkalis and Titrations Paper 2
Acids, Alkalis and Titrations Paper 2
Uploaded by
DinangaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acids, Alkalis and Titrations Paper 2
Acids, Alkalis and Titrations Paper 2
Uploaded by
DinangaCopyright:
Available Formats
(b) The diagrams show the readings on the thermometer before and after one of the
students added a portion of acid.
°C 20 °C 25
15 20
before adding acid after adding acid
Write down the thermometer readings and calculate the temperature change.
(3)
Temperature before adding acid ................................................................ °C
Temperature after adding acid . .................................................................... °C
Temperature change . . . . . . . . . . . . . . . . . . . . . . . . . . . .................................................................... °C
(c) In a titration using solutions of the same acid and alkali but of different concentrations,
she recorded these results.
volume of sulfuric acid = 25.0 cm3
concentration of sulfuric acid = 0.107 mol/dm3
average (mean) volume of lithium hydroxide solution = 22.85 cm3
The equation for the reaction is
2LiOH + H2SO4 o Li2SO4 + 2H2O
(i) Calculate the amount, in moles, of H2SO4 in 25.0 cm3 of 0.107 mol/dm3 sulfuric acid.
(2)
amount of H2SO4 = ................................................. . . . . . . . . . . . . . . . mol
(ii) Calculate the amount, in moles, of LiOH in the 22.85 cm3 of lithium hydroxide solution.
(1)
amount of LiOH = ................................................. . . . . . . . . . . . . . . . mol
(iii) Calculate the concentration, in mol/dm3, of LiOH in the lithium hydroxide solution.
(2)
concentration of LiOH = ............................................................... . mol/dm3
(b) The diagram shows the thermometer readings in one experiment.
°C 20 °C 20
15 15
before adding alkali after adding alkali
Write down the thermometer readings and calculate the temperature change.
(3)
temperature after adding alkali .................................................................... °C
temperature before adding alkali ............................................................... °C
temperature change . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .................................................................... °C
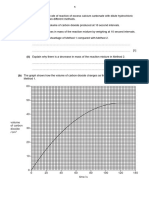
(d) Plot the results of experiment 3 on the grid.
Draw a straight line of best fit through the first four points, and another straight
line of best fit through the last three points. Make sure that the two lines cross.
(4)
25.0 –
Temperature
in °C 20.0 –
15.0 –
–
–
0 10.0 20.0 30.0
Volume of alkali in cm3
(e) The point where the lines cross indicates the volume of alkali added to exactly neutralise
the acid and also the maximum temperature reached.
Record these values.
(2)
volume of alkali. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .................................................... cm3
maximum temperature. . . . . . . . . . . . . . . . . . . . . . .................................................... °C
(f ) Another student used sulfuric acid instead of nitric acid in her experiments.
She started with 25.0 cm3 of sulfuric acid of concentration 0.650 mol/dm3.
She added 0.500 mol/dm3 sodium hydroxide solution until the acid was
completely neutralised.
The equation for this reaction is
2NaOH + H2SO4 o Na2SO4 + 2H2O
(i) Calculate the amount, in moles, of sulfuric acid used.
(2)
amount = .................. . . . . . . . . . . . . . . . mol
(ii) Calculate the amount, in moles, of sodium hydroxide needed to neutralise this
amount of sulfuric acid.
(1)
amount = .................. . . . . . . . . . . . . . . . mol
(iii) Calculate the volume, in cm3, of sodium hydroxide solution needed to neutralise
this amount of sulfuric acid.
(2)
volume = .................. . . . . . . . . . . . . . . . cm3
(Total for Question 4 = 18 marks)
You might also like
- Ib Physics HL Review Packet - ThermodynamicsDocument19 pagesIb Physics HL Review Packet - ThermodynamicsGhenwa DimachkiNo ratings yet
- MT 75.3 Determination of PH Values: Miscellaneous TechniquesDocument2 pagesMT 75.3 Determination of PH Values: Miscellaneous TechniquesDennis TranquilNo ratings yet
- IB - Ch6 - 16 Past PapersDocument33 pagesIB - Ch6 - 16 Past Papersrejymol100% (1)
- Rounding Upper and Lower BoundsDocument1 pageRounding Upper and Lower BoundsDinangaNo ratings yet
- Molarity Molality Osmolality Osmolarity Worksheet and Key CurrentDocument13 pagesMolarity Molality Osmolality Osmolarity Worksheet and Key CurrentPark GwynethNo ratings yet
- Sample Problem #3Document1 pageSample Problem #3DozdiNo ratings yet
- Chlorhexidine Gluconate Scrub Solution - ProtocolDocument16 pagesChlorhexidine Gluconate Scrub Solution - ProtocolMy bookNo ratings yet
- ChemCollective Autograded Labs PDFDocument13 pagesChemCollective Autograded Labs PDFYuri M. BandaNo ratings yet
- Acids, Alkalis and Titrations and Paper 1Document26 pagesAcids, Alkalis and Titrations and Paper 1DinangaNo ratings yet
- Acids, Alkalis and Titrations 1 QPDocument14 pagesAcids, Alkalis and Titrations 1 QPSaad AnsariNo ratings yet
- IGCSE Edexcel Chemistry SQ 3 Energetics 1CDocument14 pagesIGCSE Edexcel Chemistry SQ 3 Energetics 1CTsang ColinNo ratings yet
- CP Enthalpy of NeutralisationDocument3 pagesCP Enthalpy of NeutralisationshivashankaranrishiNo ratings yet
- Acids, Alkalis and Titrations 1 QPDocument15 pagesAcids, Alkalis and Titrations 1 QPAbdullah SheikhNo ratings yet
- Acids, Alkalis and Titrations 1 QPDocument14 pagesAcids, Alkalis and Titrations 1 QPkhalil rehmanNo ratings yet
- Chemical Energetics Unit3 FCDocument31 pagesChemical Energetics Unit3 FCIbrahim AbidNo ratings yet
- Revision Heat of Neutralisation 123Document6 pagesRevision Heat of Neutralisation 123Ariyan ShahmieNo ratings yet
- 2 1 A Student Investigates Two Fuels: Hexane and Octane.: 5070/42/M/J/18 © UCLES 2018Document14 pages2 1 A Student Investigates Two Fuels: Hexane and Octane.: 5070/42/M/J/18 © UCLES 2018Mujeeb SiddiqueNo ratings yet
- Concentration CalculationsDocument5 pagesConcentration CalculationsSiyah HashTagNo ratings yet
- Unit 4 Rate of Reaction QuestionsDocument101 pagesUnit 4 Rate of Reaction Questionskeyur100100% (1)
- Rate of Reaction 3 QPDocument14 pagesRate of Reaction 3 QPNeelam MakkarNo ratings yet
- Q2 Chem Lecture 4Document4 pagesQ2 Chem Lecture 4Laiba ImtiazNo ratings yet
- Amount of Substance QPDocument22 pagesAmount of Substance QPshahs784.316No ratings yet
- Acids, Alkalis and Titrations 1 QP AnswersDocument14 pagesAcids, Alkalis and Titrations 1 QP AnswersIman DOTNo ratings yet
- Quiz 1Document23 pagesQuiz 1FIKRIYE ONDEROLNo ratings yet
- C8 Chemistry Rates and Equilibrium HomeworkDocument8 pagesC8 Chemistry Rates and Equilibrium HomeworkChloeYapYanQiNo ratings yet
- t2 Chem Revision Ex 17Document16 pagest2 Chem Revision Ex 17Nicholas OwNo ratings yet
- Year 11 Physical ChemistryDocument23 pagesYear 11 Physical Chemistrytechninja256No ratings yet
- Paper-3 Class Test IBY2Document9 pagesPaper-3 Class Test IBY2VALLINAYAGAM SNo ratings yet
- 1 Limewater Is A Saturated Solution of Calcium Hydroxide, Ca (OH)Document3 pages1 Limewater Is A Saturated Solution of Calcium Hydroxide, Ca (OH)Mushfiqur RahmanNo ratings yet
- Quiz 1 ChemDocument8 pagesQuiz 1 ChemFIKRIYE ONDEROLNo ratings yet
- Chemistry Test: Substances Which Donate/ Give H Ions in A Reaction. +Document7 pagesChemistry Test: Substances Which Donate/ Give H Ions in A Reaction. +Azra JabeenNo ratings yet
- Physical Chemistry QuestionsDocument22 pagesPhysical Chemistry QuestionshanaNo ratings yet
- Stoichiometry 4 QPDocument8 pagesStoichiometry 4 QPraghavi luthraNo ratings yet
- cls9 qp1 Sec TermDocument20 pagescls9 qp1 Sec TermShebin PaulNo ratings yet
- Chapter 3 - The Mole ConceptDocument15 pagesChapter 3 - The Mole ConceptNOOR Ahmed 8bNo ratings yet
- 9701/23/M/J/20 © Ucles 2020Document10 pages9701/23/M/J/20 © Ucles 2020Fire stormNo ratings yet
- 03 0620 42 3RP - InddDocument5 pages03 0620 42 3RP - InddIzzati AnuarNo ratings yet
- Acids, Bases and Salt Preparations Paper 2Document15 pagesAcids, Bases and Salt Preparations Paper 2DinangaNo ratings yet
- TOPIC: 4.2 Equilibria: Answer All Questions Total 40 MarksDocument4 pagesTOPIC: 4.2 Equilibria: Answer All Questions Total 40 MarksrokuNo ratings yet
- Rate of Reaction: Caco (S) + 2Hcl (Aq) Cacl (Aq) + H O (L) + Co (G)Document5 pagesRate of Reaction: Caco (S) + 2Hcl (Aq) Cacl (Aq) + H O (L) + Co (G)eismatmidahNo ratings yet
- Rate of Reaction 1 QPDocument11 pagesRate of Reaction 1 QPjunaidsaeed200No ratings yet
- Rate of Reaction 1 QPDocument11 pagesRate of Reaction 1 QPRobert EdwardsNo ratings yet
- Redox TitrationDocument5 pagesRedox TitrationchristinaNo ratings yet
- Evaluación Pruebas 1, 2 y 3 Mayo15Document8 pagesEvaluación Pruebas 1, 2 y 3 Mayo15yanezmariafernanda09No ratings yet
- Rates and EquilibriumDocument26 pagesRates and EquilibriumtapiwanashejakaNo ratings yet
- My TestDocument20 pagesMy TestHidayah TeacherNo ratings yet
- Equilibria AsDocument39 pagesEquilibria AsyousafNo ratings yet
- Stoichiometry AFL QPDocument4 pagesStoichiometry AFL QPdrake lordNo ratings yet
- Chemical Reactions WorksheetDocument10 pagesChemical Reactions Worksheetuvigodage2008No ratings yet
- DraftDocument12 pagesDraftArooj AbidNo ratings yet
- Energetics Exam QsDocument5 pagesEnergetics Exam Qszadinova.tereza16No ratings yet
- Year 10 Chemistry - Practice Exam Questions Rates and REversible ReactionsDocument4 pagesYear 10 Chemistry - Practice Exam Questions Rates and REversible ReactionsAshleyn Mary SandersNo ratings yet
- Chemical Tests QPDocument17 pagesChemical Tests QPnova katuneriyaNo ratings yet
- Acid and BaseDocument6 pagesAcid and BaseFenNo ratings yet
- Ch-5 Mole Calc HWDocument5 pagesCh-5 Mole Calc HWsahikalam458No ratings yet
- 1.2 Assessed HomeworkDocument8 pages1.2 Assessed HomeworkNavine NavNo ratings yet
- My TestDocument33 pagesMy TestqusaielnoorNo ratings yet
- Chemistry Second Homework SosoDocument5 pagesChemistry Second Homework SosoSakinah HassanNo ratings yet
- t2 Chem Revision Ex 17 Answer SchemeDocument16 pagest2 Chem Revision Ex 17 Answer SchemeNicholas Ow100% (1)
- Equilibria Part 1Document131 pagesEquilibria Part 1Raafi Mian Year 13No ratings yet
- Heat Transfer in Polymer Composite Materials: Forming ProcessesFrom EverandHeat Transfer in Polymer Composite Materials: Forming ProcessesNicolas BoyardNo ratings yet
- 2021 Jan 2CDocument16 pages2021 Jan 2CDinangaNo ratings yet
- 2021 Jun 1CDocument21 pages2021 Jun 1CDinangaNo ratings yet
- 2021 Jun 2CDocument15 pages2021 Jun 2CDinangaNo ratings yet
- IGCSE Files&documents Telegram ChannelDocument176 pagesIGCSE Files&documents Telegram ChannelDinangaNo ratings yet
- 4MA1 1H Que 20220111Document28 pages4MA1 1H Que 20220111DinangaNo ratings yet
- 4HB1 01 Rms 20220303Document10 pages4HB1 01 Rms 20220303DinangaNo ratings yet
- 4MA1 1HR Rms 20220303Document20 pages4MA1 1HR Rms 20220303DinangaNo ratings yet
- International Gcse English Language A Young Dyslexic Lesson PlanDocument3 pagesInternational Gcse English Language A Young Dyslexic Lesson PlanDinangaNo ratings yet
- 4MA1 1HR Que 20220111Document32 pages4MA1 1HR Que 20220111Dinanga100% (1)
- 4HB1 01 Que 20220108Document24 pages4HB1 01 Que 20220108DinangaNo ratings yet
- COMPARISON Prayer Before Birth and War PhotographerDocument1 pageCOMPARISON Prayer Before Birth and War PhotographerDinangaNo ratings yet
- 4HB1 02 Rms 20220303Document10 pages4HB1 02 Rms 20220303DinangaNo ratings yet
- Rubycitalan - Chem MT 1 6Document3 pagesRubycitalan - Chem MT 1 6DinangaNo ratings yet
- 4HB1 02 Que 20220114Document24 pages4HB1 02 Que 20220114DinangaNo ratings yet
- Fongrsy - Acids Bases and AlkalisDocument2 pagesFongrsy - Acids Bases and AlkalisDinangaNo ratings yet
- Fongrsy Salt-PreparationDocument1 pageFongrsy Salt-PreparationDinangaNo ratings yet
- Kiserbj241 - Grade 8 ScienceDocument3 pagesKiserbj241 - Grade 8 ScienceDinangaNo ratings yet
- Romeo and Juliet KO AQA 2018Document3 pagesRomeo and Juliet KO AQA 2018DinangaNo ratings yet
- Caa9b7b6 1Document3 pagesCaa9b7b6 1DinangaNo ratings yet
- Unit 1 - Algebra1Document2 pagesUnit 1 - Algebra1DinangaNo ratings yet
- Information and Communication Technology (ICT) : Pearson Edexcel International GCSEDocument24 pagesInformation and Communication Technology (ICT) : Pearson Edexcel International GCSEDinangaNo ratings yet
- Still I Rise WorksheetDocument2 pagesStill I Rise WorksheetDinangaNo ratings yet
- ElectricityDocument6 pagesElectricityDinangaNo ratings yet
- An Inspector CallsDocument68 pagesAn Inspector CallsDinangaNo ratings yet
- Still I Rise Lesson Planv2Document3 pagesStill I Rise Lesson Planv2DinangaNo ratings yet
- Out Out Scasi Essay FinalDocument3 pagesOut Out Scasi Essay FinalDinangaNo ratings yet
- Inverse PercentagesDocument1 pageInverse PercentagesDinangaNo ratings yet
- Structure of Living OrganismsDocument28 pagesStructure of Living OrganismsDinangaNo ratings yet
- The Nature and Variety of Living OrganismsDocument1 pageThe Nature and Variety of Living OrganismsDinangaNo ratings yet
- Properties of Acids & Bases (7.1.1) CIE IGCSE Chemistry Revision Notes 2023 Save My ExamsDocument1 pageProperties of Acids & Bases (7.1.1) CIE IGCSE Chemistry Revision Notes 2023 Save My ExamsgkrauelNo ratings yet
- Comparative Study of Chelating Ion Exchange Resins For The Recovery of Nickel and Cobalt From Laterite Leach Tailings PDFDocument5 pagesComparative Study of Chelating Ion Exchange Resins For The Recovery of Nickel and Cobalt From Laterite Leach Tailings PDFRodrigoNo ratings yet
- 1112 Grade 12 Chemistry Revision Sheet Final Term 2Document32 pages1112 Grade 12 Chemistry Revision Sheet Final Term 2aalharthy_1No ratings yet
- Chemistry ReportDocument5 pagesChemistry ReportAngel Trisha Mae DelMundoNo ratings yet
- Wa0002.Document24 pagesWa0002.eeshaahmed83No ratings yet
- Lesson 3 - Partition - Adsorption Chromatography Columns - Shodex - HPLC Columns, Detectors, StandardsDocument2 pagesLesson 3 - Partition - Adsorption Chromatography Columns - Shodex - HPLC Columns, Detectors, StandardslgNo ratings yet
- Chapter 12: Liquid-Liquid and Fluid-Solid Separation ProcessesDocument40 pagesChapter 12: Liquid-Liquid and Fluid-Solid Separation ProcessesAmit Yadav60% (5)
- Characterization of Essential Oils by Gas Chromatography Characterization of Essential Oils by Gas ChromatographyDocument4 pagesCharacterization of Essential Oils by Gas Chromatography Characterization of Essential Oils by Gas ChromatographyKevin GuzmanNo ratings yet
- Types of ElectrolytesDocument24 pagesTypes of ElectrolytesPranoy Baishya100% (1)
- Derivatization of Formaldehyde With Dimedone and Subsequent Sorption On Sodium Dodecylsulfate/Alumina Admicelles For Fluorometric AnalysisDocument2 pagesDerivatization of Formaldehyde With Dimedone and Subsequent Sorption On Sodium Dodecylsulfate/Alumina Admicelles For Fluorometric Analysishariharan rNo ratings yet
- DistillatnDocument149 pagesDistillatnVinayak ThalangeNo ratings yet
- Titration Lab Report 1Document28 pagesTitration Lab Report 1api-607048551No ratings yet
- Magnesium Oxide AnalysisDocument3 pagesMagnesium Oxide AnalysisAnupriya Oberoi100% (1)
- 1.ionic Equilibrium (1-26)Document26 pages1.ionic Equilibrium (1-26)Ayush KumarNo ratings yet
- GcmsDocument3 pagesGcmsRista Susanti100% (1)
- مطالعہ پاکستان نوٹس مختصر سوالات جماعت نہمDocument24 pagesمطالعہ پاکستان نوٹس مختصر سوالات جماعت نہمasadahmed492No ratings yet
- Chapter 15 Practice QuestionsDocument17 pagesChapter 15 Practice QuestionsKim LeeNo ratings yet
- Lab 4 - Soda Titrations 1Document9 pagesLab 4 - Soda Titrations 1api-385516219No ratings yet
- Lewis Concept of Acids and BasesDocument3 pagesLewis Concept of Acids and BasesMaku MichaelNo ratings yet
- Preparation of Buffers For Use in Enzyme Studies (By G. Gomori)Document9 pagesPreparation of Buffers For Use in Enzyme Studies (By G. Gomori)navoditgoel1985No ratings yet
- 1 - An Introduction To Analytical ChemistryDocument23 pages1 - An Introduction To Analytical ChemistryBismah SaeedNo ratings yet
- Bac Lab Rep 1Document16 pagesBac Lab Rep 1Peach BabyNo ratings yet
- Mettler Toledo Application M615-2010: Potentiometric Titration of A Betaine in ShampooDocument4 pagesMettler Toledo Application M615-2010: Potentiometric Titration of A Betaine in ShampooHasan Zeki BayrakNo ratings yet
- 3 Potentiometric TitrationDocument2 pages3 Potentiometric TitrationDelin Shaji JohnNo ratings yet
- Sample Preparation - Resolution SystemsDocument52 pagesSample Preparation - Resolution SystemsResolution Systems, Inc.No ratings yet