Professional Documents
Culture Documents
2019 FMSS p2
2019 FMSS p2
Uploaded by
nigelOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2019 FMSS p2
2019 FMSS p2
Uploaded by
nigelCopyright:
Available Formats
Name: __________________________________ ( ) Class: __________
(ii) Discuss how the concentration of hydrochloric acid affects the rate of
reaction.
As concentration of
hydrochloric acid increases,
…………………………………………………………………………...........
rate of
reaction increases as thereis more
…………………………………………………………………………...........
volume which reacts with[2]
molecules
per unit
……………………………………………………………………………
the calcium carbonate
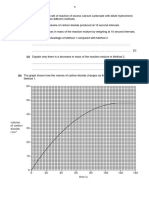
(iii) In Figure 5.1, sketch a graph to show the results if 1.00 moldm −3 dilute
-
sulfuric acid was reacted with 5.0g of marble chips instead.
-
[1]
calcium sulfate famed
(c) 450cm3 of gas was produced in Experiment X.
Yr⑥p
Calculate the percentage purity of calcium carbonate in the marble chips.
law } Ks ) + 21-1 cereal → fakllaql + coil g) -11-120141
} 450cm>
2<59 I met 1dm
3
÷ 24dm
no .
otmoeot =
COs
= 0.0187 5m01
[3]
no Otmo
of
'
.
Cults
=
u¥ÉÑ
= 0.025 Mul
more ratio of law } : coz
1 : I
0 . 01875
Percentage = ✗ 10% =
purity
=
-75%
FMS(S) Sec 4 Express Preliminary Examination 2019 9
Chemistry (6092/02)
www.KiasuExamPaper.com
180
You might also like
- Solutions Fundamentals of Semiconductor FabricationDocument85 pagesSolutions Fundamentals of Semiconductor Fabricationgarmsirian0% (2)
- HDM-250 Manual - 042808Document35 pagesHDM-250 Manual - 042808Hakkı YılmazNo ratings yet
- The Cnidaria, Past, Present and Future: Stefano Goff Redo Zvy Dubinsky EditorsDocument842 pagesThe Cnidaria, Past, Present and Future: Stefano Goff Redo Zvy Dubinsky Editorscuentas cuentasNo ratings yet
- Askeland Chap 4 SolutionDocument10 pagesAskeland Chap 4 SolutionDamita de Peña50% (2)
- 05 Askeland ChapDocument10 pages05 Askeland ChapWeihanZhang100% (2)
- Sample Concrete Mix DesignDocument7 pagesSample Concrete Mix DesignNabendu Lodh0% (1)
- CHEMISTRY-23-07 - 11th (J-Batch)Document8 pagesCHEMISTRY-23-07 - 11th (J-Batch)Raju SinghNo ratings yet
- Mass Spectra Worksheet 1Document5 pagesMass Spectra Worksheet 13estherNo ratings yet
- KineticsDocument7 pagesKineticsInês AlmeidaNo ratings yet
- F325 How Far How Fast TestDocument14 pagesF325 How Far How Fast TestSigourney MarshNo ratings yet
- Determination of Rate Equation - 2 QPDocument12 pagesDetermination of Rate Equation - 2 QPSalman ZaidiNo ratings yet
- Assignment (Chemical Kinetics and Chemical Equilibrium)Document4 pagesAssignment (Chemical Kinetics and Chemical Equilibrium)Mapalo faith ChamaNo ratings yet
- Essentials of Materials Science and Engineering Si Edition 3rd Edition Askeland Solutions ManualDocument11 pagesEssentials of Materials Science and Engineering Si Edition 3rd Edition Askeland Solutions Manualjeffreyhayesagoisypdfm100% (13)
- (CR (Acac) 3) ....Document14 pages(CR (Acac) 3) ....qamaralmahseriNo ratings yet
- Dwnload Full Essentials of Materials Science and Engineering Si Edition 3rd Edition Askeland Solutions Manual PDFDocument36 pagesDwnload Full Essentials of Materials Science and Engineering Si Edition 3rd Edition Askeland Solutions Manual PDFinurerejoice7xt8mh100% (12)
- CHM2 Kinetics and Equilibria QDocument41 pagesCHM2 Kinetics and Equilibria QHakim AbbasNo ratings yet
- Eee L-1, T-2 (2016-2017) PDFDocument26 pagesEee L-1, T-2 (2016-2017) PDFআশিক পালোয়ানNo ratings yet
- Chemistry PaperDocument38 pagesChemistry Paperabdulhamam974No ratings yet
- Rate (F) (Clo) 1.2X10 M/ S 0.10M 0.010M: Kinetics ProblemsDocument6 pagesRate (F) (Clo) 1.2X10 M/ S 0.10M 0.010M: Kinetics ProblemsFiyan HidayatNo ratings yet
- AS - CHEM - 2024 March Mock - Structured III - 75 Mins MsDocument5 pagesAS - CHEM - 2024 March Mock - Structured III - 75 Mins Msliuyuhui972No ratings yet
- Research LetterDocument5 pagesResearch LetterAmeelaDNo ratings yet
- A Level Chemistry Practice Paper 4Document19 pagesA Level Chemistry Practice Paper 4Myra Joy B MonteroNo ratings yet
- AS KineticsDocument41 pagesAS Kineticsvintu pvNo ratings yet
- CHEMISTRY-17-09-11th (PQRS)Document9 pagesCHEMISTRY-17-09-11th (PQRS)Raju SinghNo ratings yet
- 6193d861716d5 Aben 147 Laboratory Exercise No. 4 Determination of Thermal Properties of Ab Materials A Computational Laboratory ExerciDocument10 pages6193d861716d5 Aben 147 Laboratory Exercise No. 4 Determination of Thermal Properties of Ab Materials A Computational Laboratory ExerciHeliNo ratings yet
- Upload 3Document9 pagesUpload 3mtyppranadaNo ratings yet
- Cy 102 Assign 4Document12 pagesCy 102 Assign 4masesena123No ratings yet
- My TestDocument33 pagesMy TestqusaielnoorNo ratings yet
- Reaction Kinetics - MediumDocument19 pagesReaction Kinetics - MediumYan Xin LuNo ratings yet
- Exampro - Acids and AlkalisDocument72 pagesExampro - Acids and AlkalisMadhavi OchaniNo ratings yet
- C4 Chemical Changes HTDocument72 pagesC4 Chemical Changes HTMadhavi OchaniNo ratings yet
- Tutorial 11 - MS3220 Rekayasa Termal (Mark Scheme)Document6 pagesTutorial 11 - MS3220 Rekayasa Termal (Mark Scheme)i need documentsNo ratings yet
- Tutorial 11 - MS3220 Rekayasa Termal (Mark Scheme)Document4 pagesTutorial 11 - MS3220 Rekayasa Termal (Mark Scheme)Th3warrior5 is bAcKNo ratings yet
- How FastDocument14 pagesHow FastBagus WicaksonoNo ratings yet
- Kinetics QuestionsDocument39 pagesKinetics Questionsmariam saikNo ratings yet
- Crystallizer DesignDocument8 pagesCrystallizer DesignPatricia MirandaNo ratings yet
- Crystallizer DesignDocument8 pagesCrystallizer DesignPrashant SinghNo ratings yet
- Mathematics EduDocument28 pagesMathematics Eduakshata.more003No ratings yet
- M25 Concrete CalculationDocument7 pagesM25 Concrete CalculationShwet SinghNo ratings yet
- Kinetics Questions (Solutions)Document11 pagesKinetics Questions (Solutions)Lee Jun HuiNo ratings yet
- Seminar Assignments - Assignment 1 Questions + Answers Seminar Assignments - Assignment 1 Questions + AnswersDocument7 pagesSeminar Assignments - Assignment 1 Questions + Answers Seminar Assignments - Assignment 1 Questions + AnswersHenry KimNo ratings yet
- U2103305 - Exp 3 - Lab ReportDocument14 pagesU2103305 - Exp 3 - Lab ReportU2103305 STUDENTNo ratings yet
- Problem Set 8Document11 pagesProblem Set 8Oluwafayosola DosunmuNo ratings yet
- 20 Dulwich 16+ ChemistryPaper 2013Document10 pages20 Dulwich 16+ ChemistryPaper 2013Thusan KanthanathanNo ratings yet
- Electrochemistry - Numerical WSDocument4 pagesElectrochemistry - Numerical WSrishima sapruNo ratings yet
- Ib Chem Answers 1Document6 pagesIb Chem Answers 1Hanh ChuNo ratings yet
- ChE101 PSDocument2 pagesChE101 PSKevin JangNo ratings yet
- Kinetics: 209 Minutes 205 MarksDocument85 pagesKinetics: 209 Minutes 205 MarksSoni SherpaNo ratings yet
- Isothermal Semi-Batch Reaction Example (See Fogler 4 Ed. Problem 4 - 9)Document4 pagesIsothermal Semi-Batch Reaction Example (See Fogler 4 Ed. Problem 4 - 9)vanesaNo ratings yet
- CHE 509 - Past Exam QuestionsDocument12 pagesCHE 509 - Past Exam QuestionsJane Eilyza Aballa100% (1)
- 1.6 Calculations Involving MassesDocument53 pages1.6 Calculations Involving MassesShriep kebabaNo ratings yet
- 8.1 Paper 2 HL Part 2Document5 pages8.1 Paper 2 HL Part 2joker83382No ratings yet
- Precam clXII qp4Document20 pagesPrecam clXII qp4GM Ali KawsarNo ratings yet
- 16 Chemistry Specimen ExaminationDocument10 pages16 Chemistry Specimen ExaminationThusan KanthanathanNo ratings yet
- Design Mix Calculation Grade 30NDocument6 pagesDesign Mix Calculation Grade 30NikhwanNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Electrochemical Processes in Biological SystemsFrom EverandElectrochemical Processes in Biological SystemsAndrzej LewenstamNo ratings yet
- Cellulose Nanocrystals: Properties, Production and ApplicationsFrom EverandCellulose Nanocrystals: Properties, Production and ApplicationsNo ratings yet
- 2019 FMSS p2Document1 page2019 FMSS p2nigelNo ratings yet
- 2019 FMSS p2Document1 page2019 FMSS p2nigelNo ratings yet
- 2019 FMSS p2Document1 page2019 FMSS p2nigelNo ratings yet
- 2019 FMSS p2Document1 page2019 FMSS p2nigelNo ratings yet
- Subject: General Chemistry Test,: Date: May 2015Document7 pagesSubject: General Chemistry Test,: Date: May 2015PHƯƠNG ĐẶNG YẾNNo ratings yet
- Crack Bottom Polymers-14-03174Document12 pagesCrack Bottom Polymers-14-03174Net SupraluckNo ratings yet
- Viton GLT-505Document12 pagesViton GLT-505Alejandro ZagalNo ratings yet
- Refining ProcessDocument22 pagesRefining Processramadoss_alwar7307No ratings yet
- Understanding Einstein: The Special Theory of RelativityDocument1 pageUnderstanding Einstein: The Special Theory of RelativityAnonymous G3DRjDMkNo ratings yet
- Earth Resources 3Document21 pagesEarth Resources 3taerachNo ratings yet
- Freezing of Foods: Mathematical and Experimental Aspects: Review ArticleDocument12 pagesFreezing of Foods: Mathematical and Experimental Aspects: Review ArticleCherise TanNo ratings yet
- General Relativity PDFDocument412 pagesGeneral Relativity PDFAndrei Avadani100% (1)
- Combined Science#1Document100 pagesCombined Science#1Tatenda ChirobeNo ratings yet
- Solution Manual For Chapter 17Document9 pagesSolution Manual For Chapter 17Kenneth LandichoNo ratings yet
- Isothermal Vapor-Liquid Equilibria For Mixtures of Ethanol, Acetone, and Diisopropyl EtherDocument16 pagesIsothermal Vapor-Liquid Equilibria For Mixtures of Ethanol, Acetone, and Diisopropyl EtherAngie AyusawaNo ratings yet
- Pressure Safety Valve Sizing Calculation Rev.01 APUDocument10 pagesPressure Safety Valve Sizing Calculation Rev.01 APUAlvin SmithNo ratings yet
- 2015 Andreiev Mat Testing TestingofpipesectionsDocument7 pages2015 Andreiev Mat Testing TestingofpipesectionsHenry PedrazaNo ratings yet
- W1.05.17 Wanda 41 Hi-Performance Clear APACv3Document3 pagesW1.05.17 Wanda 41 Hi-Performance Clear APACv3Jumadi AlkutsNo ratings yet
- Theoretical and Experimental Evidences of Defects in LiMgPO4 - 2018Document32 pagesTheoretical and Experimental Evidences of Defects in LiMgPO4 - 2018Ricardo DanielNo ratings yet
- Air Pressure and Hot Air Balloons!Document12 pagesAir Pressure and Hot Air Balloons!rishiNo ratings yet
- Oil Field Chemicals: RX Marine InternationalDocument35 pagesOil Field Chemicals: RX Marine Internationalmelvinkuri100% (1)
- Refractive Index and Thickness Determination of Thin-Films Using Lloydõs InterferometerDocument8 pagesRefractive Index and Thickness Determination of Thin-Films Using Lloydõs InterferometerLuis MestaNo ratings yet
- DPP Alkyl Halide PDFDocument33 pagesDPP Alkyl Halide PDFSandy 05No ratings yet
- IFY Chemistry Exam 2122 V3Document13 pagesIFY Chemistry Exam 2122 V3kushiperera80fmNo ratings yet
- Chapter 6. Equilibrium Based Separation Processes: 6.1 Study ObjectivesDocument17 pagesChapter 6. Equilibrium Based Separation Processes: 6.1 Study ObjectivesMaarifa KidogeNo ratings yet
- List of Figures and Tables 1 Chapter1 Introduction 2Document16 pagesList of Figures and Tables 1 Chapter1 Introduction 2PawanNo ratings yet
- 410002G Fuel Requirements TRDocument18 pages410002G Fuel Requirements TRichitaka_setoNo ratings yet
- Aero 3477 PDFDocument9 pagesAero 3477 PDFLUIS XV100% (1)
- Es 103 - Module 11 - Economic SectionsDocument37 pagesEs 103 - Module 11 - Economic SectionsMinari ChaengNo ratings yet
- Chemicals From Coal CokingDocument29 pagesChemicals From Coal CokingNatalia MayaNo ratings yet
- Effects of MN, P, S, Si & V On The Mechanical Properties of SteelDocument2 pagesEffects of MN, P, S, Si & V On The Mechanical Properties of SteelMohit SunnyNo ratings yet
- Boundary Layer Theory Stability Turbulent ModelingDocument20 pagesBoundary Layer Theory Stability Turbulent ModelingTsegaye GetachewNo ratings yet