Professional Documents
Culture Documents
Ch113wk1 2
Ch113wk1 2
Uploaded by
kent AldayOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ch113wk1 2
Ch113wk1 2
Uploaded by
kent AldayCopyright:
Available Formats
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
Week 1: Thermochemistry
I. Introduction
[1] Thermochemistry refers to the study of the heat flow that accompanies chemical
reactions. The sun’s rays, campfires and rubbing your hands together all produce heat. However,
other activities like boiling water and melting ice, absorb heat. Thermochemistry focuses on the
heat changes that occur during chemical reactions. In this chapter, you will examine heat and its
effects on a number of chemical and physical processes. It will be important for us to first
understand the relationship between heat and energy.
II. Objectives
At the end of the week, you should be able to:
1. Identify energy as potential or kinetic and understand the units of energy.
2. Explain the relationship between energy and heat.
3. Distinguish between heat capacity and specific heat.
4. Use specific heat to calculate the heat loss or gain, temperature change or mass of a
sample.
5. Draw, label and know the functions of common laboratory apparatus.
III. Energy
Energy is involved in almost everything we do. We use energy when we walk, engage in
sports or when we breathe. We also use energy when we cook food, turn on lights, warm water,
use a washing machine and drive our cars or ride a bicycle. In our bodies, the food that we eat
provides us energy. When water evaporates, it forms a gas or vapor. Water changes state by
gaining or losing energy.
[1] Energy is defined as the ability to do work. When you are running, walking or dancing,
you are using energy to do work. [1] All energy can be classified as potential energy or kinetic
energy. Potential energy is stored energy, while kinetic energy is the energy of motion.
Any object that is moving has kinetic energy. A boulder resting on top of a mountain has potential
energy because of its location. If the boulder rolls down the mountain, the potential energy
becomes kinetic energy. Water stored in a reservoir has potential energy. When it flows over
the dam and falls to the stream below, its potential energy is converted to kinetic energy. Foods
and fossil fuels have potential energy stored in their molecules. When you digest food or burn
gasoline in your car, potential energy is converted into kinetic energy to do work. Different
substances store different amounts of energy. The kinds of atoms and their arrangement in the
substance determine the amount of energy stored in the substance.
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 7
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
[1] Heat, represented by q, is energy that transfers from one object to another because of
a temperature difference between them. Heat, itself, cannot be detected by the senses or by
instruments. Only changes caused by heat can be detected. One of the effects of adding heat is a
rise in the temperature of objects. It is the heat of the sun’s rays that makes a summer day hot.
In this example, air is the object that absorbs heat and increases in temperature. Heat always
flows from a warmer object to a cooler object, until the temperature of both objects is the same.
IV. Exothermic and Endothermic Processes
All chemical reactions and changes in physical state involve the release or absorption of
heat. In the study of heat changes, it is useful to define a system as the part of the universe on
which you focus your attention. The surroundings include everything else in the universe. And
together the system and its surroundings constitute the universe. A major goal of studying
thermochemistry is to examine the flow of heat from the surroundings to the system or flow of
heat from the system to its surroundings. [1] The Law of Conservation of Energy states that in a
chemical or physical process, energy is neither created nor destroyed. All of the energy involved in
a process can be accounted for as work, stored energy, or heat.
In thermochemical calculations, the direction of heat flow is given from the point of view
of the system. Heat flowing into a system from its surroundings is defined as positive; q has a
positive value. [1] A process that absorbs heat from the surroundings is called an endothermic
process. In an endothermic process, the system gains heat as the surroundings cool down. When
heat flows out of the system to its surroundings, heat flow is negative; q is negative because the
system is losing heat. [1] A process that releases heat to its surroundings is called an exothermic
process. In an exothermic process, the system loses heat as the surroundings heat up.
Table 1.1 Heat Change Sign Convention
Direction of Heat Flow Sign Reaction Type
Heat flows out of the system Heat change ‹ 0 (negative) Exothermic
Heat flows into the system Heat change › 0 (positive) Endothermic
V. Units of Energy
During exercise, your body generates heat, and this heat is measured in units called
calories. The heat is generated as your body breaks down sugars and fats into carbon dioxide and
water. In breaking down 10 grams of sugar, for example, your body generates a certain amount
of heat. The same amount of heat would be produced if 10 grams of sugar were completely
burned in fire, producing carbon dioxide and water.
[1] A calorie is defined as the quantity of heat needed to raise the temperature of 1 gram
of pure water 10C. The calorie written in small c is used except when referring to the energy
contained in food. One dietary Calorie is equal to one kilocalorie, or 1000 calories.
1 Calorie = 1 kilocalorie = 1000 calories
The statement “10 grams of sugar has 41 Calories” means that 10 g of sugar releases 41
kilocalories of heat when completely burned to produce CO2 and H2O.
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 8
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
The calorie is also related to the joule, the SI unit of heat and energy [1] named after the
English physicist James Prescott Joule (1818-1889). One joule of heat raises the temperature of 1
g of pure water 0.23900C.
1 J = 0.2390 cal 1 cal = 4.184J
Example 1.1
When 1.0 g of octane fuel burns in an automobile engine, 48,000 J are released. Convert this
energy to the following units: a.) calories b.) kilojoules
Solution:
a.) calories
using : 1 cal = 4.184 J
( 48,000 J ) x = 11,000 cal multiplying the given data by the
.
conversion factor ( J cancels out)
= 1.1 x 104 cal expressing answer in scientific
Notation
Answer: 11,000 cal or 1.1 x 104 cal
b.) kilojoules
using : 1 kJ = 1000 J
(48,000 J ) x = 48 kJ multiplying given data by the
conversion factor ( J cancels out)
Answer: 48 kJ
VI. Learning Activity 1.1
1. Indicate whether each statement describes potential or kinetic energy:
a. Water at the top of a waterfall ______________________________
b. Kicking a ball ______________________________
c. the energy in a lump of coal ______________________________
d. a skier at the top of a hill ______________________________
e. an earthquake ______________________________
2. A person uses 750 kcal to run a race. Convert the energy used for the race to the following
energy units:
a. calories
b. Joules
c. kilojoules
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 9
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
VII. Heat Capacity and Specific Heat
[1] The amount of heat needed to increase the temperature of the object exactly 1 0C is
the heat capacity of that object. The heat capacity of an object depends on its mass as well as its
chemical composition. The greater the mass of the object, the greater its heat capacity. A
massive steel girder, for example, requires much more heat to raise its temperature 1 0C than a
small steel nail does. Similarly, a cup of water has a much greater heat capacity than a drop of
water. Heat capacity also depends on its chemical composition. Different substances with the
same mass may have different heat capacities.
[1] The specific heat capacity or simply the specific heat, of a substance is the amount of
heat it takes to raise the temperature of 1 gram of the substance 10C.
In general, the relationship between the magnitude of heat flow, q, and the temperature
change, Δt , is given by the equation
q = C x Δt
where Δt = tfinal - tinitial
C = heat capacity of the system ( units: J/ 0C )
For a pure substance of a certain mass, the expression for q can be written as
q = mass x c x Δt
where c is the specific heat of the substance (units: J/ g 0C )
Table 1.2 Specific Heats of Some Common Substances
c in J/ g 0C c in J/ g 0C
Br2 (l) 0.474 Cu (s) 0.387
Cl2 (g) 0.478 Fe (s) 0.446
C2H5OH (l) 2.46 H2O (g) 1.87
C6H6 (l) 1.72 H2O (l) 4.18
CO2 (g) 0.843 NaCl (s) 0.866
Al (s) 0.90 Ag (s) 0.24
From the table, liquid water has a very high specific heat compared with the other
substances. Metals, however, have low specific heats. Specific heat like melting point or density,
is an intensive property that can be used to identify a substance or determine its purity.
Example 1.2
The temperature of a piece of copper with a mass of 95.4 g increases from 25.0 0C to 48.00C when
the metal absorbs 849J of heat. What is the specific heat of copper?
Solution:
Using the formula: q = mass x c x Δt
or q = mc Δt
𝒒
c = deriving the formula for c
𝒎𝜟𝒕
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 10
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
c = q = 849 J
.
m= 95.4 g
Δt = ( 480C – 250C ) = 230C
c = 0.387 J/g 0C note: no units were cancelled out
Answer: c = 0.387 J/g0C
Example 1.3
How many joules are absorbed by 45.2 g of Aluminum ( Al ) if its temperature rises from 12.5 0C to
76.80C? From Table 1.2, c for Al is 0.90 J/g0C
Solution:
Using the formula: q = mass x c x Δt
or q = mc Δt
q = 45.2 g x 0.90 J/g0C x x 64.30C m = 45.2g
c = 0.90 J/g0C
Δt = (76.80C – 12.50C) = 64.30C
q = 2615.7 J Note: units g and 0C cancels out
Answer: q = 2615.7 J
Example 1.4
Ethanol, C2H5OH has a specific heat of 2.46 J/g0C. When 655 J are added to a sample of ethanol,
its temperature rises from 18.20C to 32.80C. What is the mass in grams of the ethanol sample?
Solution:
Using the formula: q = mass x c x Δt
or q = mc Δt
m = derive formula for m
𝒄𝜟𝒕
m = . substitute into the equation
.
q = 655 J
c = 2.46 J/g 0C
Δt =(32.8 0C – 18.20C) = 14.60C
m = 18.2 g note: the units J and 0C cancels out
Answer: m = 18.2 g
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 11
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
VIII. Exercise 1.1
1. If the same amount of heat is supplied to samples of 10.0 g each of aluminum, iron and
copper, all at 150C, which sample would reach the highest temperature?
2. Calculate the specific heat ( in J/g0C ) for each of the following:
a. a 13.5 g sample of Zinc heated from 24.20C to 83.60C that absorbs 312 J of heat.
b. a 48.2 g sample of a metal that absorbs 345 J when temperature increases from 35.0 0C to
57.90C.
3. What is the amount of heat required in each of the following?
a. calories to heat 25 g of water from 150C to 250C
b. calories to heat 150 g of water from 00C to 750C
c. Kilocalories to heat 150 g of water in a kettle from 150C to 770C
4. Calculate the energy in joules and in calories
a. Required to heat 25.0 g of water from 12.50C to 25.70C
b. Required to heat 38.0 g of Copper from 1220C to 2460C
c. Lost when 15.0 g ethanol, C2H5OH, cools from 60.50C to -42.00C
5. Calculate the mass in grams for each of the following:
a. A gold sample ( c = 0.129 J/g0C) that absorbs 225 J to change its temperature from 15.00C
to 47.00C.
b. A sample of Titanium (c = 0.523 J/g0C ) that loses 14,200 J when it cools from 1850C to
420C.
6. Using the heat equation, solve for each of the following:
a. The mass in grams, of water that absorbs 8250 J when its temperature rises from 18.3 0C
to 92.60C.
b. The rise in temperature, Δt, when a 20 g sample of iron absorbs 1580 J.
c. The specific heat in cal/g0C and in J/g0C of a metal when 8.5 g of the metal absorbs 28 cal
and temperature rises from 120C to 240C.
IX. Quiz
Submit test booklets to the testing center before taking the examination. Schedule will be
announced to the class group. Coverage for Quiz 1 will be Energy, Heat Capacity and Specific Heat.
X. Laboratory
Read and understand the laboratory safety rules to be given by your instructor. Initial activities at
the lab will include common laboratory operations and identifying laboratory apparatus to be used in
the different lab activities. The best way to identify and remember the different lab apparatus is by
drawing them and indicating their functions.
Pre-Lab Activity
Draw, label and give functions of the different common laboratory apparatus, in a short size bond
paper. The hand-written report will be submitted together with the answer to Quiz 1. The due date
for submission will be announced to the group.
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 12
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
Week 2 : Calorimetry
I. Introduction
Energy changes occur in many systems and most of the chemical and physical changes occur at
constant atmospheric pressure. By defining a thermodynamic variable called enthalpy, we can measure
the energy changes that accompany chemical and physical processes.
II. Objectives
At the end of the week, you should be able to:
. 1. Construct equations that show heat changes for chemical and physical processes.
2. Calculate heat changes In chemical processes.
III. Calorimetry
Heat that is released or absorbed during many chemical reactions can be measured by
calorimetry. [1] Calorimetry is the accurate and precise measurement of heat change for chemical and
physical processes. In calorimetry, the heat released by the system is equal to the heat absorbed by its
surroundings. To measure heat changes accurately and precisely, the process must be carried out in an
insulated container. The insulated device used to measure the absorption or release of heat in processes
is called the calorimeter. The apparatus contains water or other materials of known heat capacity. We
use an insulated container so that there will be no exchange of heat with the surrounding air. The only
heat flow is between the reaction system and the calorimeter. [1] The heat flow for the reaction system is
equal in magnitude but opposite in sign to that of the calorimeter:
qreaction = - qcalorimeter
Foam cups are excellent heat insulators, and because they do not let much heat in or out, they
can be used as simple calorimeters. Because most chemical reactions and physical changes carried out in
the laboratory are open to the atmosphere, these changes occur at constant pressure. [1] For systems at
constant pressure, the heat content is the same as a property called the enthalpy (H) of the system. Heat
changes for reactions carried out at constant pressure are the same as changes in enthalpy, represented
by ΔH ( delta H ). Heat and enthalpy may be used interchangeably, and this can also be applied to the
heat equation.
q = ΔH = m x c x Δt
where ΔH is the heat change; m is the mass of water; c is the specific heat of water and Δt = tf – ti . The
sign of ΔH is negative for an exothermic reaction and positive for an endothermic reaction.
Table 1.3 Enthalpy Sign Convention
Exothermic reaction ΔH is negative (ΔH ‹ 0 )
Endothermic reaction ΔH is positive (ΔH › 0 )
To measure the heat change for a reaction in aqueous solution in a foam cup calorimeter, you
dissolve the reacting chemicals (the system) in known volumes of water (the surroundings). Then you
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 13
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
measure the initial temperature of each solution before you mix them in the foam cup. After the reaction
is complete, measure the final temperature of the mixed solutions. Because you know the initial and final
temperatures and the specific heat of water, you can now calculate the heat released or absorbed in the
reaction using the equation q = ΔH = m c Δt.
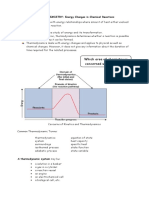
Source: https://www.ddscalorimeters.com/the-difference-between-a-coffee-cup-calorimeter-and-a-bomb-calorimeter/
Figure 1.1 A simple constant- pressure calorimeter. The thermometer measures the temperature change
of the substances as they react in water. The stirrer is used to keep the solution at a uniform temperature.
The chemical substances that react in solution constitute the system, with water as the surroundings.
Example 1.5
To study the heat released during a neutralization reaction, 25.0 ml of water containing 0.025 mol HCl is
added to 25.0 ml of water containing 0.025 mol NaOH in a foam cup calorimeter. At the start, the
solutions and the calorimeter are all at 25.00C. During the reaction, the highest temperature observed is
32.00C. Calculate the heat (in kJ) released during this reaction. Assume the densities of the solutions are
1.00 g/ml.
Solution:
Given: HCl solution VHCl = 25.0 ml
solution contains 0.025 mol HCl
NaOH solution VNaOH = 25.0 ml
solution contains 0.025 mol NaOH
Final Volume = VHCl + VNaOH = 25 ml + 25 ml = 50 ml adding the volumes of the
two solutions in the mixture
ti = 250C
tf = 320C
c for water = 4.18 J/g 0C
density of solution = 1.00 g/ml
Solve for: ΔH in kJ
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 14
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
Solving for mass of water: m = V x density applying d = m/V; m = Vd
m = (50 ml) ( 1.00 g/ml)
m = 50 g the unit ml cancels out
Using the formula: ΔH = m c Δt
ΔH = (50 g) (4.18 J/g0C) ( 70C) substituting given data to the equation
Δt = (320C – 250C) = 70C
the units g and 0C cancels out
ΔH = 1463 J = 1.5 x 103 J
converting joules to kilojoules
ΔH = (1.5 x 103 J ) ( ) applying conversion factor: 1 kJ = 1000 J
the unit J cancels out
ΔH = 1.5 kJ
Answer: ΔH = 1.5 kJ
Note: The sign of ΔH for the water is positive. The water absorbs 1.5J of heat. Therefore this
neutralization reaction releases 1.5 kJ of heat into the water in the calorimeter, so the sign for ΔH for the
reaction is negative. [1] qreaction = - qwater
Example 1.6
A small pebble is heated and placed in a foam cup calorimeter containing 25 ml water at 25 0C. The water
reaches a maximum temperature of 26.40C. How many joules of heat were released by the pebble?
Solution:
Using the formula: ΔH = m c Δt
ΔH = ( 25 g ) ( 4.18 J/g 0C ) ( 1.4 0C ) mwater = dV = (1 g/ml)(25ml) = 25 g
Cwater = 4.18 J/g0C
Δt = (26.40C – 250C ) = 1.40C
ΔH = 146.3 J the units g and 0C cancels out
IV. Heat in Changes of State
Matter undergoes a change of state when it is converted from one state to another state. When
Heat is added to a solid, the particles in the rigid structure move faster. At a temperature called the
melting point, the particles in the solid gain sufficient energy to overcome the attractive forces that hold
them together. The particles in the solid separate and move about in random patterns. The substance is
melting, changing from solid to a liquid.
If the temperature of a liquid is lowered, the reverse process takes place. Kinetic energy is lost,
the particles slow down, and attractive forces pull the particles close together. The substance is freezing.
A liquid changes to a solid at its freezing point, which is the same temperature as the melting point. Every
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 15
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
substance has its own freezing (melting) point: Water freezes (melts) at 0 0C, golf freezes (melts) at
10640C, and nitrogen freezes (melts) at -2100C.
When a change of state occurs, the temperature of a substance remains constant. If you have a
glass containing ice and water, the ice melts when heat is added at 0 0C, forming more liquid. The liquid
when heat is removed at 00C.
During melting, energy called the heat of fusion is added to separate the particles of a solid. For
example, [1] 334 joules ( or 80 calories) of heat are needed to melt 1 g of ice at its melting point ( 0 0C).
Heat of Fusion for water:
The heat of fusion is also the amount of heat that must be removed to freeze 1 g of water at its freezing
point (00C). To determine the amount of heat needed to melt a sample of ice, multiply the mass of ice by
its heat of fusion. There is no temperature change in the calculation because temperature remains
constant as long as the ice is melting.
Calculating Heat to Melt ( or Freeze ) Water:
Heat = mass x heat of fusion
Example 1.7
Ice cubes at 00C with a mass of 26 g are added to your juice.
a. How much heat (joules) must be added to melt all the ice at 00C?
b. What happens to the temperature of your juice? Why?
Solution:
a. Using the equation: Heat = mass x heat of fusion
Heat = (26 g water) 𝑥 heat of fusion = 334 J/1g H2O
= 8700 J unit g water cancels out
Answer: 8700 J
b. The juice will be colder because heat from the juice is providing the energy to melt the ice.
In the process called sublimation, the particles on the surface of a solid change directly to a gas with
no temperature change and without going through the liquid state. In the reverse process of sublimation
called deposition, gas particles change directly to solid.
𝟐𝟓𝟗𝟎 𝑱 𝟔𝟐𝟎 𝒄𝒂𝒍
Heat of Sublimation for Water:
𝟏 𝒈 𝒐𝒇 𝒘𝒂𝒕𝒆𝒓 𝟏 𝒈 𝒘𝒂𝒕𝒆𝒓
For example, dry ice, which is solid carbon dioxide (CO2), undergoes sublimation at -780C. It is
called “ dry” because it does not form a liquid as it warms. In extremely cold areas, snow does not melt
but sublimes directly to water vapor.
The energy that must be added to vaporize exactly 1 g of liquid to gas at its boiling point is called
the heat of vaporization. For water, 540 cal or 2260 J are needed to convert 1 g of water to vapor at
1000C. This amount of heat is released when 1 g of water vapor (gas) changes to liquid at 100 0C.
Therefore, 540 cal/g or 2260 J/g is also called the heat of condensation.
𝟐𝟐𝟔𝟎 𝑱
Heat of Vaporization for Water:
𝟏 𝒈 𝒘𝒂𝒕𝒆𝒓
To calculate the amount of heat added to vaporize ( or removed to condense ) a sample of water, the
mass of the sample is multiplied by the heat of vaporization. No temperature change occurs during the
change of state.
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 16
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
Calculating Heat to Vaporize ( or Condense ) Water:
Heat = mass x heat of vaporization
Just as substances have different melting and boiling points, they also have different heats of
fusion and heats of vaporization, shown in the table below:
Table 1.4 : Heats of Fusion and Heats of Vaporization of Some Substances
Liquid Formula Melting Heat of Fusion (J/g) Boiling Point Heat of Vaporization
0
Point ( C ) (0C) (J/g)
Water H2O 0 334 100 2260
Ethanol C2H5OH -114 109 78 841
Ammonia NH3 -78 351 -33 1380
Acetone C3H6O -95 98 56 335
Mercury Hg -39 11 357 294
Acetic Acid C2H4O2 17 192 118 390
Example 1.8
In a sauna, 150 g of water is converted to steam at 100 0C. How many kilocalories of heat are needed?
Solution:
Using the equation: Heat = mass x heat of vaporization
Heat = (150 g water) x heat of vaporization=540cal/gH2O
= 81,000 cal unit g water cancels out
Heat in kilocalories:
Heat = 81,000 cal x using 1 kcal = 1000 cal
= 81 kcal unit cal cancels out
Answer: 81 kcal
V. Learning Activity 1.2
1. Identify each of the following changes of state as melting, freezing, sublimation or deposition:
a. The solid structure of a substance breaks down
as liquid forms. ______________________
b. Coffee is freeze dried ______________________
c. Water on the street turns into ice during winter ______________________
d. Crystals of ice form on a gallon pack of ice cream ______________________
e. Dry ice in a popsicle cart disappears. ______________________
2. Calculate the heat needed at 00C to make each of the following changes of state. Indicate whether
heat was absorbed or released.
a. calories to melt 65 g of ice
b. calories to melt 17 g of ice
c. kilocalories to freeze 225 g of water
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 17
UNIVERSITY OF NUEVA CACERES COLLEGE OF ENGINEERING AND ARCHITECTURE
VI. Exercise 1.2
1. Sodium chloride is added in cooking to enhance the flavor of food. When 10 g of NaCl are
dissolved in 200 ml of water at 250C in a coffee cup calorimeter, 669 J of heat is absorbed. Use
the following assumptions about the solution: volume = 200 ml, density = 1 g/ml, specific heat =
4.18 J/g 0C.
a. Is the solution process exothermic?
b. What is qwater ?
c. What is the final temperature of the solution?
2. Identify each of the following changes of state as evaporation, boiling or condensation:
a. The water vapor in the clouds changes to rain.
b. Wet clothes dry on a clothesline.
c. Lava flows into the ocean, and steam forms.
d. After a hot shower, your bathroom mirror is covered with water droplets.
3. Calculate the heat change at 1000C in each of the following problems. Indicate whether heat was
absorbed or released.
a. calories to vaporize 10.0 g of water
b. Kilocalories to vaporize 50.0 g of water
c. Kilocalories to condense 8.0 kg of steam
VII. Quiz
Submit test booklets to the testing center before taking the examination. Schedule will be announced to
the class group. Coverage for Quiz 2 will be Calorimetry and heat in changes of state .
CHEMISTRY for ENGINEERS v1.0 by GINA E.DIOCOS, ChE 18
You might also like
- Thermodynamics by Praveen KulkarniDocument142 pagesThermodynamics by Praveen KulkarniAniket kumar sahu100% (3)
- Heat and TemperatureDocument52 pagesHeat and TemperatureEazel SolanaNo ratings yet
- Slide 1Document30 pagesSlide 1abdulqader.nizarNo ratings yet
- Energy Is The Ability To Do Work or To Produce Heat Light, Heat, Electricity EtcDocument83 pagesEnergy Is The Ability To Do Work or To Produce Heat Light, Heat, Electricity Etcramavtaragrawal2018No ratings yet
- ThermochemistryDocument36 pagesThermochemistryJayadevi ShanmugamNo ratings yet
- First LawDocument23 pagesFirst Lawnoah.sibulo2014No ratings yet
- Chapter 2 - Energy and MatterDocument33 pagesChapter 2 - Energy and Mattermiaka96No ratings yet
- Energy and ChemistryDocument27 pagesEnergy and ChemistryterantejkNo ratings yet
- "Thermochemistry": Chemistry Charles Page High School Stephen L. CottonDocument25 pages"Thermochemistry": Chemistry Charles Page High School Stephen L. CottonAminNo ratings yet
- Chemistry For Engineers - ThermochemDocument3 pagesChemistry For Engineers - ThermochemCharles Augustus100% (2)
- G10 Ch7 Measurement of Heat11May07Document9 pagesG10 Ch7 Measurement of Heat11May07Tun Lin AungNo ratings yet
- HEATDocument3 pagesHEATCrystal Blue Sapphire Silver Ace FleminixNo ratings yet
- Lesson 1. EnergyDocument26 pagesLesson 1. EnergyCelape CabanesNo ratings yet
- Section 3 - Physical Chemistry - Ch19-21Document44 pagesSection 3 - Physical Chemistry - Ch19-21Devi SatyaraniNo ratings yet
- Thermochemistry PowerPointDocument25 pagesThermochemistry PowerPointShinpay doodlesNo ratings yet
- 1 - Energy - Chemistry - For - Engineers - Topic - 01 - Intro - To - EnergyDocument6 pages1 - Energy - Chemistry - For - Engineers - Topic - 01 - Intro - To - EnergyJay Grijaldo100% (1)
- Gwen Gwen GwenDocument33 pagesGwen Gwen Gwenzablan.jsNo ratings yet
- ThermochemDocument29 pagesThermochemNash MartinezNo ratings yet
- Chapter 11Document2 pagesChapter 11api-373649599No ratings yet
- Cady B - Take Home Test Background Information For Calorimetry LabDocument3 pagesCady B - Take Home Test Background Information For Calorimetry Labapi-298329103No ratings yet
- ThermodynamicsDocument9 pagesThermodynamicsapi-249060862No ratings yet
- Ch06 SlidesDocument73 pagesCh06 SlidesbeelzeburtonNo ratings yet
- What We Have Already LearntDocument13 pagesWhat We Have Already Learnt'Shyam SinghNo ratings yet
- AP Chapter 5 Powerpoint For WebsiteDocument56 pagesAP Chapter 5 Powerpoint For Websitenyphia CruzNo ratings yet
- Heat (Add Science) OkDocument35 pagesHeat (Add Science) OkJaswardi Anwar Bin Md Yaacob� IPGKKBNo ratings yet
- Definition of Heat CapacityDocument48 pagesDefinition of Heat CapacityPunitha NagappanNo ratings yet
- Heat & Thermal MeasurementsDocument47 pagesHeat & Thermal Measurementskriston khanNo ratings yet
- Thermodynamics: Chapter 2 Topic 1Document9 pagesThermodynamics: Chapter 2 Topic 1April Galope OlaliaNo ratings yet
- Chapter1a ThermochemistryDocument27 pagesChapter1a ThermochemistryRin, Trisha Angelica MitraNo ratings yet
- Decentralized Poly-Generation of Energy: Basic ConceptsDocument28 pagesDecentralized Poly-Generation of Energy: Basic ConceptsMogahed OsmanNo ratings yet
- Ns U6 - Energy Changes-18Document4 pagesNs U6 - Energy Changes-18api-368121935No ratings yet
- Mod 5Document17 pagesMod 5S M AkashNo ratings yet
- L23 WorksheetDocument2 pagesL23 WorksheetmaplecookieNo ratings yet
- 5.1 EnergeticsDocument8 pages5.1 EnergeticsEldin EnggNo ratings yet
- Lesson 1 - First Law of Thermodynamics and CalorimetryDocument4 pagesLesson 1 - First Law of Thermodynamics and CalorimetryJeff ValdezNo ratings yet
- Heat CapacityDocument11 pagesHeat Capacitymohammed merkhasNo ratings yet
- Melc 124 127 ThermochemistryDocument44 pagesMelc 124 127 ThermochemistryFmae antoinette100% (1)
- Which Area of Chemistry Is Concerned With The Topic?: THERMOCHEMISTRY: Energy Changes in Chemical ReactionsDocument19 pagesWhich Area of Chemistry Is Concerned With The Topic?: THERMOCHEMISTRY: Energy Changes in Chemical ReactionsSahada KanapiyaNo ratings yet
- Unit 3 - Physical ChemistryDocument94 pagesUnit 3 - Physical ChemistryUmair HibatullahNo ratings yet
- Heat and TempDocument74 pagesHeat and TempPortia A. EgkenNo ratings yet
- Larry D. Buban, PH.D Science Education - Physics Cas - WvsuDocument49 pagesLarry D. Buban, PH.D Science Education - Physics Cas - WvsuHannah Grace Romano ViceralNo ratings yet
- HeatDocument4 pagesHeatGaber HassanNo ratings yet
- Measurement of HeatDocument56 pagesMeasurement of Heatkoromamoses235No ratings yet
- Week 007 Module ThermochemistryDocument12 pagesWeek 007 Module ThermochemistryFigh terNo ratings yet
- P 7.4 ThermochemistryDocument32 pagesP 7.4 ThermochemistryFelicia GunawanNo ratings yet
- Heat-I: Theory and Exercise BookletDocument39 pagesHeat-I: Theory and Exercise BookletMD CHHIMPANo ratings yet
- Lecture Notes On CE HeatDocument44 pagesLecture Notes On CE HeatRichard WongNo ratings yet
- 3Document7 pages3chikeruNo ratings yet
- Heat and EnthalpyDocument4 pagesHeat and EnthalpytuvvacNo ratings yet
- ThermochemistryDocument38 pagesThermochemistryAris GProNo ratings yet
- Chemistry 3-Wk3-IntroThermoDocument29 pagesChemistry 3-Wk3-IntroThermoJezelflor Frias50% (2)
- Heat and TemperatureDocument66 pagesHeat and TemperatureMark Francis HernandezNo ratings yet
- CalorimetryDocument25 pagesCalorimetryAbhijit Kar Gupta100% (11)
- ThermodynamicsDocument25 pagesThermodynamicsJean BesanaNo ratings yet
- THERMOCHEMISTRYDocument20 pagesTHERMOCHEMISTRYdeegemite_24100% (1)
- 2 All About Heat (Students - Copy)Document54 pages2 All About Heat (Students - Copy)Crystal HuffNo ratings yet
- Specific Heat CapacityDocument14 pagesSpecific Heat CapacityAidan KNo ratings yet
- What Is WorkDocument11 pagesWhat Is WorkviveknarayanNo ratings yet
- Lecture 1Document20 pagesLecture 1Sadika Afrin SnigdhaNo ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- HVC1 P 1Document20 pagesHVC1 P 1ahmilove135No ratings yet
- Physics SSC I Solution of 2nd Set Model Question PaperDocument9 pagesPhysics SSC I Solution of 2nd Set Model Question PaperFaisal SamiNo ratings yet
- Chapter 1 Thermodynamics and EquilibriumDocument23 pagesChapter 1 Thermodynamics and EquilibriumJakeNK94No ratings yet
- Heat Engines Vol 22Document1 pageHeat Engines Vol 22prasanthiNo ratings yet
- Thermal Transmittance Measurement With The Hot BoxDocument9 pagesThermal Transmittance Measurement With The Hot BoxLuca Scarsella100% (1)
- Energy Conservation & ManagementDocument3 pagesEnergy Conservation & Managementnavneetkpatil8409No ratings yet
- Chapter 3Document30 pagesChapter 3Nashit AhmedNo ratings yet
- Physics NomenclatureDocument4 pagesPhysics NomenclaturesmithastellaNo ratings yet
- MIN-305 Heat & Mass Transfer Tutorial - 1Document2 pagesMIN-305 Heat & Mass Transfer Tutorial - 1Ayush JaiswalNo ratings yet
- DVI143 Neatpump EN 1209 PDFDocument4 pagesDVI143 Neatpump EN 1209 PDFHamza BaraketNo ratings yet
- RAC Syllabus - MandiDocument2 pagesRAC Syllabus - MandiGupta JayeshNo ratings yet
- SP41Document214 pagesSP41Umar MohammadNo ratings yet
- B.pharmacy 3rd Sem To 8th Sem New SchemeDocument65 pagesB.pharmacy 3rd Sem To 8th Sem New SchemePremendra YadawNo ratings yet
- Chem Ia FinalDocument13 pagesChem Ia FinalAngelina TomacNo ratings yet
- TRANSPORT PHENOMENA - Unit 1 NotesDocument150 pagesTRANSPORT PHENOMENA - Unit 1 NotesRathi ManiNo ratings yet
- Magnetic Field Effect On Double-Diffusion With Magnetic and Non-Magnetic Nano UidsDocument21 pagesMagnetic Field Effect On Double-Diffusion With Magnetic and Non-Magnetic Nano UidsShafqat HussainNo ratings yet
- World Journal of Engineering Research and Technology WjertDocument8 pagesWorld Journal of Engineering Research and Technology Wjertammar fahmiNo ratings yet
- Ejectors Applications in Refrigeration TechnologyDocument27 pagesEjectors Applications in Refrigeration TechnologyRizwan Ali100% (1)
- Physics SeminarDocument15 pagesPhysics SeminarNivesh Programming and GamingNo ratings yet
- 08 Specific Heat CapacityDocument11 pages08 Specific Heat CapacityZoelFikar Fahmi67% (3)
- Lightnin: Fermentation: Critical Process Phenomena and New Technology Developments That Affect Yield and ProductivityDocument6 pagesLightnin: Fermentation: Critical Process Phenomena and New Technology Developments That Affect Yield and ProductivityAkash PagareNo ratings yet
- Course Outline Phy107-Spring-2023Document4 pagesCourse Outline Phy107-Spring-2023Mohammed Arif Mainuddin 2211577042No ratings yet
- Thermodynamics Question Solve 2010Document10 pagesThermodynamics Question Solve 2010MD SR ShantoNo ratings yet
- Tutorial Sol CH 20Document6 pagesTutorial Sol CH 20Abraham wisdomNo ratings yet
- Applied Sciences: Study On Residual Stress of Welded Hoop StructureDocument16 pagesApplied Sciences: Study On Residual Stress of Welded Hoop StructureChandrasekarNo ratings yet
- Obtencion de AcetonaDocument7 pagesObtencion de AcetonaLiz Laureano RodriguezNo ratings yet
- SYLLABUS 2021-2022: Standard: 11 Subject: PhysicsDocument8 pagesSYLLABUS 2021-2022: Standard: 11 Subject: Physicschandran chandranNo ratings yet
- CHE-314: Lecture 4. Section 2: The Heat Diffusion Equation and Transport PropertiesDocument26 pagesCHE-314: Lecture 4. Section 2: The Heat Diffusion Equation and Transport PropertiesAkib ImtihanNo ratings yet
- International Journal of Heat and Fluid Flow: A. Buczek, T. TelejkoDocument7 pagesInternational Journal of Heat and Fluid Flow: A. Buczek, T. TelejkoRanjith RNNo ratings yet