Professional Documents
Culture Documents
Chem - Rates of Reaction Problems
Chem - Rates of Reaction Problems
Uploaded by
lyagobaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chem - Rates of Reaction Problems
Chem - Rates of Reaction Problems
Uploaded by
lyagobaCopyright:
Available Formats
ORDINARY LEVEL CHEMISTRY PROBLEMS
PART 13: RATES OF REACTION
(1) Curve Y in the diagram below shows the results that were obtained during the
investigation of the rate of reaction between iron and dilute hydrochloric acid under
normal conditions. Curve X and Z were obtained when some of the conditions of the
experiment were changed
Mass of flask Y
and contents (g)
Z
X
M
Time (s)
(a) (i) List three conditions that were changed to obtain curve X
(ii) state what point M represents
(b) some conditions you have listed in (a) (i) were changed to obtain curve Z
(i) state the conditions changed
(ii) give a reason for your answer
(2) Sodium thiosulphate reacts with dilute acids according to the equation
𝑆2 𝑂32− (𝑎𝑞) + 2𝐻 + (𝑎𝑞) → 𝑆𝑂2 (𝑔) + 𝑆(𝑠) + 𝐻2 𝑂(𝑙)
(a) State what would be observed if dilute hydrochloric acid was added to sodium
thiosulphate
(b) The rate of reaction is affected by concentration of thiosulphate
(i) State one factor other than concentration that can affect the rate of
reaction
(ii) Briefly explain the effect of the factor on the rate of reaction you have
named
(iii) Describe an experiment that can be carried out in the laboratory to show
the effect of the factor you have named on the rate on the rate of the
reaction
(c) Table below shows the variation in the concentration of sodium thiosulphate
with time
Time (s) 200 100 40 20 10
Concentration of thiosulphate (moldm3) 0.00 0.09 0.14 0.20 0.25
1
(𝑑𝑚3 𝑚𝑜𝑙 −1 )
𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑡ℎ𝑖𝑜𝑠𝑢𝑙𝑝ℎ𝑎𝑡𝑒
1
(i) Complete the table by determining the values of
𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑡ℎ𝑖𝑜𝑠𝑢𝑙𝑝ℎ𝑎𝑡𝑒
1
(ii) Plot a graph of against time
𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑡ℎ𝑖𝑜𝑠𝑢𝑙𝑝ℎ𝑎𝑡𝑒
(iii) State any conclusion you can draw from the shape from the graph
Success has a catalyst; it is called hard work © Ssekyejwe A. Ronald Page 1
(3) State and explain the effect of each of the following conditions on the rate of a chemical
reaction
(a) Temperature
(b) Particle size
(c) Concentration
(4) (a) (i) Write an equation for the reaction between dilute nitric acid and calcium
carbonate
(ii) State how temperature can affect the rate of the reaction
(iii) Give a reason why a large surface area of calcium carbonate can speed
up the rate of reaction in (a) (i)
(b) Magnesium can react with hydrochloric acid to form hydrogen. State the
conditions and write an ionic equation for the reaction
(c) The table below shows the volumes of hydrogen evolved when various lengths of
magnesium ribbon were reacted with fixed volumes of hydrochloric acid
Length of ribbon (cm) 1.0 2.0 3.0 5.0 6.0
Volume of hydrogen (cm3/min) 2.2 3.6 5.2 9.2 10.8
(i) Plat a graph of volume of hydrogen against length of magnesium ribbon
(ii) Explain the shape of the graph
(iii) Using the graph determine the rate of the reaction if 4.0cm of
magnesium ribbon was used.
(5) (a) Define the term rate of reaction
(b) The table below shows the variation in volume of hydrogen evolved with time
when dilute hydrochloric acid was added to excess zinc.
Volume of hydrogen (cm3) 0 20 35 46 56 72 79 79
Time (s) 0 10 20 30 40 60 80 90
Plot a graph of volume of hydrogen evolved against time.
(c) Using the graph determine the time taken to collect 60cm 3 of hydrogen gas
(d) (i) Draw tangents on your graph at points when the time is 20 60 seconds
and determine the gradient
(ii) Compare the rate of the reaction at 20 seconds and 60 seconds
(e) Comment on the rate of the reaction after 20 seconds and 60 seconds. Explain.
(6) The graph below shows the variation of volume of hydrogen with time when excess
magnesium was added to 100cm3 of 1.0M sulphuric acid ar room temperature
Volume of
hydrogen (cm3)
Time (s)
Success has a catalyst; it is called hard work © Ssekyejwe A. Ronald Page 2
(a) Calculate the number of moles of hydrogen ions contained in 100cm 3 of
(i) 0.5M sulphuric acid
(ii) 1.0M sulphuric acid
(b) (i) Sketch on the same axes of the graph in the figure, the graph that would
be obtained if the same mass of magnesium was added to 100cm3 of
0.5M sulphuric acid at room temperature
(ii) Mark on the graphs the times when the two reactions reach completion
(iii) Compare the time the reaction took to reach completion when 0.5M
sulphuric acid was used to that when 1.0M sulphuric acid was used
(7) (a) Describe an experiment to show how surface area can affect the rate of the
reaction between calcium carbonate and 2M hydrochloric acid
(b) Briefly explain why when a 4M hydrochloric acid was used instead of the 2M
acid, the rate of reaction was faster
(c) State one factor other than those mentioned above than can affect the rate of the
reaction between hydrochloric acid and calcium carbonate
(8) (a) What is meant by rate of chemical reaction
(b) State how the following factors affect the rate of a chemical reaction
(i) Temperature
(ii) Surface area of the reactants
(c) The table below shows the volume of hydrogen collected at various time intervals
when magnesium was reacted with a 2M hydrochloric acid
Time (s) 0 1 2 3 4 5 6 7
Volume of hydrogen (cm3) 0 25 45 60 70 75 77 77
(i) Plot a graph of hydrogen against time
(ii) Determine the rate of the reaction at 3s
(iii) Determine the volume of hydrogen evolved at 3.5s
(d) State how the rate of a chemical reaction at 3s would be affected if a 1M
hydrochloric acid was used
(9) When a certain volume of 0.1M hydrochloric acid was reacted at room temperature with
excess iron fillings. 120cm3 of a gas were produced
(a) Draw a diagram to show how the rate of reaction was determined.
(b) Write equation for the reaction that took place
(c) Calculate the
(i) Volume of 0.1M hydrochloric acid required to produce 120cm 3 of the gas.
(ii) Mass of iron filings reacted
(d) Draw a sketch graph of the volume against time.
(e) State how the rate of reaction would change if the reaction was carried out at a
temperature above room temperature
(10) (a) State three factors that can affect the rate of a chemical reaction
Success has a catalyst; it is called hard work © Ssekyejwe A. Ronald Page 3
(b) A mixture of a known mass of magnesium and a certain volume of 2M
hydrochloric was put in a conical flask and the mass of the mixture was
recorded at various intervals. The results of the experiment are as shown
Mass of the
mixture (g)
Time (s)
On the same axes, draw a graph that would be obtained when the same mass of
magnesium was reacted with the same volume of 1M hydrochloric acid
(c) 5.0g of calcium carbonate was reacted with 20cm 3 of 2M hydrochloric acid
(i) Write equation for the reaction
(ii) Calculate the mass of calcium carbonate that was left
(11) (a) (i) What is rate of reaction
(ii) How does particle size affect rate of reaction. Explain
(b) The table below shows the time taken for sulphur to form various concentration
of thiosulphate, 𝑆2 𝑂32− , were used
Concentration of 𝑆2 𝑂32− (M) 0.2 0.6 0.8 1.2 1.6
Time for sulphur to form (s) 60 20 15 10 7.5
1
Plot a graph of against concentration of 𝑆2 𝑂32−
𝑡𝑖𝑚𝑒
1
(c) (i) explain the relationship between rate of reaction and
𝑡𝑖𝑚𝑒
(ii) deduce from the graph, how the rate of reaction varies with
concentration of 𝑆2 𝑂32−
(12) In an experiment to determine the rate of reaction between zinc and sulphuric, dilute
sulphuric acid was reacted with zinc granules to which some copper(II) sulphate
solution was added. The volumes of hydrogen gas collected at various time intervals
were measured. Results were as shown.
Time (minutes) 0 5 10 15 20 25 30
Volume of gas (cm3) 0 10 20 25.5 29.5 32 32
(a) (i) What is the role of copper(II) sulphate
(ii) Write an ionic equation for the reaction
(iii) Explain what would happen if zinc granules were replaced with zinc
powder
(b) (i) Plot a graph of volume of hydrogen evolved against time
(ii) Describe how you would determine the rate of reaction at 12 minutes
Success has a catalyst; it is called hard work © Ssekyejwe A. Ronald Page 4
(iii) Compare the rates of reaction at 12 minutes with that at 20 minutes.
Give a reason for your answer.
(iv) What happens to the shape of the graph after 25 minutes? Explain your
answer.
(13) A certain mass of zinc powder was reacted with hydrochloric acid at room temperature.
(a) (i) Write equation for the reaction.
(ii) Draw a graph to show how
• The volume of the gaseous product varied with time
• The mass of the reaction flask and its contents varied with time.
(b) What would be the effect on the rate of reaction of
(i) Adding copper(II) sulphate solution to the reaction mixture at room
temperature.
(ii) Using the same mass of zinc granules instead of zinc powder
(c) Give a reason for your answer in b(i) and b(ii) above
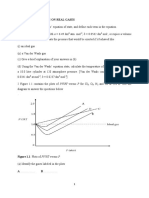
(14) The graphs below show the effect of temperature of the rate of reaction between marble
chips of the same mass and excess 2M hydrochloric acid.
Mass of flask A
and contents (g)
C B
Time (s)
(a) If curve B is for the reaction at 40ºC, which curve shows the reaction taking
place at
(i) 20ºC
(ii) 60ºC
(b) Explain why the curves eventually end at the same level
(c) State one other method that can be used to determine the rate of reaction
between marble and hydrochloric acid.
(15) 8g of zinc powder was added to 50cm 3 of 1M hydrochloric acid in a conical flask.
(a) Write equation for the reaction
(b) (i) Describe how the rate of the reaction can be determined. Draw a diagram
to illustrate your answer
(ii) Sketch a graph to show the rate of the reaction. Label this X
(c) In another experiment, 8g of zinc powder was added to 100cm 3 of 0.5M
hydrochloric acid.
(i) Sketch a graph for the rate of reaction using the same axes in b(ii).
Label this Y
(ii) Explain the shape of the two graphs
Success has a catalyst; it is called hard work © Ssekyejwe A. Ronald Page 5
(16) Oxygen is formed from hydrogen peroxide in the presence of manganese(IV) oxide
according to the equation
2𝐻2 𝑂2 (𝑎𝑞) → 2𝐻2 𝑂(𝑙) + 𝑂2 (𝑔)
(a) In an experiment, a certain volume of hydrogen peroxide was used to prepare
oxygen at room temperature. With aid of a suitable diagram, describe how the
following can be determined
(i) Volume of oxygen evolved
(ii) The rate of evolution of oxygen
(b) (i) In another experiment, one half of the volume of hydrogen peroxide used in
(a) was diluted with an equal volume of water. On the same axes, draw
graphs to show the variation of the volume of oxygen with time for
experiments in (a) and (b)
(ii) Explain the differences in the shapes of the curve
(iii) Comment of the time taken for the reactions to reach completion
(c) Oxygen produced from 200cm3 of 0.5M hydrogen peroxide was completely
reacted with magnesium. Calculate the mass of magnesium that reacted.
17. (a). Hydrogen peroxide produces gas bubbles slowly when exposed to air, but when
aqueous iron(III) chloride is added, the production of bubbles becomes more rapid.
(i). Name the gas produced when hydrogen peroxide is exposed to air
(ii). Write the equation for the reaction that takes place
(iii). State the role of iron(III) chloride in the reaction
(iv). Name another substance that can affect the production pf the gas in the
same way as iron(III) chloride
(b). The table below shows the variation if the concentration of hydrogen peroxide with
time when a sample of hydrogen peroxide was mixed with iron(III) chloride at room
temperature
𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 ℎ𝑦𝑑𝑟𝑜𝑔𝑒𝑛 𝑝𝑒𝑟𝑜𝑥𝑖𝑑𝑒 (𝑚𝑜𝑙𝑑𝑚−3 ) 0.05 0.10 0.15 0.20 0.25
𝑇𝑖𝑚𝑒, 𝑡 (𝑠) 53 26 17 13 10.5
1 −1
(𝑠 )
𝑡
1
(i). Copy and complete the table above by computing and filling in the values of
𝑡
1
(ii). Plot a graph of against concentration of hydrogen peroxide
𝑡
(iii). Using your graph, deduce how the rate of reaction varies with the
concentration of hydrogen peroxide
(iv). Determine the slope of the graph
(v). State two ways by which the rate of the reaction in (b) could be made faster.
END
Success has a catalyst; it is called hard work © Ssekyejwe A. Ronald Page 6
You might also like
- MT 181 Solubility in Organic Solvents: Miscellaneous TechniquesDocument4 pagesMT 181 Solubility in Organic Solvents: Miscellaneous TechniquesFelipe Navarrete100% (1)
- IB - Ch6 - 16 Past PapersDocument33 pagesIB - Ch6 - 16 Past Papersrejymol100% (1)
- Chapter 16-Acids and BasesDocument33 pagesChapter 16-Acids and BasesGörkem DamdereNo ratings yet
- 4.3 Reaction Rates and Reversible ReactionsDocument18 pages4.3 Reaction Rates and Reversible ReactionsVictor VC100% (6)
- Rates of Reaction o LevelDocument19 pagesRates of Reaction o Leveljosephmadrara2No ratings yet
- ALEVELREVISIONQUESTIONSDocument7 pagesALEVELREVISIONQUESTIONSAnthony AndyNo ratings yet
- L6 Chem ExamDocument8 pagesL6 Chem Examhmatara8No ratings yet
- S.4 Chem Revision Questions 2020 Revision & Past PapersDocument5 pagesS.4 Chem Revision Questions 2020 Revision & Past Papersmoggadavid480No ratings yet
- Chapter 1: Rate of Reaction: Larning Task 1.2 Problem SolvingDocument29 pagesChapter 1: Rate of Reaction: Larning Task 1.2 Problem Solvingamin_zamanNo ratings yet
- Uneb Uace Chemistry Paper 2 2018Document3 pagesUneb Uace Chemistry Paper 2 2018basilkens200061No ratings yet
- Chemistry - Higher Level: Pre-Leaving Certifi Cate Examination, 2014 Triailscrúdú Na Hardteistiméireachta, 2014Document8 pagesChemistry - Higher Level: Pre-Leaving Certifi Cate Examination, 2014 Triailscrúdú Na Hardteistiméireachta, 2014Diaa SaberNo ratings yet
- Level 2 (Some Things Missing)Document4 pagesLevel 2 (Some Things Missing)Viviana PlacentinoNo ratings yet
- Chemistry Take-Home Test-Set A, 2020. Answer All Questions. Q. 1 Solid Sodium Carbonate Reacts With Aqueous Hydrochloric Acid To Form Aqueous SodiumDocument2 pagesChemistry Take-Home Test-Set A, 2020. Answer All Questions. Q. 1 Solid Sodium Carbonate Reacts With Aqueous Hydrochloric Acid To Form Aqueous SodiumMuyatwa LiksNo ratings yet
- Chemistry F4 Set 13 14Document7 pagesChemistry F4 Set 13 14samwelaldoboyNo ratings yet
- S1 Chemistry Seminar OneDocument5 pagesS1 Chemistry Seminar Onesolomonogenrwot704No ratings yet
- Chem Cgce 2011 A/lDocument9 pagesChem Cgce 2011 A/lmengotNo ratings yet
- 2017 Chemistry TheoryDocument3 pages2017 Chemistry TheoryEffNo ratings yet
- JUNE 2013 Section A: Physical and General Chemistry (Answer Only TWO Questions in This Section)Document10 pagesJUNE 2013 Section A: Physical and General Chemistry (Answer Only TWO Questions in This Section)Zozo FozaoNo ratings yet
- National 5 Exam Style QuestionsDocument24 pagesNational 5 Exam Style Questionsmilneda31No ratings yet
- VVDocument5 pagesVVLisaam De YesteNo ratings yet
- Chemistry Question and Answer 2013-2017Document52 pagesChemistry Question and Answer 2013-2017Chikuta ShingaliliNo ratings yet
- A Level Chemistry Paper 2 Exam 14Document4 pagesA Level Chemistry Paper 2 Exam 14Anthony AndyNo ratings yet
- CHEMISTRY-PP2-Form-4-END TERMDocument10 pagesCHEMISTRY-PP2-Form-4-END TERMKevinNo ratings yet
- s6 Chemistry Pp2Document5 pagess6 Chemistry Pp2ANYWAR SIMONNo ratings yet
- Tutorial Questions For CHME2201Document4 pagesTutorial Questions For CHME2201Peguy FotsoNo ratings yet
- CHEMISTRYset 1 P3Document7 pagesCHEMISTRYset 1 P3Yu YanNo ratings yet
- A Level Chemistry Paper 2 Exam 10Document4 pagesA Level Chemistry Paper 2 Exam 10Anthony AndyNo ratings yet
- A Level Chemistry 2 Mocks UMTADocument7 pagesA Level Chemistry 2 Mocks UMTAmakueimadol17No ratings yet
- ST - Julian Gayaza Chemistry Olevel Seminor QuestionDocument43 pagesST - Julian Gayaza Chemistry Olevel Seminor QuestionMajanga Johnny0% (2)
- S3CHEMISTRY3Document3 pagesS3CHEMISTRY3HASHIMU BWETENo ratings yet
- S.6 Chem Seminar Questions March 2020 Revision Past PapersDocument9 pagesS.6 Chem Seminar Questions March 2020 Revision Past PapersMaama PhionaNo ratings yet
- Genetics ReportDocument4 pagesGenetics ReportMukisa EliasNo ratings yet
- Al 2015 Chem 2Document4 pagesAl 2015 Chem 2Sand FossohNo ratings yet
- Chapter 1Document11 pagesChapter 1kenenathNo ratings yet
- Chemistry P2 S6 Aceiteka 2023Document6 pagesChemistry P2 S6 Aceiteka 2023williamesilu3No ratings yet
- 2022 Uace Chemistry SeminarDocument11 pages2022 Uace Chemistry Seminarmakueimadol17No ratings yet
- Form 4 Acids Bases and Salts Questions Teacher - Co .KeDocument9 pagesForm 4 Acids Bases and Salts Questions Teacher - Co .KeMikeNo ratings yet
- Model Question Grade XI Chemistry (Theory) F.M: 75 Attempt All Questions Group "A"Document4 pagesModel Question Grade XI Chemistry (Theory) F.M: 75 Attempt All Questions Group "A"Supriya Rai0% (1)
- Acids, BAIS AND SALTS QDocument10 pagesAcids, BAIS AND SALTS Qexan14431No ratings yet
- A Level Chemistry Paper 2 Exam 4Document4 pagesA Level Chemistry Paper 2 Exam 4kitookebarnabasNo ratings yet
- Chemistry Form 4 PP2Document12 pagesChemistry Form 4 PP2jmwalimu81No ratings yet
- AL Chemistry 1995-1998 Paper 1Document18 pagesAL Chemistry 1995-1998 Paper 1api-3734333No ratings yet
- Test Ten Paper TwoDocument7 pagesTest Ten Paper TwoWanje MichaelNo ratings yet
- 6 Chemical Kinetics Practice Questions.Document2 pages6 Chemical Kinetics Practice Questions.Greg The MedicNo ratings yet
- Chemistry Form 4 Paper 2Document12 pagesChemistry Form 4 Paper 2nuhrramah2912No ratings yet
- SPM Kimia Tingkatan, 5 Rate of Reaction ExerciseDocument7 pagesSPM Kimia Tingkatan, 5 Rate of Reaction Exerciseryder1man6433No ratings yet
- Ch36 Written AssignmentDocument4 pagesCh36 Written AssignmentHau Hei, Matthew LinNo ratings yet
- A Level Chemistry Paper 2 Exam 6Document4 pagesA Level Chemistry Paper 2 Exam 6majanga johnNo ratings yet
- Revision STPM Term 1Document15 pagesRevision STPM Term 1Wong WengSiongNo ratings yet
- 1996 2009 Kcse Chemistry 1Document177 pages1996 2009 Kcse Chemistry 1W GNo ratings yet
- Madaraka F4 Discussion QuestionsDocument15 pagesMadaraka F4 Discussion Questionsshad kojwangNo ratings yet
- A Level Chemistry Paper 2 Exam 26Document5 pagesA Level Chemistry Paper 2 Exam 26Anthony AndyNo ratings yet
- Ndejje Senior Secondary School: 525/2 Chemistry Paper 2 July/August 2006 2 HoursDocument9 pagesNdejje Senior Secondary School: 525/2 Chemistry Paper 2 July/August 2006 2 HoursntambiNo ratings yet
- A Level Chemistry Paper 2 Exam 28Document3 pagesA Level Chemistry Paper 2 Exam 28Anthony AndyNo ratings yet
- A Level Chemistry Paper 2 Exam 3Document6 pagesA Level Chemistry Paper 2 Exam 3Anthony AndyNo ratings yet
- How FastDocument54 pagesHow FastKaushal Silva RanpatabendigeNo ratings yet
- Ok A Level Chem Seminar Questions 2023-1Document17 pagesOk A Level Chem Seminar Questions 2023-1ashaba mosesNo ratings yet
- Chemistry 1 - Exam N Answers - Dyampaye - Co.tzDocument18 pagesChemistry 1 - Exam N Answers - Dyampaye - Co.tzErick MwalukasaNo ratings yet
- Kcse Chemistry Marking SchemeDocument174 pagesKcse Chemistry Marking SchemeDavid Musila ToywaNo ratings yet
- RTS Chemistry SPM Question Bank Chapter 10Document8 pagesRTS Chemistry SPM Question Bank Chapter 10Scorched ZenNo ratings yet
- Practice Questions On CHM 212Document4 pagesPractice Questions On CHM 212Help HandNo ratings yet
- CE 6237 Durability of Structures: ConcreteDocument27 pagesCE 6237 Durability of Structures: Concreteraju_420034520No ratings yet
- 0620 Chemistry: MARK SCHEME For The October/November 2012 SeriesDocument4 pages0620 Chemistry: MARK SCHEME For The October/November 2012 SeriesCamilo CahuanaNo ratings yet
- 1ST Summative Test-Science ViDocument4 pages1ST Summative Test-Science ViDell Nebril SalaNo ratings yet
- Fatty Acid Synthesis by Prof DR Abdalla Jarari 2nd Year For VIDEOSDocument66 pagesFatty Acid Synthesis by Prof DR Abdalla Jarari 2nd Year For VIDEOSnoran alfaitoryNo ratings yet
- 2chemicalbrochure 11jan2023Document8 pages2chemicalbrochure 11jan2023Maksudur RahmanNo ratings yet
- Avacado OilDocument16 pagesAvacado OilDeshan ManodyaNo ratings yet
- BP Sylab TCDocument73 pagesBP Sylab TCamithg33No ratings yet
- PCR Issue Fall 2021Document64 pagesPCR Issue Fall 2021Kristin OsikaNo ratings yet
- Taconic TSM-DS3M - All The T's and I'sDocument7 pagesTaconic TSM-DS3M - All The T's and I'sjackNo ratings yet
- Gas Absorption and Gas StrippingDocument14 pagesGas Absorption and Gas StrippingDozdi86% (7)
- Ejercicio 11 Electrotecnia ResueltoDocument3 pagesEjercicio 11 Electrotecnia Resueltobrayan23No ratings yet
- Chemical Technology Self Study Topic: Polyvinyl Chloride (PVC)Document11 pagesChemical Technology Self Study Topic: Polyvinyl Chloride (PVC)Paridhi GargNo ratings yet
- Solid-Liquid Equilibrium Data of Amoxicillin and HDocument10 pagesSolid-Liquid Equilibrium Data of Amoxicillin and HTouatiNo ratings yet
- Excretory SystemDocument37 pagesExcretory SystemBlister CountNo ratings yet
- Microstructure of Concrete: By: Dr. Hana AljewifiDocument18 pagesMicrostructure of Concrete: By: Dr. Hana Aljewifigen ridanNo ratings yet
- Chapter 11 Post-Emulsified Fluorescent (Hydrophilic & Lipophilic)Document13 pagesChapter 11 Post-Emulsified Fluorescent (Hydrophilic & Lipophilic)maxpan maxNo ratings yet
- PolyethyleneDocument51 pagesPolyethyleneiiphyd2403No ratings yet
- Anquamine® 401 Curing Agent: DescriptionDocument5 pagesAnquamine® 401 Curing Agent: DescriptionrosarioNo ratings yet
- API 510 Question Bank Sets IDocument4 pagesAPI 510 Question Bank Sets IMd Asif FaizanNo ratings yet
- FScGenGardGN7300 ENDocument1 pageFScGenGardGN7300 ENengr.shahid041No ratings yet
- Fiber Matrix Properties and Fiber Reinforced PropertiesDocument85 pagesFiber Matrix Properties and Fiber Reinforced PropertiesTrina DominiqueNo ratings yet
- Xu Princeton 0181D 10615Document175 pagesXu Princeton 0181D 10615Sailendra MeherNo ratings yet
- HPLCdetectorsDocument34 pagesHPLCdetectorsShiva Kumar RamachandraNo ratings yet
- 01-08-2021 SR - Super60 (In Coming) Jee-Adv 2017 P2 WTA-38 Question PaperDocument16 pages01-08-2021 SR - Super60 (In Coming) Jee-Adv 2017 P2 WTA-38 Question Paperdasari srinidhiNo ratings yet
- Presentation 2Document18 pagesPresentation 2rayonneeemdyNo ratings yet
- Redox and Equivalent ConceptDocument6 pagesRedox and Equivalent ConceptajaxNo ratings yet
- Chemistry Salt Trial AnswersDocument16 pagesChemistry Salt Trial AnswersArchanaa DorairajNo ratings yet
- Inorganic Material Chemistry - Class 3 - Lecture NotesDocument41 pagesInorganic Material Chemistry - Class 3 - Lecture NotesPrabash RathnayakaNo ratings yet