Professional Documents
Culture Documents
Jaundice How To Evaluate
Jaundice How To Evaluate
Uploaded by
Novita ApramadhaCopyright:
Available Formats
You might also like
- Fluid, Electrolyte, and Acid-Base Disorders Robert B Schonberger 2018Document18 pagesFluid, Electrolyte, and Acid-Base Disorders Robert B Schonberger 2018Akhmad Fadhiel Noor100% (1)
- Anatomy, Lecture 12, Blood Supply of The Gastrointestinal Tract (Slides)Document27 pagesAnatomy, Lecture 12, Blood Supply of The Gastrointestinal Tract (Slides)Ali Al-Qudsi100% (1)
- LIVERDocument24 pagesLIVERFredIanBantolioNo ratings yet
- Transient Hepatic Attenuation DifferencesDocument6 pagesTransient Hepatic Attenuation Differencesademar milton de souza filhoNo ratings yet
- Puentes Portosistémicos: Reporte de Un Caso ClínicoDocument5 pagesPuentes Portosistémicos: Reporte de Un Caso ClínicobumetroNo ratings yet
- Diagnosis and Classification of Vascular Liver DisDocument16 pagesDiagnosis and Classification of Vascular Liver DisKata TölgyesiNo ratings yet
- Veterinary Internal Medicne - March 1993 - Leveille - Pathophysiology and Pharmacologic Modulation of Hepatic FibrosisDocument13 pagesVeterinary Internal Medicne - March 1993 - Leveille - Pathophysiology and Pharmacologic Modulation of Hepatic FibrosisAna Paula SeminarioNo ratings yet
- Glomerular Filtration: An Overview: Mary Jo HolechekDocument8 pagesGlomerular Filtration: An Overview: Mary Jo HolechekagaasfaNo ratings yet
- Imagen Hepática Vascular Hígado 2022Document15 pagesImagen Hepática Vascular Hígado 2022Oscar BushNo ratings yet
- Tubular Transport 2010Document27 pagesTubular Transport 2010Maria Fernanda Pineda FëirethdNo ratings yet
- Portal Hypertension: Historical BackgroundDocument15 pagesPortal Hypertension: Historical BackgroundDiego Andres Garcia RochaNo ratings yet
- Review: Hematopoiesis: An Evolving Paradigm For Stem Cell BiologyDocument14 pagesReview: Hematopoiesis: An Evolving Paradigm For Stem Cell Biologywildan pragaNo ratings yet
- Mechanisms of Disease: V - B - S H H SDocument10 pagesMechanisms of Disease: V - B - S H H SMedranoReyesLuisinNo ratings yet
- Astra-Module-38 Pedia NephroDocument20 pagesAstra-Module-38 Pedia NephroenzocruzinNo ratings yet
- The Anatomical Record - 2020 - Mak - Hepatic Sinusoids Versus Central Veins Structures Markers Angiocrines and Roles inDocument31 pagesThe Anatomical Record - 2020 - Mak - Hepatic Sinusoids Versus Central Veins Structures Markers Angiocrines and Roles inKata TölgyesiNo ratings yet
- Koagulasi CKDDocument12 pagesKoagulasi CKDmuhammad RidoNo ratings yet
- Vascular Remodeling in Hypertension: Roles of Apoptosis, Inflammation, and FibrosisDocument8 pagesVascular Remodeling in Hypertension: Roles of Apoptosis, Inflammation, and FibrosisAmirul JannahNo ratings yet
- Renal Failure en 2Document103 pagesRenal Failure en 2ezz sharabyNo ratings yet
- Broodbank & Christian. Renal Tubular Disorders. 2018.Document10 pagesBroodbank & Christian. Renal Tubular Disorders. 2018.Jéssica Hilário BonomoNo ratings yet
- Rta PDFDocument4 pagesRta PDFtraceyNo ratings yet
- Liver CirrhosisDocument13 pagesLiver Cirrhosisshashi mittalNo ratings yet
- Embolization of Bronchial Arteries For The Treatment of HEMOPTYSIS. Update and Literature ReviewDocument20 pagesEmbolization of Bronchial Arteries For The Treatment of HEMOPTYSIS. Update and Literature ReviewHai SheikhNo ratings yet
- 04 Mar 1999 Renal-ReplacementDocument12 pages04 Mar 1999 Renal-ReplacementAFH12No ratings yet
- Difference Between Hematopoiesis and ErythropoiesisDocument7 pagesDifference Between Hematopoiesis and Erythropoiesisrsnsl7410No ratings yet
- Gap-Junction-Mediated Cell-To-Cell Communication: ReviewDocument11 pagesGap-Junction-Mediated Cell-To-Cell Communication: Reviewdornaz69No ratings yet
- Manov 2020Document8 pagesManov 2020kanaNo ratings yet
- Pietrangelo NEJM2004Document16 pagesPietrangelo NEJM2004Shahid HussainNo ratings yet
- Role of Ion Channels in The Mechanism of ProteinuriaDocument10 pagesRole of Ion Channels in The Mechanism of ProteinuriaM Dwi SuprayogiNo ratings yet
- Gut Microbiota Sustains Hematopoiesis - BloodDocument3 pagesGut Microbiota Sustains Hematopoiesis - BloodFlávia UchôaNo ratings yet
- Chapter 5Document22 pagesChapter 5diah apriliaNo ratings yet
- LIVER TRANSIENT HEPATIC ATTENUATION DEFECT ExplainedDocument6 pagesLIVER TRANSIENT HEPATIC ATTENUATION DEFECT Explainedcalustre2016No ratings yet
- Fistula ArteriovenosaDocument14 pagesFistula ArteriovenosaJoshua CajinaNo ratings yet
- Haemophilia A: A Review of Clinical Manifestations, Treatment, Mutations, and The Development of InhibitorsDocument21 pagesHaemophilia A: A Review of Clinical Manifestations, Treatment, Mutations, and The Development of InhibitorsGeorge VicolNo ratings yet
- Intengan Schiffrin 2001 Vascular Remodeling in HypertensionDocument7 pagesIntengan Schiffrin 2001 Vascular Remodeling in HypertensionManilkkaNo ratings yet
- Acta PhlebologicaDocument14 pagesActa PhlebologicaDomenico Feleppa100% (1)
- Portal Hypertension in Cirrhosis: Pathophysiological Mechanisms and TherapyDocument14 pagesPortal Hypertension in Cirrhosis: Pathophysiological Mechanisms and TherapyAisyaah imranNo ratings yet
- SYOK HipovolemikDocument31 pagesSYOK HipovolemikAmaliahHarumiKarimNo ratings yet
- Hematology & Blood Bank TechniqueDocument125 pagesHematology & Blood Bank Techniquemandawa786No ratings yet
- Serous Fluid AnalysisDocument4 pagesSerous Fluid AnalysisanonacadsNo ratings yet
- Coagulation Falanga 4Document1 pageCoagulation Falanga 4s3lki3No ratings yet
- First Aid For The USMLE Step 1 2020Document13 pagesFirst Aid For The USMLE Step 1 2020valerieefeNo ratings yet
- Pathology of The Urinary Tract (Handout)Document12 pagesPathology of The Urinary Tract (Handout)Razel PerezNo ratings yet
- Cirrhosis 2008. LANCETDocument14 pagesCirrhosis 2008. LANCETMaría José Klinger Del VillarNo ratings yet
- Diagnostic Approach To Inherited Bleeding Disorders: Clinical Chemistry and Laboratory Medicine February 2007Document12 pagesDiagnostic Approach To Inherited Bleeding Disorders: Clinical Chemistry and Laboratory Medicine February 2007Mohamed MounirNo ratings yet
- Portal BiliopathyDocument7 pagesPortal BiliopathyAsif.N.IqbalNo ratings yet
- 27.liver and Gastrointestinal PhysiologyDocument12 pages27.liver and Gastrointestinal PhysiologyAnggreany AshariNo ratings yet
- 0320 Histology II Consitt: o Dual Blood SupplyDocument8 pages0320 Histology II Consitt: o Dual Blood SupplyYvonne ChuehNo ratings yet
- Portal Hypertension PDFDocument2 pagesPortal Hypertension PDFKedar SainiNo ratings yet
- Acknowledgement ReceiptDocument21 pagesAcknowledgement ReceiptdeevoncNo ratings yet
- From Normal To Pathological HemostasisDocument10 pagesFrom Normal To Pathological HemostasisDsm DsmNo ratings yet
- Pries Et Al 1992 Redistribution of Red Blood Cell Flow in Microcirculatory Networks by HemodilutionDocument9 pagesPries Et Al 1992 Redistribution of Red Blood Cell Flow in Microcirculatory Networks by HemodilutionThomas PodgorskiNo ratings yet
- 3 - Hepato-Pancreato-Biliary Diseases 2.4 (2024)Document60 pages3 - Hepato-Pancreato-Biliary Diseases 2.4 (2024)R. nounNo ratings yet
- Dilatasi VenaDocument14 pagesDilatasi Venaworo winantiNo ratings yet
- Hierarchical Modeling of The Liver Vascular SystemDocument12 pagesHierarchical Modeling of The Liver Vascular SystemKofi DehNo ratings yet
- High Acuity Fluid and Electrolytes Chapter 25Document19 pagesHigh Acuity Fluid and Electrolytes Chapter 25AANo ratings yet
- Pathophysiology of Coagulopathy in Hematological.3Document7 pagesPathophysiology of Coagulopathy in Hematological.3Daniela Mihaela ConstantinNo ratings yet
- Materi Jurding AnestesiDocument8 pagesMateri Jurding AnestesiM.Farhan AkhyarNo ratings yet
- Haematopoiesis and Red Blood Cells 2013 Surgery OxfordDocument6 pagesHaematopoiesis and Red Blood Cells 2013 Surgery OxfordSanjaya SenevirathneNo ratings yet
- Regulation of Renal HemodynamicsDocument15 pagesRegulation of Renal HemodynamicsallerasicNo ratings yet
- Hepatic Segments and Vasculature - Projecting CT Anatomy Onto Angiograms1Document23 pagesHepatic Segments and Vasculature - Projecting CT Anatomy Onto Angiograms1Veronica AlexanderNo ratings yet
- Detecting Pediatric Malnutrition Webinar 2 Updated Feb 2020Document49 pagesDetecting Pediatric Malnutrition Webinar 2 Updated Feb 2020Novita ApramadhaNo ratings yet
- Linsenmeier 2020Document6 pagesLinsenmeier 2020Novita ApramadhaNo ratings yet
- Campmans-Kuijpers 2021 IBDDocument34 pagesCampmans-Kuijpers 2021 IBDNovita ApramadhaNo ratings yet
- John Hopkins IbdDocument38 pagesJohn Hopkins IbdNovita ApramadhaNo ratings yet
- Stanford IbdDocument37 pagesStanford IbdNovita ApramadhaNo ratings yet
- Using ProbioticDocument11 pagesUsing ProbioticNovita ApramadhaNo ratings yet
- Buku Panduan Kolitis UlseratifDocument17 pagesBuku Panduan Kolitis UlseratifNovita ApramadhaNo ratings yet
- Rayner 2006Document5 pagesRayner 2006Novita ApramadhaNo ratings yet
- Elevated BilirubinDocument5 pagesElevated BilirubinNovita ApramadhaNo ratings yet
- Zhao Glisirizin For Liver DiseaseDocument46 pagesZhao Glisirizin For Liver DiseaseNovita ApramadhaNo ratings yet
- Obs Jaundice AcceptedDocument8 pagesObs Jaundice AcceptedNovita ApramadhaNo ratings yet
- Penelitian GlyserizinDocument9 pagesPenelitian GlyserizinNovita ApramadhaNo ratings yet
- Gizi Pada IBDDocument15 pagesGizi Pada IBDNovita ApramadhaNo ratings yet
- Guideline Terbaru ACLFDocument28 pagesGuideline Terbaru ACLFNovita ApramadhaNo ratings yet
- Dispepsia Manajemen Dan TatalaksanaDocument8 pagesDispepsia Manajemen Dan TatalaksanaNovita ApramadhaNo ratings yet
- Therapy Insight: Gastrointestinal Complications of Diabetes-Pathophysiology and ManagementDocument10 pagesTherapy Insight: Gastrointestinal Complications of Diabetes-Pathophysiology and ManagementNovita ApramadhaNo ratings yet
- A Practical Approach To Gastrointestinal Complications of DiabetesDocument8 pagesA Practical Approach To Gastrointestinal Complications of DiabetesNovita ApramadhaNo ratings yet
- Guest Editorial: The Surgical NeonateDocument2 pagesGuest Editorial: The Surgical NeonateNovita ApramadhaNo ratings yet
- Nat Med 26, 1017-1032 (2020)Document2 pagesNat Med 26, 1017-1032 (2020)Novita ApramadhaNo ratings yet
- Liver Diseases Anesthesia Exam Notes by Dr. ZeeshanDocument99 pagesLiver Diseases Anesthesia Exam Notes by Dr. ZeeshanNida NazimNo ratings yet
- Gastrointestinal SystemDocument74 pagesGastrointestinal SystemakshitaNo ratings yet
- Valores Normales Eco HofferDocument3 pagesValores Normales Eco HofferViviana MartínezNo ratings yet
- Full Ebook of Color Atlas of Ultrasound Anatomy 2Nd Edition Berthold Block Online PDF All ChapterDocument69 pagesFull Ebook of Color Atlas of Ultrasound Anatomy 2Nd Edition Berthold Block Online PDF All Chaptermaxyjektonoporkins100% (5)
- Alcoholic Live DiseaseDocument53 pagesAlcoholic Live DiseaseKuldeep SinghNo ratings yet
- Medical MCQ Center - Liver Mcqs For Mccee, Aipgmee, Pgiee, Jipmer, Fmge, State PG EntranceDocument3 pagesMedical MCQ Center - Liver Mcqs For Mccee, Aipgmee, Pgiee, Jipmer, Fmge, State PG Entrancenarendrakumar94100% (1)
- DC-70 Specification Ver 1.0 20140808Document17 pagesDC-70 Specification Ver 1.0 20140808miljenkoNo ratings yet
- Portal HypertensionDocument25 pagesPortal HypertensionJainab SiddiquiNo ratings yet
- Identification Data:: Chief Complaioon of My ClientDocument30 pagesIdentification Data:: Chief Complaioon of My ClientNyagawa GodkNo ratings yet
- Human Metabolism by Michael PalmerDocument400 pagesHuman Metabolism by Michael PalmerPedro Henrique Cesar100% (1)
- Nihal Reddy CAHW-0000298026 M / 8M 23D Dr. Otclab - OeDocument25 pagesNihal Reddy CAHW-0000298026 M / 8M 23D Dr. Otclab - Oesandeep reddyNo ratings yet
- Liver 1Document35 pagesLiver 1Ebraheam HadiNo ratings yet
- NLR Blood Neutrophil To Lymphocyte Count As A Prognostic Marker in Liver Cirrhosis PDFDocument124 pagesNLR Blood Neutrophil To Lymphocyte Count As A Prognostic Marker in Liver Cirrhosis PDFDumitru RadulescuNo ratings yet
- Portal Hypertension - A Case ReportDocument65 pagesPortal Hypertension - A Case ReportTEAM K 1920No ratings yet
- Portal Vein - WikipediaDocument23 pagesPortal Vein - WikipediaNaqeeb AhmadNo ratings yet
- LECTURES Liver PathophysiologyDocument118 pagesLECTURES Liver PathophysiologyTarik100% (1)
- Liver and Portal Vein: 3 Major 3 Minor Couin 4 StructuresDocument3 pagesLiver and Portal Vein: 3 Major 3 Minor Couin 4 StructuresprashanthNo ratings yet
- Portal HypertensionDocument4 pagesPortal HypertensiondrstalamNo ratings yet
- 300719Document70 pages300719Vinod GuptaNo ratings yet
- Portal Hypertension and Ascites: Table 1Document7 pagesPortal Hypertension and Ascites: Table 1Hưng Nguyễn KiềuNo ratings yet
- Superior & Inferior Mesenteric Arteries Portal Vein & Its TributariesDocument19 pagesSuperior & Inferior Mesenteric Arteries Portal Vein & Its TributariesHevin GokulNo ratings yet
- Case Study CLD 3Document18 pagesCase Study CLD 3MoonNo ratings yet
- LI-RADS Lesiones HepáticasDocument24 pagesLI-RADS Lesiones HepáticasvictoriaNo ratings yet
- Portal SystemDocument11 pagesPortal Systemlitziya7894No ratings yet
- Diagnosis and Classification of Vascular Liver DisDocument16 pagesDiagnosis and Classification of Vascular Liver DisKata TölgyesiNo ratings yet
- Comparative Anatomy of Heart, Circulation Aortic Arches EtcDocument36 pagesComparative Anatomy of Heart, Circulation Aortic Arches EtcNarasimha Murthy100% (4)
- The Tamil Nadu Dr. M.G.R Medical University: Dissertation Submitted ToDocument129 pagesThe Tamil Nadu Dr. M.G.R Medical University: Dissertation Submitted ToAmmar AlnajjarNo ratings yet
- Khanna 2018Document20 pagesKhanna 2018wahid akbarNo ratings yet
- ESR Ebook For Undergraduate Education in Radiology - 08a Liver Imaging PDFDocument80 pagesESR Ebook For Undergraduate Education in Radiology - 08a Liver Imaging PDFANAS ALINo ratings yet
Jaundice How To Evaluate
Jaundice How To Evaluate
Uploaded by
Novita ApramadhaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Jaundice How To Evaluate
Jaundice How To Evaluate
Uploaded by
Novita ApramadhaCopyright:
Available Formats
Morphometric changes and imaging findings of diffuse liver disease in relation to intrahepatic hemodynamics
Kumi Ozaki1, Kazuto Kozaka2, Yasuo Kosaka2, Osamu Matsui2, Hirohiko Kimura1, Toshifumi Gabata2
Department of Radiology1, University of Fukui, Department of Human Pathology2 and Radiology3, Kanazawa University Graduate School of Medicine
Diffuse hepatic diseases have a variety of etiologies, and any underlying pathophysiologic condition affects the intrahepatic hemodynamics.
A temporal disorder of intrahepatic hemodynamics is compensated for by vascular communication, while long-term alteration of the
intrahepatic hemodynamics results in an increase in the total hepatic vascular resistance. Blood flow disorders induced by this increased
vascular resistance elicit hepatic cellular necrosis and fibrosis, and in the advanced stage, morphometric changes to the whole liver occur
due to the combination of selective atrophy and compensatory hypertrophy (atrophy-hypertrophy complex). In addition to the alteration
of microhemodynamics, several factors including the specific pathophysiology of each etiology affect the morphometric changes. Each
diffuse hepatic disease shows characteristic morphometric changes, and these changes are clearly depicted by CT and MR imaging.
In this presentation, we review each mico- or macro-intrahepatic hemodynamic factor and characteristic imaging findings of
morphometric changes and accompanying accessory findings of each etiology.
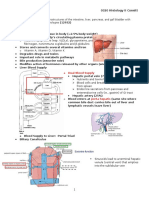
Blood flow of the liver Hepatic lobule and zonation Potential communications There are several potential communications between the
There is a heterogeneity of terminal hepatic arterioles and the terminal portal venules,
hepatocytes with respect to including
their metabolic activities, transsinusoidal routes
resulting in a zonal transversal routes (vasa vasorum of the portal vein)
differentiation of hepatocytes. transplexal routes (peribiliary plexus)
Which zone will When a vascular disorder occurs, these vascular
predominantly be affected communications play an important role in the balance of
depends on the etiology. blood perfusion to the liver parenchyma.
The normal liver receives a unique Blood flow from the hepatic artery and portal vein both drain
dual blood supply from the hepatic into the hepatic sinusoids, and collect in a central vein that
artery (30%) and portal vein (70%). drains into the hepatic vein.
Balance of blood perfusion
Hepatic perfusion disorders can be classified by their pathophysiological mechanisms involving the arterial inflow, portal venous inflow, hepatic
sinusoidal microcirculation, and hepatic venous outflow. The response is an important compensatory mechanism to maintain perfusion of the liver
parenchyma through an increase of hepatic arterial flow and a reduction of portal venous flow, which is known as the hepatic arterial buffer response.
Hepatic sinusoidal microcirculation disorder A variety of mediators (such as endotoxins) reach the liver and
The sinusoids are lined with endothelial cells and flanked by cause significant alterations in the regulation of blood flow. In the
plates of hepatocytes. The space between a hepatocyte and a response to liver injury, extensive changes occur in the sinusoids.
sinusoid is called the space of Disse, and contains hepatic Endothelin and nitric oxide (NO) are potent vasoconstricting and
stellate cells. Hepatic sinusoidal microcirculation disorder is relaxing agents, respectively, and are mainly related to sinusoidal
elicited by multiple conditions (systemic inflammation, drug vascular disorder (reduced portal venous inflow and hepatic blood flow. Other factors may also interact with the activation of
intake, etc.) in addition to the aforementioned direct hepatic venous outflow obstruction). stellate cells and sinusoidal blood flow..
Asymmetric and complicating morphometrical structures of the liver Central and peripheral zones In the area adjacent to the hepatic hilum (central
Histologically, the hepatic lobule has an identical structure throughout In the area adjacent to the hepatic surface zone), the portal blood flow is preserved through
the liver parenchyma. However, non-homogeneous morphometric (peripheral zone), the vascular resistance small collaterals, and the hepatic venous drainage is
alternation of the liver occurs due to the intricate morphology of the is higher, and the parenchyma can easily less affected due to the short distance. The area tends
liver and characteristics of the vascular anatomy. become atrophic. to show hypertrophy.
Anatomy and inhomogeneous blood content of portal vein Asymmetric hepatic venous anatomy and anatomical variation Atrophy-hypertrophy complex
Several factors influence the portal flow resistance and There are three main hepatic veins, that drain de-oxygenated blood into Any morphometric change is based on the
content, which might result in loss of the cytoplasmic the inferior vena cava (IVC). Several factors involve the difference of the atrophy-hypertrophy complex, which is the
volume of hepatocytes and consequent necrosis. venous outflow pressure, which has an association with the length and controlled restoration following hepatocyte loss.
diameter of Selective atrophy of the damaged area occurs
The hepatic initially, followed by compensatory hypertrophy
vein and the
volume of of other areas. Peripheral atrophy and central
drainage area. hypertrophy are the most significant.
Diffuse hepatic diseases
The parenchymal hepatic diseases presented in this review have been categorized as inflammatory, vascular, bile duct, and deposition-
and-storage diseases, depending on the initially-predominant pathophysiologic processes. Liver cirrhosis is the end stage of a wide variety
of chronic diffuse liver diseases. In addition, some other conditions can show similar morphological changes to cirrhosis (*).
Role of imaging
CT and MR imaging, thus play important roles in the evaluation of diffuse liver disease, offering supporting information on the following
factors in a non-invasive manner: configuration of the entire liver, nodular liver surface, regenerative nodules (size), anatomic relationships
between the liver and adjacent organs, fibrosis, fat and iron deposits, extrahepatic lesions.
Normal liver
Liver cirrhosis A
Cirrhosis
Cirrhosis is the end-stage disease of various chronic liver diseases, and is pathologically defined as multiple regenerative nodules surrounded by fibrous tissue.
Although fibrosis is a common change in chronic liver disease, its histological pattern varies depending on the underlying etiology (Table 1). Different fibrous
patterns result in different sizes of regenerative nodules, classified as macro-, micro-, and mixed-nodular cirrhosis based on the size of regenerative nodules.
B C D E Characteristic morphometric changes of cirrhosis commonly demonstrate atrophy of the right lobe
and medial segment and hypertrophy of the caudate lobe and lateral segment. The common
morphometric changes of cirrhosis have been expressed in various ways; such as modified caudate right lobe ratio ( 0.55
suggests cirrhosis) [A], expanded gallbladder fossa [B], enlarged periportal space [C], right posterior hepatic notch sign [D], and
selective atrophy of the MHV drainage area [E]. All of them reflect common characteristic morphometric changes. In addition,
nodularity of hepatic surface representing the regenerative nodules is specific for cirrhosis. The patterns of hepatic morphometric
changes overlap among different etiologies of cirrhosis and show common tendencies in their appearances, although there are some difference and specificity of each etiology.
Hepatitis virus-related disease Biliary diseases There are several biliary diseases at the Hepatic venous outflow obstruction Noncirrhotic chronic diffuse liver diseases
The fibrosis in viral hepatitis and autoimmune hepatitis Several noncirrhotic liver conditions can lead to morphologic changes mimicking
following different levels: Hepatic venous outflow obstruction can cirrhosis.
begin in periportal area (zone 1), eventually leading to Dilatation of peripheral
macronodular cirrhosis. 3
1. large intra- and extrahepatic bile ducts occur at the following levels: Congenital hepatic fibrosis intrahepatic bile ducts ( )
2
60-year-old man with Hepatitis B virus 60-year-old man with Hepatitis C virus related disease (PSC, recurrent pyogenic cholangitis) 1. the heart (congestive heart failure) -old female with congenital hepatic fibrosis
Medial segment is
related disease 2. medium sized bile ducts 2. IVC (Budd-Chiaris syndrome) normal sized or
1 enlarged ( )
The nodular (PSC, recurrent pyogenic cholangitis) 3. hepatic veins (Budd-Chiaris syndrome)
liver surface Atrophy of the
caused by 3. intrahepatic small bile ducts (PBC) 4. sinusoids and central vein right lobe and
regenerative
nodules tends 4 4. interlobular bile ducts (Alagille syndrome) (sinusoidal obstruction syndrome) hypertrophy of the
left lobe and the
× ×
to be distinct in
Interlobular
bile duct
×× Although these biliary diseases differ in Hepatic venous outflow obstruction leads Arterial phase CT Portal phase CT
caudate lobe MRCP
Central vein
hepatitis B
to atrophy of hepatocytes in zone 3, Idiopathic portal hypertension
Portal vein
×
virus-related × their clinical and imaging characteristics,
cirrhosis. ( )
Multiple hypovascular nodules spread whole liver,
Zone1
they commonly lead to cirrhosis. following fibrosis. The development of -old female -old female
Hepatocyte Central
which are regenerative nodules in macronodular Hepatic artery Sinusoid fibrous septa that characteristically bridge hypertrophy
cirrhosis.
Primary sclerosing cholangitis the central hepatic veins (reversed lobuli).
Autoimmune hepatitis -old male with PSC Congestive heart failure Relatively large
portal branches
-year-old man with autoimmune hepatitis (chronic phased after acute onset) are located in
-old female with congestive heart failure due to idiopathic pulmonary arterial hypertension the subcapsular
Irregularly atrophy and Non-segmental
Reflex of contrast material into hepatic veins -old female area( ).
confluent fibrosis (arrows) are
predominantly spread in lateral segment Equilibrium phase CT Fat Sat T2
Periportal halo sign ( )
and central area (arrows) .
Pseudocirrhosis
Dullness of hepatic edge (arrowheads) and -year-old woman with breast cancer (scirrhous carcinoma) has presented with hepatic metastasis,
central hypertrophy (asterisks) are and has treated with chemotherapy with shifting drug-regimen.
depicte( ) 3 years after mastectomy
Distinct hypertrophy of the caudate lobe ( ) Pruned tree appearance ( )
Recurrent pyogenic cholangitis Arterial phase CT Equilibrium phase CT
Alcoholic cirrhosis -old male with recurrent cholangitis
Atrophy of the lateral or posterior hepatic segments,
Dilated hepatic vein and IVC Mosaic pattern of enhancement ( ) Perivascular lymphedema ( )
Fibrosis in alcoholic hepatitis and NASH begins adjacent to
the central veins (zone 3), eventually leading to
hypertrophy of the caudate lobe ( )
Arterial phase CT images depict multiple arterioportal shunts, which
Budd-Chiaris syndrome Non contrast CT Non contrast CT Equilibrium phase CT
represent the intrahepatic cholangitis ( ). -old female -old female Deformities mimicking cirrhosis.
micronodular cirrhosis. Hypertrophy
-old female with PBC (stage 4)
of the Hepatic necrosis and regeneration after severe hepatitis
-old male -old male caudate lobe -year-old male with etiology unknown fulminant hepatic necrosis
Distinct hypertrophy of Periportal hyperintensity observed can
( ) Iron deposition
the caudate lobe ( ) be seen in any diffuse liver disease, but is
significantly more prevalent in cases with
Primary biliary cholangitis PBC ( ).
Intrahepatic
collateral
-old female with PBC (stage 4) (Child-Pugh class A) veins ( )
Entire enlargement of the The morphometric Equilibrium
Equilibrium phase
phase CT
CT Equilibrium phase CT
Spleen
liver due to abundant fibrosis changes of PBC resemble Diffuse, inhomogeneous, patchy enhancement ( ) Central hypertrophy and peripheral atrophy
is clearly depicted as ill- those seen in other forms Equilibrium phase CT Fat sat T2 Hepatobiliary phase
defined hypointensity.
Arterial phase CT
-old male
Hepatobiliary phase
-old male
of cirrhosis.
Lymphadenopathy ( )
Sinusoidal obstruction syndrome Non-segmental deformity Right lobe atrophy Obstruction of posterior branch of portal vein
Splenomegaly ( )
Hepatobiliary phase
-old male with colorectal hepatic metastasis after 6 cycles of FOLFOX
Before chemotherapy Multiple metastasis decrease in size.
Portal vein obstruction with Cavernous transformation
Portal phase CT
Portosystemic shunt -old male with portal vein obstruction
Nodularity of hepatic surface is not observed.
Alagille syndrome The dorsal part of the medial segment does not
-old female show atrophy.
The relatively large portal branch is located in
subcapsular area ( ) Metastasis
Portal phase CT Fat sat T2WI Portal phase CT
The area of hypertrophy or hyperplasia shows slightly Central hypertrophy ( )
Confluent hepatic fibrosis (arrows) Obscure expanded gallbladder fossa sign
hyperintense compared with the background liver ( ). Portal phase CT Hepatobiliary phase Hepatobiliary phase Hepatobiliary phase Reticular hypointensity Portal phase CT
Conclusion Morphometric changes in diffuse liver diseases are related to several factors including micro-and macro- hemodynamics. Each diffuse hepatic disease shows characteristic
You might also like
- Fluid, Electrolyte, and Acid-Base Disorders Robert B Schonberger 2018Document18 pagesFluid, Electrolyte, and Acid-Base Disorders Robert B Schonberger 2018Akhmad Fadhiel Noor100% (1)
- Anatomy, Lecture 12, Blood Supply of The Gastrointestinal Tract (Slides)Document27 pagesAnatomy, Lecture 12, Blood Supply of The Gastrointestinal Tract (Slides)Ali Al-Qudsi100% (1)
- LIVERDocument24 pagesLIVERFredIanBantolioNo ratings yet
- Transient Hepatic Attenuation DifferencesDocument6 pagesTransient Hepatic Attenuation Differencesademar milton de souza filhoNo ratings yet
- Puentes Portosistémicos: Reporte de Un Caso ClínicoDocument5 pagesPuentes Portosistémicos: Reporte de Un Caso ClínicobumetroNo ratings yet
- Diagnosis and Classification of Vascular Liver DisDocument16 pagesDiagnosis and Classification of Vascular Liver DisKata TölgyesiNo ratings yet
- Veterinary Internal Medicne - March 1993 - Leveille - Pathophysiology and Pharmacologic Modulation of Hepatic FibrosisDocument13 pagesVeterinary Internal Medicne - March 1993 - Leveille - Pathophysiology and Pharmacologic Modulation of Hepatic FibrosisAna Paula SeminarioNo ratings yet
- Glomerular Filtration: An Overview: Mary Jo HolechekDocument8 pagesGlomerular Filtration: An Overview: Mary Jo HolechekagaasfaNo ratings yet
- Imagen Hepática Vascular Hígado 2022Document15 pagesImagen Hepática Vascular Hígado 2022Oscar BushNo ratings yet
- Tubular Transport 2010Document27 pagesTubular Transport 2010Maria Fernanda Pineda FëirethdNo ratings yet
- Portal Hypertension: Historical BackgroundDocument15 pagesPortal Hypertension: Historical BackgroundDiego Andres Garcia RochaNo ratings yet
- Review: Hematopoiesis: An Evolving Paradigm For Stem Cell BiologyDocument14 pagesReview: Hematopoiesis: An Evolving Paradigm For Stem Cell Biologywildan pragaNo ratings yet
- Mechanisms of Disease: V - B - S H H SDocument10 pagesMechanisms of Disease: V - B - S H H SMedranoReyesLuisinNo ratings yet
- Astra-Module-38 Pedia NephroDocument20 pagesAstra-Module-38 Pedia NephroenzocruzinNo ratings yet
- The Anatomical Record - 2020 - Mak - Hepatic Sinusoids Versus Central Veins Structures Markers Angiocrines and Roles inDocument31 pagesThe Anatomical Record - 2020 - Mak - Hepatic Sinusoids Versus Central Veins Structures Markers Angiocrines and Roles inKata TölgyesiNo ratings yet
- Koagulasi CKDDocument12 pagesKoagulasi CKDmuhammad RidoNo ratings yet
- Vascular Remodeling in Hypertension: Roles of Apoptosis, Inflammation, and FibrosisDocument8 pagesVascular Remodeling in Hypertension: Roles of Apoptosis, Inflammation, and FibrosisAmirul JannahNo ratings yet
- Renal Failure en 2Document103 pagesRenal Failure en 2ezz sharabyNo ratings yet
- Broodbank & Christian. Renal Tubular Disorders. 2018.Document10 pagesBroodbank & Christian. Renal Tubular Disorders. 2018.Jéssica Hilário BonomoNo ratings yet
- Rta PDFDocument4 pagesRta PDFtraceyNo ratings yet
- Liver CirrhosisDocument13 pagesLiver Cirrhosisshashi mittalNo ratings yet
- Embolization of Bronchial Arteries For The Treatment of HEMOPTYSIS. Update and Literature ReviewDocument20 pagesEmbolization of Bronchial Arteries For The Treatment of HEMOPTYSIS. Update and Literature ReviewHai SheikhNo ratings yet
- 04 Mar 1999 Renal-ReplacementDocument12 pages04 Mar 1999 Renal-ReplacementAFH12No ratings yet
- Difference Between Hematopoiesis and ErythropoiesisDocument7 pagesDifference Between Hematopoiesis and Erythropoiesisrsnsl7410No ratings yet
- Gap-Junction-Mediated Cell-To-Cell Communication: ReviewDocument11 pagesGap-Junction-Mediated Cell-To-Cell Communication: Reviewdornaz69No ratings yet
- Manov 2020Document8 pagesManov 2020kanaNo ratings yet
- Pietrangelo NEJM2004Document16 pagesPietrangelo NEJM2004Shahid HussainNo ratings yet
- Role of Ion Channels in The Mechanism of ProteinuriaDocument10 pagesRole of Ion Channels in The Mechanism of ProteinuriaM Dwi SuprayogiNo ratings yet
- Gut Microbiota Sustains Hematopoiesis - BloodDocument3 pagesGut Microbiota Sustains Hematopoiesis - BloodFlávia UchôaNo ratings yet
- Chapter 5Document22 pagesChapter 5diah apriliaNo ratings yet
- LIVER TRANSIENT HEPATIC ATTENUATION DEFECT ExplainedDocument6 pagesLIVER TRANSIENT HEPATIC ATTENUATION DEFECT Explainedcalustre2016No ratings yet
- Fistula ArteriovenosaDocument14 pagesFistula ArteriovenosaJoshua CajinaNo ratings yet
- Haemophilia A: A Review of Clinical Manifestations, Treatment, Mutations, and The Development of InhibitorsDocument21 pagesHaemophilia A: A Review of Clinical Manifestations, Treatment, Mutations, and The Development of InhibitorsGeorge VicolNo ratings yet
- Intengan Schiffrin 2001 Vascular Remodeling in HypertensionDocument7 pagesIntengan Schiffrin 2001 Vascular Remodeling in HypertensionManilkkaNo ratings yet
- Acta PhlebologicaDocument14 pagesActa PhlebologicaDomenico Feleppa100% (1)
- Portal Hypertension in Cirrhosis: Pathophysiological Mechanisms and TherapyDocument14 pagesPortal Hypertension in Cirrhosis: Pathophysiological Mechanisms and TherapyAisyaah imranNo ratings yet
- SYOK HipovolemikDocument31 pagesSYOK HipovolemikAmaliahHarumiKarimNo ratings yet
- Hematology & Blood Bank TechniqueDocument125 pagesHematology & Blood Bank Techniquemandawa786No ratings yet
- Serous Fluid AnalysisDocument4 pagesSerous Fluid AnalysisanonacadsNo ratings yet
- Coagulation Falanga 4Document1 pageCoagulation Falanga 4s3lki3No ratings yet
- First Aid For The USMLE Step 1 2020Document13 pagesFirst Aid For The USMLE Step 1 2020valerieefeNo ratings yet
- Pathology of The Urinary Tract (Handout)Document12 pagesPathology of The Urinary Tract (Handout)Razel PerezNo ratings yet
- Cirrhosis 2008. LANCETDocument14 pagesCirrhosis 2008. LANCETMaría José Klinger Del VillarNo ratings yet
- Diagnostic Approach To Inherited Bleeding Disorders: Clinical Chemistry and Laboratory Medicine February 2007Document12 pagesDiagnostic Approach To Inherited Bleeding Disorders: Clinical Chemistry and Laboratory Medicine February 2007Mohamed MounirNo ratings yet
- Portal BiliopathyDocument7 pagesPortal BiliopathyAsif.N.IqbalNo ratings yet
- 27.liver and Gastrointestinal PhysiologyDocument12 pages27.liver and Gastrointestinal PhysiologyAnggreany AshariNo ratings yet
- 0320 Histology II Consitt: o Dual Blood SupplyDocument8 pages0320 Histology II Consitt: o Dual Blood SupplyYvonne ChuehNo ratings yet
- Portal Hypertension PDFDocument2 pagesPortal Hypertension PDFKedar SainiNo ratings yet
- Acknowledgement ReceiptDocument21 pagesAcknowledgement ReceiptdeevoncNo ratings yet
- From Normal To Pathological HemostasisDocument10 pagesFrom Normal To Pathological HemostasisDsm DsmNo ratings yet
- Pries Et Al 1992 Redistribution of Red Blood Cell Flow in Microcirculatory Networks by HemodilutionDocument9 pagesPries Et Al 1992 Redistribution of Red Blood Cell Flow in Microcirculatory Networks by HemodilutionThomas PodgorskiNo ratings yet
- 3 - Hepato-Pancreato-Biliary Diseases 2.4 (2024)Document60 pages3 - Hepato-Pancreato-Biliary Diseases 2.4 (2024)R. nounNo ratings yet
- Dilatasi VenaDocument14 pagesDilatasi Venaworo winantiNo ratings yet
- Hierarchical Modeling of The Liver Vascular SystemDocument12 pagesHierarchical Modeling of The Liver Vascular SystemKofi DehNo ratings yet
- High Acuity Fluid and Electrolytes Chapter 25Document19 pagesHigh Acuity Fluid and Electrolytes Chapter 25AANo ratings yet
- Pathophysiology of Coagulopathy in Hematological.3Document7 pagesPathophysiology of Coagulopathy in Hematological.3Daniela Mihaela ConstantinNo ratings yet
- Materi Jurding AnestesiDocument8 pagesMateri Jurding AnestesiM.Farhan AkhyarNo ratings yet
- Haematopoiesis and Red Blood Cells 2013 Surgery OxfordDocument6 pagesHaematopoiesis and Red Blood Cells 2013 Surgery OxfordSanjaya SenevirathneNo ratings yet
- Regulation of Renal HemodynamicsDocument15 pagesRegulation of Renal HemodynamicsallerasicNo ratings yet
- Hepatic Segments and Vasculature - Projecting CT Anatomy Onto Angiograms1Document23 pagesHepatic Segments and Vasculature - Projecting CT Anatomy Onto Angiograms1Veronica AlexanderNo ratings yet
- Detecting Pediatric Malnutrition Webinar 2 Updated Feb 2020Document49 pagesDetecting Pediatric Malnutrition Webinar 2 Updated Feb 2020Novita ApramadhaNo ratings yet
- Linsenmeier 2020Document6 pagesLinsenmeier 2020Novita ApramadhaNo ratings yet
- Campmans-Kuijpers 2021 IBDDocument34 pagesCampmans-Kuijpers 2021 IBDNovita ApramadhaNo ratings yet
- John Hopkins IbdDocument38 pagesJohn Hopkins IbdNovita ApramadhaNo ratings yet
- Stanford IbdDocument37 pagesStanford IbdNovita ApramadhaNo ratings yet
- Using ProbioticDocument11 pagesUsing ProbioticNovita ApramadhaNo ratings yet
- Buku Panduan Kolitis UlseratifDocument17 pagesBuku Panduan Kolitis UlseratifNovita ApramadhaNo ratings yet
- Rayner 2006Document5 pagesRayner 2006Novita ApramadhaNo ratings yet
- Elevated BilirubinDocument5 pagesElevated BilirubinNovita ApramadhaNo ratings yet
- Zhao Glisirizin For Liver DiseaseDocument46 pagesZhao Glisirizin For Liver DiseaseNovita ApramadhaNo ratings yet
- Obs Jaundice AcceptedDocument8 pagesObs Jaundice AcceptedNovita ApramadhaNo ratings yet
- Penelitian GlyserizinDocument9 pagesPenelitian GlyserizinNovita ApramadhaNo ratings yet
- Gizi Pada IBDDocument15 pagesGizi Pada IBDNovita ApramadhaNo ratings yet
- Guideline Terbaru ACLFDocument28 pagesGuideline Terbaru ACLFNovita ApramadhaNo ratings yet
- Dispepsia Manajemen Dan TatalaksanaDocument8 pagesDispepsia Manajemen Dan TatalaksanaNovita ApramadhaNo ratings yet
- Therapy Insight: Gastrointestinal Complications of Diabetes-Pathophysiology and ManagementDocument10 pagesTherapy Insight: Gastrointestinal Complications of Diabetes-Pathophysiology and ManagementNovita ApramadhaNo ratings yet
- A Practical Approach To Gastrointestinal Complications of DiabetesDocument8 pagesA Practical Approach To Gastrointestinal Complications of DiabetesNovita ApramadhaNo ratings yet
- Guest Editorial: The Surgical NeonateDocument2 pagesGuest Editorial: The Surgical NeonateNovita ApramadhaNo ratings yet
- Nat Med 26, 1017-1032 (2020)Document2 pagesNat Med 26, 1017-1032 (2020)Novita ApramadhaNo ratings yet
- Liver Diseases Anesthesia Exam Notes by Dr. ZeeshanDocument99 pagesLiver Diseases Anesthesia Exam Notes by Dr. ZeeshanNida NazimNo ratings yet
- Gastrointestinal SystemDocument74 pagesGastrointestinal SystemakshitaNo ratings yet
- Valores Normales Eco HofferDocument3 pagesValores Normales Eco HofferViviana MartínezNo ratings yet
- Full Ebook of Color Atlas of Ultrasound Anatomy 2Nd Edition Berthold Block Online PDF All ChapterDocument69 pagesFull Ebook of Color Atlas of Ultrasound Anatomy 2Nd Edition Berthold Block Online PDF All Chaptermaxyjektonoporkins100% (5)
- Alcoholic Live DiseaseDocument53 pagesAlcoholic Live DiseaseKuldeep SinghNo ratings yet
- Medical MCQ Center - Liver Mcqs For Mccee, Aipgmee, Pgiee, Jipmer, Fmge, State PG EntranceDocument3 pagesMedical MCQ Center - Liver Mcqs For Mccee, Aipgmee, Pgiee, Jipmer, Fmge, State PG Entrancenarendrakumar94100% (1)
- DC-70 Specification Ver 1.0 20140808Document17 pagesDC-70 Specification Ver 1.0 20140808miljenkoNo ratings yet
- Portal HypertensionDocument25 pagesPortal HypertensionJainab SiddiquiNo ratings yet
- Identification Data:: Chief Complaioon of My ClientDocument30 pagesIdentification Data:: Chief Complaioon of My ClientNyagawa GodkNo ratings yet
- Human Metabolism by Michael PalmerDocument400 pagesHuman Metabolism by Michael PalmerPedro Henrique Cesar100% (1)
- Nihal Reddy CAHW-0000298026 M / 8M 23D Dr. Otclab - OeDocument25 pagesNihal Reddy CAHW-0000298026 M / 8M 23D Dr. Otclab - Oesandeep reddyNo ratings yet
- Liver 1Document35 pagesLiver 1Ebraheam HadiNo ratings yet
- NLR Blood Neutrophil To Lymphocyte Count As A Prognostic Marker in Liver Cirrhosis PDFDocument124 pagesNLR Blood Neutrophil To Lymphocyte Count As A Prognostic Marker in Liver Cirrhosis PDFDumitru RadulescuNo ratings yet
- Portal Hypertension - A Case ReportDocument65 pagesPortal Hypertension - A Case ReportTEAM K 1920No ratings yet
- Portal Vein - WikipediaDocument23 pagesPortal Vein - WikipediaNaqeeb AhmadNo ratings yet
- LECTURES Liver PathophysiologyDocument118 pagesLECTURES Liver PathophysiologyTarik100% (1)
- Liver and Portal Vein: 3 Major 3 Minor Couin 4 StructuresDocument3 pagesLiver and Portal Vein: 3 Major 3 Minor Couin 4 StructuresprashanthNo ratings yet
- Portal HypertensionDocument4 pagesPortal HypertensiondrstalamNo ratings yet
- 300719Document70 pages300719Vinod GuptaNo ratings yet
- Portal Hypertension and Ascites: Table 1Document7 pagesPortal Hypertension and Ascites: Table 1Hưng Nguyễn KiềuNo ratings yet
- Superior & Inferior Mesenteric Arteries Portal Vein & Its TributariesDocument19 pagesSuperior & Inferior Mesenteric Arteries Portal Vein & Its TributariesHevin GokulNo ratings yet
- Case Study CLD 3Document18 pagesCase Study CLD 3MoonNo ratings yet
- LI-RADS Lesiones HepáticasDocument24 pagesLI-RADS Lesiones HepáticasvictoriaNo ratings yet
- Portal SystemDocument11 pagesPortal Systemlitziya7894No ratings yet
- Diagnosis and Classification of Vascular Liver DisDocument16 pagesDiagnosis and Classification of Vascular Liver DisKata TölgyesiNo ratings yet
- Comparative Anatomy of Heart, Circulation Aortic Arches EtcDocument36 pagesComparative Anatomy of Heart, Circulation Aortic Arches EtcNarasimha Murthy100% (4)
- The Tamil Nadu Dr. M.G.R Medical University: Dissertation Submitted ToDocument129 pagesThe Tamil Nadu Dr. M.G.R Medical University: Dissertation Submitted ToAmmar AlnajjarNo ratings yet
- Khanna 2018Document20 pagesKhanna 2018wahid akbarNo ratings yet
- ESR Ebook For Undergraduate Education in Radiology - 08a Liver Imaging PDFDocument80 pagesESR Ebook For Undergraduate Education in Radiology - 08a Liver Imaging PDFANAS ALINo ratings yet